

高等学校化学学报 ›› 2025, Vol. 46 ›› Issue (8): 20250067.doi: 10.7503/cjcu20250067
罗淑芳1, 赵远进1, 王硕1, 周润川2, 杨霞2, 贺爱华1( )
)
收稿日期:2025-03-07
出版日期:2025-08-10
发布日期:2025-05-19
通讯作者:
贺爱华
E-mail:ahhe@qust.edu.cn
基金资助:
LUO Shufang1, ZHAO Yuanjin1, WANG Shuo1, ZHOU Runchaun2, YANG Xia2, HE Aihua1( )
)
Received:2025-03-07
Online:2025-08-10
Published:2025-05-19
Contact:
HE Aihua
E-mail:ahhe@qust.edu.cn
Supported by:摘要:
端基官能化橡胶在改善填料分散性, 增加橡胶与填料之间相互作用力, 进而影响橡胶复合材料性能方面发挥重要作用. 本文采用非均相TiCl4/MgCl2型Ziegler-Natta催化剂, 以二环己胺(DCHA)为链转移剂, 通过配位链转移聚合法一步合成了组成和结构可控的胺端基官能化的高反式-1,4-丁二烯-异戊二烯共聚橡胶 (F-TBIR). 研究了DCHA和助催化剂三乙基铝(AlEt3)的用量对催化活性、 胺端基官能化效率(CE, %)和F-TBIR链微观结构的影响. 结果表明, DCHA不改变催化剂的定向能力, DCHA用量增加时, 催化活性及聚合物的分子量降低, CE显著提高; 随着AlEt3用量增加, 催化活性先增加后降低, CE与聚合物分子量均逐渐降低. 计算得到本文实验条件下DCHA的链转移常数为0.0537, AlEt3的链转移常数为0.016. 结合密度泛函理论(DFT)模拟, 讨论了DCHA和AlEt3在非均相Ziegler-Natta催化剂催化二烯烃配位聚合中的链转移机理, 为制备端基官能化的合成橡胶提供了一种简便可行的策略.
中图分类号:
TrendMD:
罗淑芳, 赵远进, 王硕, 周润川, 杨霞, 贺爱华. 胺端基官能化反式丁戊共聚橡胶的制备及配位链转移机理. 高等学校化学学报, 2025, 46(8): 20250067.
LUO Shufang, ZHAO Yuanjin, WANG Shuo, ZHOU Runchaun, YANG Xia, HE Aihua. Preparation of Amine-capped Functionalized Trans-1,4-poly(butadiene-co-isoprene) Rubber and Coordination Chain Transfer Mechanism. Chem. J. Chinese Universities, 2025, 46(8): 20250067.
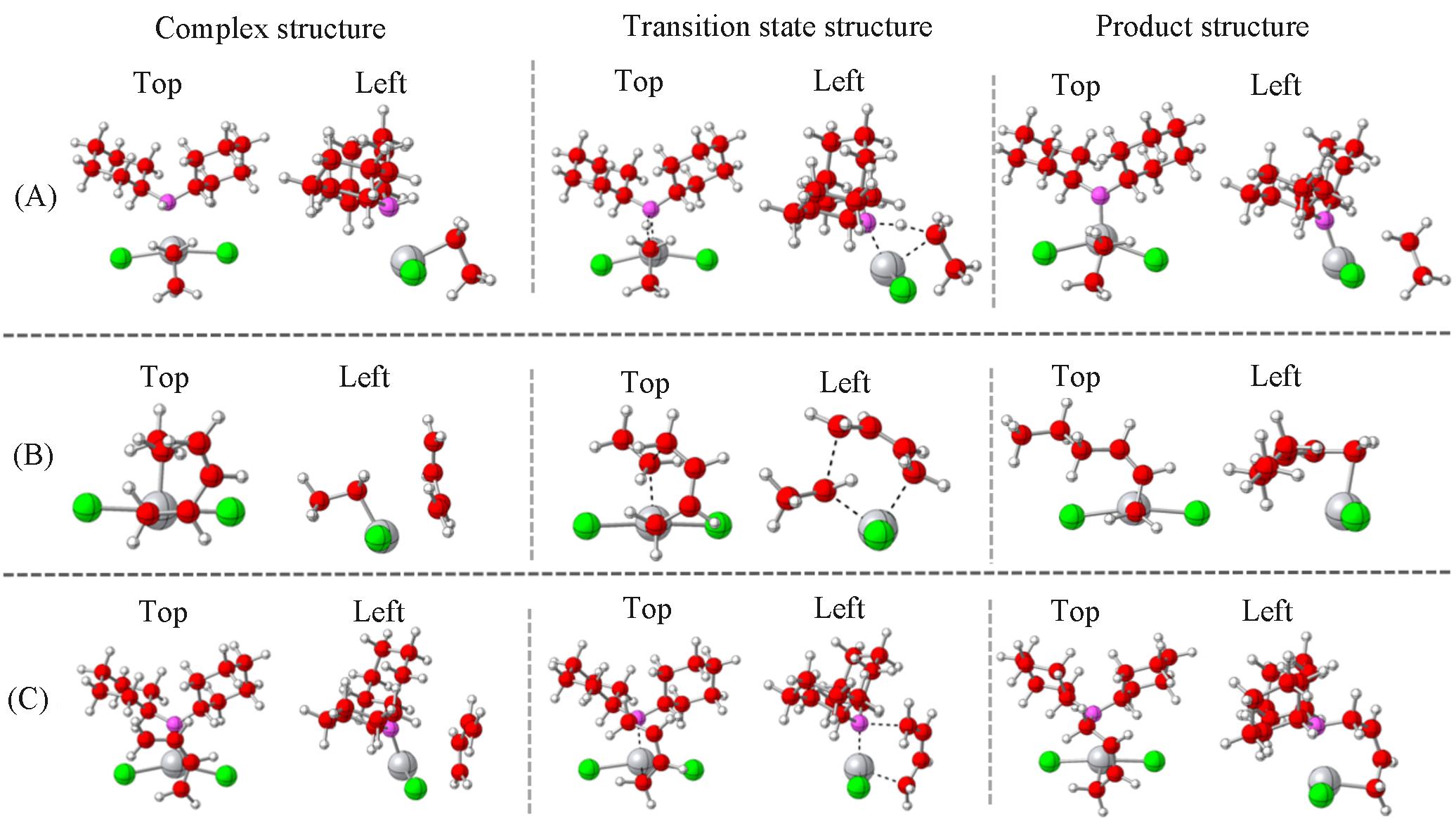
Fig.2 Top and left views of the complex, transition state and product structures(A) Chain transfer from C*-1 to C*-2; (B) chain growth of C*-1; (C) chain growth of C*-2.Colors: C, red; Cl, green; H, white; Ti, gray; N, pink.
| Sample | n(DCHA)/n(M) | n(Al)/ n(Ti) | Conv. (%) | CA/ (gP∙g | CE b (%) | Molar fraction of trans⁃1,4⁃units(%) b | FBd | T | T | ΔH (J∙g-1) | 10-4M | Mw/M | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ip unit c | Bd unit c | ||||||||||||
| TBIR | 0 | 50 | 21.4 | 4051 | 0 | 96.5 | 96.8 | 23.5 | -75.5 | 23.5 | 25.5 | 94 | 2.2 |
| F⁃TBIR⁃1 | 0.0012 | 50 | 17.3 | 3930 | 21.4 | 96.6 | 95.2 | 23.8 | -75.9 | 20.5 | 26.2 | 75 | 2.4 |
| F⁃TBIR⁃2 | 0.0015 | 50 | 18.0 | 3208 | 38.7 | 96.1 | 96.7 | 22.9 | -75.6 | 24.0 | 27.8 | 72 | 2.5 |
| F⁃TBIR⁃3 | 0.0020 | 50 | 15.7 | 2913 | 49.6 | 95.8 | 96.9 | 24.1 | -75.1 | 23.1 | 24.8 | 67 | 2.7 |
| F⁃TBIR⁃4 | 0.0020 | 100 | 16.2 | 3067 | 46.1 | 96.3 | 95.4 | 24.3 | -77.0 | 23.8 | 23.8 | 74 | 3.1 |
| F⁃TBIR⁃5 | 0.0020 | 200 | 15.6 | 2843 | 42.5 | 96.0 | 97.4 | 27.3 | -77.0 | 16.9 | 21.4 | 56 | 3.3 |
Table 1 Copolymerization results of butadiene with isoprene catalyzed by heterogeneous Ziegle-Natta catalyst a
| Sample | n(DCHA)/n(M) | n(Al)/ n(Ti) | Conv. (%) | CA/ (gP∙g | CE b (%) | Molar fraction of trans⁃1,4⁃units(%) b | FBd | T | T | ΔH (J∙g-1) | 10-4M | Mw/M | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ip unit c | Bd unit c | ||||||||||||
| TBIR | 0 | 50 | 21.4 | 4051 | 0 | 96.5 | 96.8 | 23.5 | -75.5 | 23.5 | 25.5 | 94 | 2.2 |
| F⁃TBIR⁃1 | 0.0012 | 50 | 17.3 | 3930 | 21.4 | 96.6 | 95.2 | 23.8 | -75.9 | 20.5 | 26.2 | 75 | 2.4 |
| F⁃TBIR⁃2 | 0.0015 | 50 | 18.0 | 3208 | 38.7 | 96.1 | 96.7 | 22.9 | -75.6 | 24.0 | 27.8 | 72 | 2.5 |
| F⁃TBIR⁃3 | 0.0020 | 50 | 15.7 | 2913 | 49.6 | 95.8 | 96.9 | 24.1 | -75.1 | 23.1 | 24.8 | 67 | 2.7 |
| F⁃TBIR⁃4 | 0.0020 | 100 | 16.2 | 3067 | 46.1 | 96.3 | 95.4 | 24.3 | -77.0 | 23.8 | 23.8 | 74 | 3.1 |
| F⁃TBIR⁃5 | 0.0020 | 200 | 15.6 | 2843 | 42.5 | 96.0 | 97.4 | 27.3 | -77.0 | 16.9 | 21.4 | 56 | 3.3 |
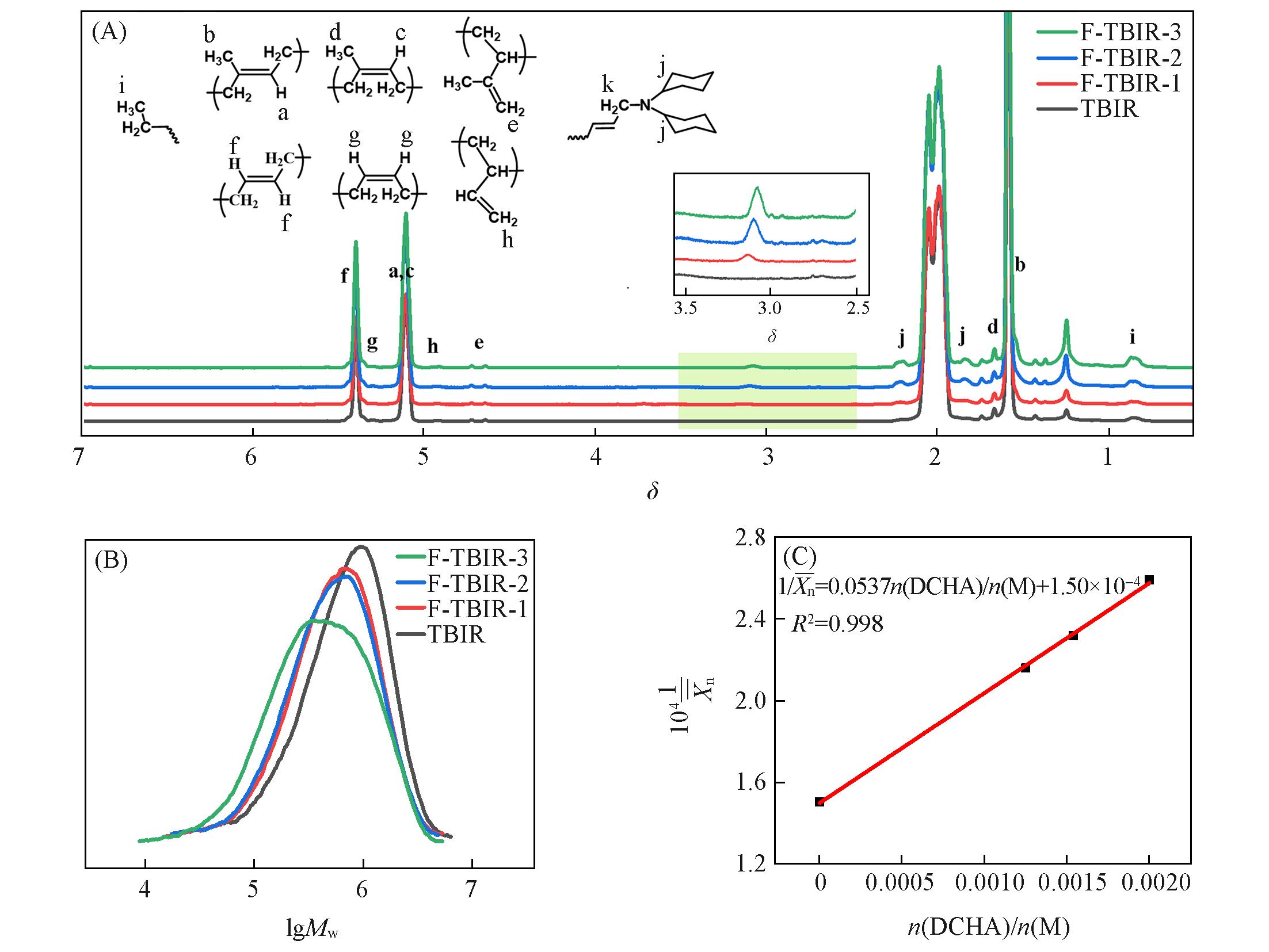
Fig.3 1H NMR spectra(A) and GPC curves(B) of TBIR and F⁃TBIR samples and diagram of 1/Xn¯versus n(DCHA)/n(M)(C)Polymerization conditions: n(Ip)/n(Bd)=94/6, n(Ti)/n(M)=5×10-5, polymerization temperature was 20 ℃, polymerization time was 1.5 h.
| Reaction | ΔGπ /(kJ∙mol-1) | ΔGA/(kJ∙mol-1) |
|---|---|---|
| Chain transfer(C*⁃1 to C*⁃2) | -57.7 | 98.7 |
| Chain growth(C*⁃1)[ | -8.8 | 149.8 |
| Chain growth(C*⁃2) | -15.9 | 155.6 |
Table 2 Complex formation energy(ΔGπ ) and activation free energy(ΔGA) calculated by DFT
| Reaction | ΔGπ /(kJ∙mol-1) | ΔGA/(kJ∙mol-1) |
|---|---|---|
| Chain transfer(C*⁃1 to C*⁃2) | -57.7 | 98.7 |
| Chain growth(C*⁃1)[ | -8.8 | 149.8 |
| Chain growth(C*⁃2) | -15.9 | 155.6 |
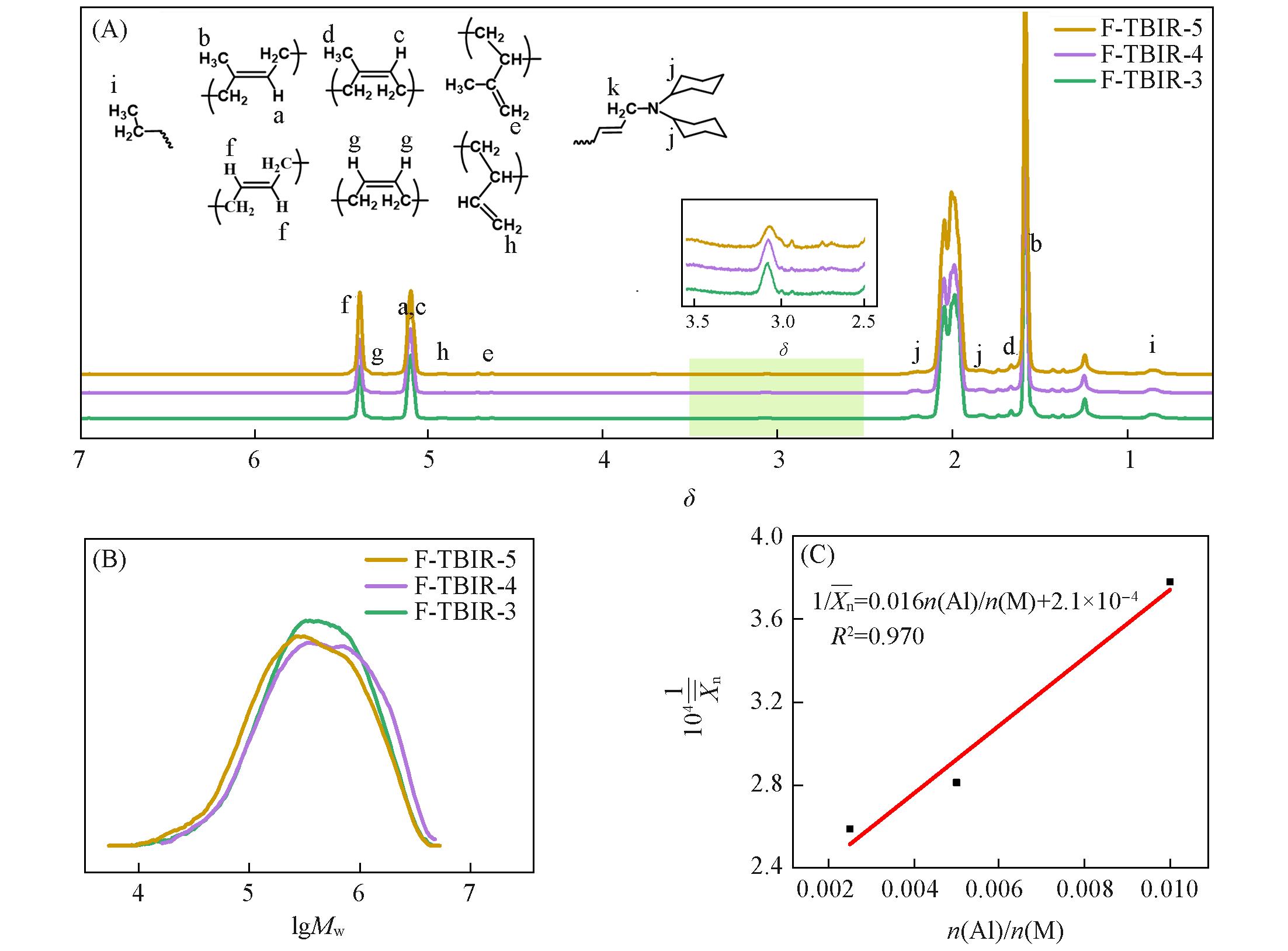
Fig.4 1H NMR spectra(A) and GPC curves(B) of F⁃TBIR samples and diagram of 1/Xn¯versusn(Al)/n(M)(C)Polymerization conditions: n(Ip)/n(Bd)=94/6, n(Ti)/n(M)=5×10-5, polymerization temperature was 20 ℃, polymerization time was 1.5 h.
| [1] | Dhanorkar R. J., Mohanty S., Gupta V. K., Ind. Eng. Chem. Res., 2021, 60(12), 4517—4535 |
| [2] | Dong K. X., He A. H., Polym. Bull., 2022, (7), 22—28 |
| 董凯旋, 贺爱华. 高分子通报, 2022, (7), 22—28 | |
| [3] | Mazumder A., Chanda J., Bhattacharyya S., Dasgupta S., Mukhopadhyay R., Bhowmick A. K., J. Appl. Polym. Sci., 2021, 138(42), 51236 |
| [4] | Xu T. W., Jia Z. X., Luo Y. F., Jia D. M., Peng Z., Appl. Surf. Sci., 2015, 328, 306—313 |
| [5] | Li M. Y., Wang K. Y., Xiong Y. Z., Materials, 2021, 14(18), 5246 |
| [6] | Ansari A., Mohanty T. R., Sarkar S., Ramakrishnan S., Amarnath S. K. P., Singha N. K., Eur. Polym. J., 2024, 213, 113069 |
| [7] | Das C., Bandyopadhyay A., Maji P. K., Kapgate B. P., Bansod N. D., Rubber Chem. Technol., 2018, 92(2), 219—236 |
| [8] | Yamada C., Yasumoto A., Matsushita T., Blume A., Funct. Compos. Mater., 2022, 3(1), 6 |
| [9] | Lee H. G., Kim H. S., Cho S. T., Jung I. T., Cho C. T., Asian J. Chem., 2013, 25(8), 5251—5256 |
| [10] | Bisschop R., Grunert F., Ilisch S., Stratton T., Blume A., Polym. Test., 2021, 99, 107219 |
| [11] | Nuinu P., Sirisinha C., Suchiva K., Daniel P., Phinyocheep P., J. Mater. Res. Technol., 2023, 24, 2155—2168 |
| [12] | Gao W., Lu J. M., Song W. N., Hu J. F., Han B. Y., RSC Adv., 2019, 9(33), 18888—18897 |
| [13] | Matic A., Schlaad H., Polym. Int., 2018, 67(5), 500—505 |
| [14] | Deepak V. D., Gungör E., Gauthier M., Polym. J., 2020, 53(2), 323—330 |
| [15] | Abdulraman D., Tiensing T., Phinyocheep P., Iran. Polym. J., 2024, 33(11), 1553—1567 |
| [16] | Qiao H., Chao M. Y., Hui D., Liu J., Zheng J. C., Lei W. W., Zhou X. X., Wang R. G., Zhang L. Q., Compos. Part B, 2017, 114, 356—364 |
| [17] | Hu G. W., Lin S. H., Zhao B. X., Pan Q. M., J. Appl. Polym. Sci., 2020, 138(8), 49899 |
| [18] | Ying W. L., Pan W. J., Gan Q., Jia X. Y., Grassi A., Gong D. R., Polym. Chem., 2019, 10(25), 3525—3534 |
| [19] | Wu L. L., Wang Y. S., Wang Y. R., Shen K. H., Li Y., Polymer, 2013, 54(12), 2958—2965 |
| [20] | Wu L. L., Ma H. W., Wang Q. Y., Li L., Wang Y. R., Li Y., J. Mater. Sci., 2014, 49(14), 5171—5181 |
| [21] | Gong D. R., Tang F. M., Xu Y. C., Hu Z. G., Luo W. W., Polym. Chem., 2021, 12(11), 1653—1660 |
| [22] | Leicht H., Göttker⁃Schnetmann I., Mecking S., Macromolecules, 2017, 50(21), 8464—8468 |
| [23] | Dong K. X., Zhang J. Y., He A. H., Polymer, 2021, 235, 124231 |
| [24] | Kang X. H., Liu S. Q., Xu L., Wang N., Macromol. Res., 2018, 26(10), 924—933 |
| [25] | Liu X., Zhao S. H., Zhang X. Y., Li X. L., Bai Y., Polymer, 2014, 55(8), 1964—1976 |
| [26] | Hassanabadi M., Najafi M., Motlagh G. H., Garakani S. S., Polym. Test., 2020, 85, 106431 |
| [27] | Amin S. B., Marks T. J., J. Am. Chem. Soc., 2007, 129(33), 10102—10103 |
| [28] | Amin S. B., Seo S., Marks T. J., Organometallics, 2008, 27, 2411—2420 |
| [29] | Hyatt M. G., Guironnet D., ACS Catal., 2017, 7(9), 5717—5720 |
| [30] | Hyatt M. G., Guironnet D., Organometallics, 2019, 38(4), 788—796 |
| [31] | Amin S. B., Marks T. J., Angew. Chem. Int. Ed., 2008, 47(11), 2006—2025 |
| [32] | Sun C. Z., Wen S. P., Ma H. G., Li Y., Chen L., Wang Z., Yuan B. B., Liu L., Ind. Eng. Chem. Res., 2018, 58(3), 1454—1461 |
| [33] | Georges S., Hashmi O. H., Bria M., Zinck P., Champouret Y., Visseaux M., Macromolecules, 2019 , 52(3), 1210—1219 |
| [34] | Ozawa Y., Takata T., J. Appl. Polym. Sci., 2019, 136, 47985 |
| [35] | Li H. Y., Zong X., Li N., Zhang X. P., He A. H., Compos. Part A, 2021, 140, 106194 |
| [36] | Zhang X. P., Cai L., He A. H., Ma H. W., Li Y., Hu Y. M., Zhang X. Q., Liu L., Compos. Sci. Technol., 2021, 203, 108601 |
| [37] | Guo Q. R., Shao H. F., He A. H., Chem. J. Chinese Universities, 2020, 41(4), 789—794 |
| 国钦瑞, 邵华锋, 贺爱华. 高等学校化学学报, 2020, 41(4), 789—794 | |
| [38] | Zong X., Wang S., Li N., Li H. Y., Zhang X. P., He A. H., Polymer, 2021, 213, 123325 |
| [39] | Wu Y. F., Li H. Y., Cai L., He A. H., Chem. J. Chinese Universities, 2020, 413), 565—571(武营飞, 李洪昱, 蔡磊, 贺爱华. 高等学校化学学报, 2020 , 41(3), 565—571 |
| [40] | Zhang J. P., Song L. Y., Wang H., Wang R. G., He A. H., Chem. J. Chinese Universities, 2018, 39(6), 1334—1341 |
| 张剑平, 宋丽媛, 王浩, 王日国, 贺爱华. 高等学校化学学报, 2018 , 39(6), 1334—1341 | |
| [41] | Shao H. F., Guo Q. R., He A. H., Polym. Test., 2022, 115, 107715 |
| [42] | Wang S., Li W. T., Li X. N., Zong X., Wang R. G., He A. H., Compos. Part A, 2023, 168, 107462 |
| [43] | Qian Z. H., Wang S., Zong X., Cai L., He A. H., Chem. J. Chinese Universities, 2023, 44(8), 20230023 |
| 钱浙濠, 王硕, 宗鑫, 蔡磊, 贺爱华. 高等学校化学学报, 2023, 44(8), 20230023 | |
| [44] | Zhang X. P., Cui H. H., Song L. Y., Ren H. C., Wang R. G., He A. H., Compos. Sci. Technol., 2018, 158, 156—163 |
| [45] | Dong K. X., Synthesis, Structure and Properties of Functionalized Polydiolefin, Qingdao University of Science and Technology, Qingdao, 2021 |
| 董凯旋. 官能化聚二烯烃的合成、 结构与性能研究, 青岛: 青岛科技大学, 2021 | |
| [46] | Luo S. F., Dong K. X., Wang S., He A. H., Compos. Sci. Technol., 2024, 258, 110899 |
| [47] | Niu Q. T., Li W. T., Liu X. Y., Wang R. G., He A. H., Polymer, 2018, 143, 173—183 |
| [48] | Lin S. A., Coordination Polymerization, Shanghai Scientific & Technical Publishers, Shanghai, 1988, 53—103 |
| 林尚安. 配位聚合, 上海: 上海科学技术出版社, 1988, 53—103 | |
| [49] | Bueno L. E., Zentel K. M., Busch M., Chem. Ing. Tech., 2024, 96(12), 1697—1708 |
| [50] | Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Keith T., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R. E., Stratmann O., Yazyev A. J., Austin R., Cammi C., Pomelli J. W., Ochterski R., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision A.1, Gaussian Inc., Wallingford CT, 2009 |
| [51] | Zhou R. C., Zhao A., Liu J., He A. H., Yang X., J. Phys. Chem. C, 2025, 129(1), 253—261 |
| [52] | Zhao A., Liu J., Zhou R. C., Yang X., He A. H., Comput. Theor. Chem., 2024, 1237, 114661 |
| [53] | Liu J., Zhao A., Zhou R. C., Yang X., He A. H., J. Phys. Chem. C, 2024, 128(16), 6658—6671 |
| [54] | Bbahri⁃Laleh N, Correa A, Mehdipour⁃Ataei S, Arabi H., Haghighi M. N., Zohuri G., Cavallo L., Macromolecules, 2011, 44(4), 778—783 |
| [55] | Becke A. D., J. Chem. Phys., 1993, 98, 5648—5652 |
| [56] | Grimme S., J. Comput. Chem., 2004, 25(12), 1463—1473 |
| [57] | Grimme S., Antony J., Ehrlich S., Krieg H., J. Chem. Phys., 2010, 132(15), 154104 |
| [58] | Ehm C., Antinucci G., Budzelaar P. H. M., Busico V., J. Organomet. Chem., 2014, 772/773, 161—171 |
| [59] | Zhao Y., Truhlar D. G., Theor. Chem. Acc., 2008, 120(1), 215—241 |
| [60] | Zhang Q. F., Jiang X. B., He A. H., Chinese J. Polym. Sci., 2014, 32(8), 1068—1076 |
| [61] | Zhang J. Y., Peng W., He A. H., Polymer, 2020, 203, 122766 |
| [62] | Peng W., Qi P. Y., Dong K. X., He A. H., Acta Chim. Sinica, 2020, 78, 1418—1425 |
| 彭伟, 戚佩瑶, 董凯旋, 贺爱华. 化学学报, 2020, 78, 1418—1425 | |
| [63] | Niu Q. T., Peng W., He A. H., Scientia Sinica Chimica, 2019, 49(8), 1047—1058 |
| 牛庆涛, 彭伟, 贺爱华. 中国科学: 化学, 2019, 49(8), 1047—1058 | |
| [64] | Liu X. Y., Li W. T., Niu Q. T., Wang R. G., He A. H., Polymer, 2018, 140, 255—268 |
| [65] | Niu Q. T., Zhang J. Y., Peng W., Fan Z. Q., He A. H., Mol. Catal., 2019, 471, 1—8 |
| [1] | 钱浙濠, 王硕, 宗鑫, 蔡磊, 贺爱华. TBIR对天然橡胶纳米复合材料结构和性能的调控[J]. 高等学校化学学报, 2023, 44(8): 20230023. |
| [2] | 周成思, 赵远进, 韩美晨, 杨霞, 刘晨光, 贺爱华. 硅烷类外给电子体对丙烯-丁烯序贯聚合的调控作用[J]. 高等学校化学学报, 2022, 43(10): 20220290. |
| [3] | 黄湃, 周光远, 刘博, 王蕾, 张佳旗. 纳米多级孔Silicalite-1分子筛负载Ziegler-Natta催化剂对聚丙烯性能的影响[J]. 高等学校化学学报, 2020, 41(9): 2025. |
| [4] | 张俊英, 彭伟, 陈子威, 贺爱华. 聚合温度对负载型Ziegler-Natta催化剂催化丁二烯-异戊二烯共聚合的影响[J]. 高等学校化学学报, 2020, 41(8): 1873. |
| [5] | 国钦瑞, 邵华锋, 贺爱华. TBIR应用于航空胎侧胶的热氧老化性能[J]. 高等学校化学学报, 2020, 41(4): 789. |
| [6] | 武营飞,李洪昱,蔡磊,贺爱华. 高耐磨低生热NBR/TBIR复合材料的结构与性能[J]. 高等学校化学学报, 2020, 41(3): 565. |
| [7] | 张新萍, 张剑平, 蔡磊, 宗鑫, 贺爱华. 高疲劳寿命氯丁橡胶基减振材料的结构与性能[J]. 高等学校化学学报, 2019, 40(7): 1571. |
| [8] | 张剑平, 宋丽媛, 王浩, 王日国, 贺爱华. TBIR改性BIIR/NR共混物的结构与性能[J]. 高等学校化学学报, 2018, 39(6): 1334. |
| [9] | 任惠成, 武营飞, 刘丹丹, 聂华荣, 贺爱华. SSBR/TPI共混体系中TPI的结晶、 成核及动力学研究[J]. 高等学校化学学报, 2018, 39(5): 1091. |
| [10] | 李欣, 倪旭峰, 沈之荃. 四氯化钛/三异丁基铝体系催化降冰片烯与异戊二烯的共聚合[J]. 高等学校化学学报, 2012, 33(05): 1095. |
| [11] | 徐德民, 马志, 宓霞, 柯毓才, 胡友良, 吴春红. 新型非对称二醚给电子体丙烯聚合催化剂研究[J]. 高等学校化学学报, 2002, 23(5): 982. |
| [12] | 王君, 刘振荣, 张向东, 贾卫国. K3[GdⅢ(nta)2(H2O)]·6H2O和(NH4)·[GdⅢ(Cydta)(H2O)2]·5H2O的合成及晶体结构[J]. 高等学校化学学报, 2002, 23(11): 2052. |
| [13] | 钱明星, 周斌, 何仁, 王梅, 黄勇, 王辉. Ind2Zr(OC6H4Me-p)2和EtnAlCl3-n催化乙烯齐聚的研究[J]. 高等学校化学学报, 2001, 22(10): 1771. |
| [14] | 陈天红, 林尚安. 等规聚苯乙烯与聚(乙烯/丙烯)嵌段共聚物的研究(Ⅰ)──一步法嵌段共聚合反应[J]. 高等学校化学学报, 1997, 18(11): 1875. |
| [15] | 李悦, 林尚安. Ti-Mg系载体催化剂乙烯加氢预聚合对乙烯气相聚合的影响[J]. 高等学校化学学报, 1996, 17(7): 1154. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||