

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (11): 2418.doi: 10.7503/cjcu20140420
• Physical Chemistry • Previous Articles Next Articles
TANG Rongzhi1,2, WANG Songlin1,2, ZHANG Yuanzhuo1,2, CHEN Tong1,*( ), WANG Gongying1
), WANG Gongying1
Received:2014-05-04
Online:2014-11-10
Published:2014-10-09
Contact:
CHEN Tong
E-mail:chentongw@sina.com.cn
Supported by:CLC Number:
TrendMD:
TANG Rongzhi, WANG Songlin, ZHANG Yuanzhuo, CHEN Tong, WANG Gongying. Catalytic Property of Titanyl Acetate in the Transesterification Reaction of Dimethyl Carbonate and Phenol†[J]. Chem. J. Chinese Universities, 2014, 35(11): 2418.
| Mass of catalyst/g | Conversion of phenol (%) | Selectivity of transesterification (%) | Yield (%) | ||
|---|---|---|---|---|---|
| MPC | DPC | AN | |||
| 0.05 | 42.2 | 99.9 | 22.3 | 19.9 | trace |
| 0.10 | 47.8 | 99.9 | 25.6 | 22.2 | trace |
| 0.15 | 47.7 | 99.9 | 25.3 | 22.4 | trace |
| 0.20 | 48.4 | 99.9 | 24.7 | 23.7 | trace |
| 0.40 | 47.1 | 99.9 | 23.3 | 23.8 | trace |
Table 1 Catalytic performance of titanyl acetate on the transesterification of DMC and phenol*
| Mass of catalyst/g | Conversion of phenol (%) | Selectivity of transesterification (%) | Yield (%) | ||
|---|---|---|---|---|---|
| MPC | DPC | AN | |||
| 0.05 | 42.2 | 99.9 | 22.3 | 19.9 | trace |
| 0.10 | 47.8 | 99.9 | 25.6 | 22.2 | trace |
| 0.15 | 47.7 | 99.9 | 25.3 | 22.4 | trace |
| 0.20 | 48.4 | 99.9 | 24.7 | 23.7 | trace |
| 0.40 | 47.1 | 99.9 | 23.3 | 23.8 | trace |
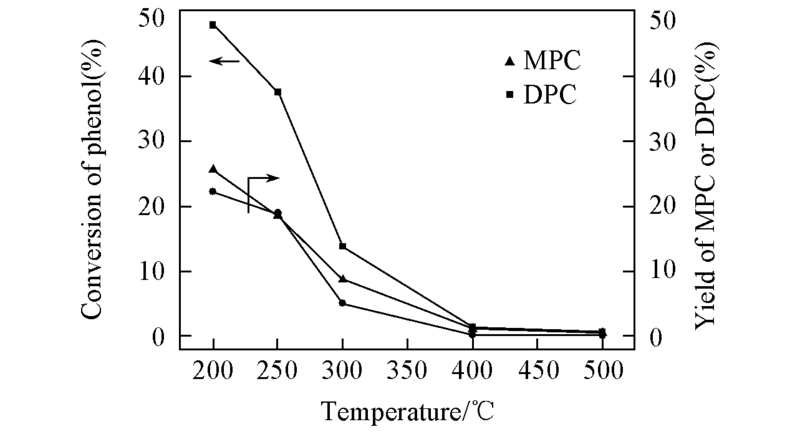
Fig.5 Effect of calcined temperature on catalytic performance of titanyl acetate Reaction condition: 160 mmol phenol, 160 mmol DMC, 0.10 g catalyst, 9 h, 150—180 ℃.
| Running time | Conversion of phenol (%) | Selectivity of transesterification (%) | Yield (%) | ||
|---|---|---|---|---|---|
| MPC | DPC | AN | |||
| 1 | 47.8 | 99.9 | 25.6 | 22.2 | trace |
| 2 | 47.5 | 99.9 | 23.2 | 24.3 | trace |
| 3 | 45.0 | 99.9 | 22.7 | 22.3 | trace |
Table 2 Reusability of catalyst*
| Running time | Conversion of phenol (%) | Selectivity of transesterification (%) | Yield (%) | ||
|---|---|---|---|---|---|
| MPC | DPC | AN | |||
| 1 | 47.8 | 99.9 | 25.6 | 22.2 | trace |
| 2 | 47.5 | 99.9 | 23.2 | 24.3 | trace |
| 3 | 45.0 | 99.9 | 22.7 | 22.3 | trace |
| [1] | Fukuoka S., Kawamura M., Komiya K., Tojo M., Hachiya H., Hasegawa K., Aminaka M., Okamoto H., Fukawa I., Konno S., Green. Chem., 2003, 5(5), 497—507 |
| [2] | Gong J. L., Ma X. B., Wang S. P., Appl. Catal. A: Gen., 2007 , 316(1), 1—21 |
| [3] | Shaikh A. G., Sivaram S., Chem. Rev., 1996, 96(3), 951—976 |
| [4] | Niu H. Y., Yao J., Wang Y., Wang G. Y., J. Mol. Catal. A: Chem., 2005, 235(1/2), 240—243 |
| [5] | Du Z. P., Kang W. K., Chen T., Yao J., Wang G. Y., J. Mol. Catal. A: Chem., 2006, 246(1/2), 200—205 |
| [6] | Lee H., Kim S. J., Ahn B. S., Lee W. K., Kim H. S., Catal. Today,2003, 87(1/4), 139—144 |
| [7] | Lee H., Bae J. Y., Kwon O. S., Kim S. J., Lee S. D., Kim H. S.M., J. Organometal. Chem., 2004, 689(10), 1816—1820 |
| [8] | Kim W. B., Lee J. S., Catal. Lett., 1999, 59(1), 83—88 |
| [9] | Zhou W. Q., Zhao X. Q., Wang Y. J., Zhang J. Y., Appl. Catal. A: Gen., 2004, 260(1), 19—24 |
| [10] | Tong D. S., Yao J., Wang Y., Niu H. Y., Wang G. Y., J. Mol. Catal. A: Chem., 2007, 268(1/2), 120—126 |
| [11] | Kim Y. T., Park E. D., Appl. Catal. A: Gen., 2009, 356(2), 211—215 |
| [12] | Zhou W. Q., Zhao X. Q., Wang S. F., Wang Y. J., J. Sichuan Univ.(Eng. Sci. Edition), 2002, 34(5), 39—41 |
| (周炜清, 赵新强, 王淑芳, 王延吉. 四川大学学报(工程科学版), 2002, 34(5), 39—41) | |
| [13] | Tang R. Z., Chen T., Chen Y., Zhang Y. Z., Wang G. Y., Chin. J. Catal., 2014, 35(4), 457—461 |
| (唐荣芝, 陈彤, 陈勇, 张元卓, 王公应. 催化学报, 2014, 35(4), 457—461) | |
| [14] | Luo S. W., Chen T., Tong D. S., Zeng Y., Lei Y. C., Wang G. Y., Chin. J. Catal., 2007, 28(11), 937—939 |
| (罗淑文, 陈彤, 童东绅, 曾毅, 雷永诚, 王公应. 催化学报, 2007, 28(11), 937—939) | |
| [15] | Li Z. H., Cheng B. W., Su K. M., Gu Y., Xi P., Guo M. L., J. Mol. Catal. A: Chem., 2008, 289(1/2), 100—105 |
| [16] | Mei F. M., Pei Z., Li G. X., Org. Process Des. Dev., 2004, 8(3), 372—375 |
| [17] | Wang S., Bai R. X., Mei F. M., Li G. X., Catal. Commun., 2009, 11(3), 202—205 |
| [18] | Chen T., Han H. J., Du Z. P., Yao J., Wang G. Y., Shi D. C., Zhang D. S., Chen Z. M., J. Nat. Gas. Chem., 2006, 15(4), 303—306 |
| [19] | Han H. J., Chen T., Yao J., Wang G. Y., Chin. J. Catal., 2006, 27(1), 7—8 |
| (韩华俊, 陈彤, 姚洁, 王公应. 催化学报, 2006, 27(1), 7—8) | |
| [20] | Chen T., Han H. J., Yao J., Wang G. Y., Catal. Commun., 2007, 8(9), 1361—1365 |
| [21] | Urlaub R., Posset U., Thull R., J. Non-Crystal. Solids., 2000, 265(3), 276—284 |
| [22] | Deacon G. B., Phillips R. J., Coord. Chem. Rev., 1980, 33(3), 227—250 |
| [23] | Griffith W.P.,J. Chem. Soc., 1964, 5248—5253 |
| [24] | Kalaivani D., Malarvizhi R., Nethaji M., J. Chem. Crystal., 2012, 42(11), 1098—1104 |
| [25] | Hsu S. T., Lin W. C., Hsiao W. F., Lee C. C., Pan T. C., Wang T. T., Huang Y. M., J. Appl. Polym. Sci., 2013, 127(1), 760—764 |
| [26] | Chen X. Q., Gu G. B., Liu H. B., Acta Chim. Sinica,2003, 61(10), 1592—1596 |
| (陈小泉, 古国榜, 刘焕斌. 化学学报,2003, 61(10), 1592—1596) | |
| [27] | Smith G. D., Caughlan C. N., Campbell J. A., Inorg. Chem., 1972, 11(12), 2989—2993 |
| [28] | Shen H. L., Hu H. H., Liang D. Y., Meng H. L., Li P. G., Tang W. H., Cui C., J. Alloys Comp., 2012, 542, 32—36 |
| [29] | Li B. J., Tang R. Z., Chen T., Wang G. Y., Chin. J. Catal., 2012, 33(4), 601—604 |
| (李碧静, 唐荣芝, 陈彤, 王公应. 催化学报, 2012, 33(4), 601—604) | |
| [30] | Fu Z. H., Ono Y., J. Mol. Catal. A: Chem., 1997, 118(3), 293—299 |
| [1] | ZHAO Yongmei, MU Yeshu, HONG Chen, LUO Wen, TIAN Zhiyong. Bis-naphthalimide Derivatives for Picronitric Acid Detection in Aqueous Solution [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210765. |
| [2] | ZHOU Ning, TANG Xiaohua, CAO Hong, ZHA Fei, LI Chun, XIE Chunyan, XU Mingping, SUN Yige. Preparation, Characterization and Degradation to BPA of Pomegranate-like Gel Microsphere Entrapmented Laccase [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210705. |
| [3] | YU Jing, WU Chao, LI Chenyang, CHEN Danfeng, DING Liuyue, MA Xiantao. Catalyst-free and Highly Efficient O-Silylation of Alcohols and Phenols [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210588. |
| [4] | MA Yukun, JIN Hui, REN Chuanli, LI Zhibo. Ring-opening Polymerization of Cyclic Esters Using Recyclable Polystyrene Supported Urea-Base Binary Catalyst [J]. Chem. J. Chinese Universities, 2021, 42(9): 2968. |
| [5] | PENG Xiaoming, WU Jianqun, DAI Hongling, YANG Zhanhong, XU Li, XU Gaoping, HU Fengping. Activation of Peroxymonosulfate by Single Atom Catalysts Ni⁃N⁃C for High Efficiency Degradation of Phenol [J]. Chem. J. Chinese Universities, 2021, 42(8): 2581. |
| [6] | WANG Man, WANG Xin, ZHOU Jing, GAO Guohua. Efficient Synthesis of Dimethyl Carbonate via Transesterification of Methanol and Ethylene Carbonate Catalyzed by Poly(ionic liquid)s [J]. Chem. J. Chinese Universities, 2021, 42(12): 3701. |
| [7] | CHEN Chen, LU Xin, ZHAO Yudi, WANG Litong, XIN Zhong. Preparation and Properties of 3-Methylcatechol/Furfurylamine Based Polybenzoxazine [J]. Chem. J. Chinese Universities, 2021, 42(12): 3757. |
| [8] | SHA Xinyi, ZHANG Changxu, WANG Yuling, ZHOU Yongfeng. High-performance Adhesive Material at Dry and Under-seawater Conditions Based on Catechol-functionalized Alternating Copolymer† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1477. |
| [9] | XIONG Junyu, WANG Shanshan, XU Yanqing, HU Changwen. Selective Oxidation of Atomically Dispersed Fe-N-C Catalyst Under Mild Conditions † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1262. |
| [10] | SHEN Yang, ZHU Fang, SHEN Wanwan, FAN Qianqian, LI Yiwen, CHENG Yiyun. Structure-function Relationship of Plant Polyphenols for Promoted siRNA Delivery † [J]. Chem. J. Chinese Universities, 2020, 41(4): 633. |
| [11] | MENG Fanxing, YU Zhiquan, JING Wenwen, WANG Yao, SUN Zhichao, WANG Anjie. Promoting Effect of Cerous Phosphate on the Phenol Catalytic Transfer Hydrogenation over Nickel Phosphide Catalyst † [J]. Chem. J. Chinese Universities, 2020, 41(4): 765. |
| [12] |
HAN Hongjing,GE Qin,CHEN Yanguang,WANG Haiying,ZHAO Hongzhi,WANG Yizhen,ZHANG Yanan,DENG Jitong,SONG Hua,ZHANG Mei.
Production of Phenolic Compounds from Bagasse Lignin via Catalytic Pyrolysis of Ca1-xPrxFe |
| [13] | ZHANG Hui, ZHANG Chenjie, XU Minmin, YUAN Yaxian, YAO Jianlin. Investigation on the Reaction of o-Aminothiophenol and 2-Iodobenzoyl Chloride Monitored by SERS-HPLC Technique [J]. Chem. J. Chinese Universities, 2020, 41(11): 2496. |
| [14] | YAN Song, ZHANG Chengwu, YUAN Fang, QIN Chuanyu. Synthesis of Nitrogen-doped Carbon Nanotubes and the Performance and Mechanism of PMS Activation [J]. Chem. J. Chinese Universities, 2020, 41(11): 2503. |
| [15] | WANG Nan,YAO Kaisheng,ZHAO Chenchen,LI Tianjin,LU Weiwei. Ionic Liquid-assisted Synthesis of AuPd Nanosponges and Their Catalytic Performance † [J]. Chem. J. Chinese Universities, 2020, 41(1): 62. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||