

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (10): 3005.doi: 10.7503/cjcu20210511
叶一桦1, 巴德良2, 刘帅磊1, 陈颍霖1, 李园园2( ), 刘金平1(
), 刘金平1( )
)
收稿日期:2021-07-16
出版日期:2021-10-10
发布日期:2021-10-10
通讯作者:
李园园
E-mail:liyynano@hust.edu.cn;liujp@whut.edu.cn
作者简介:刘金平, 男, 博士, 教授, 主要从事新能源材料与器件研究. E-mail: 基金资助:
YE Yihua1, BA Deliang2, LIU Shuailei1, CHEN Yinglin1, LI Yuanyuan2( ), LIU Jinping1(
), LIU Jinping1( )
)
Received:2021-07-16
Online:2021-10-10
Published:2021-10-10
Contact:
LI Yuanyuan
E-mail:liyynano@hust.edu.cn;liujp@whut.edu.cn
Supported by:摘要:
锂离子储能器件具有高能量密度与绿色环保等优点, 在未来新能源汽车和大规模储能领域中将显示出巨大的潜力. 然而, 由于传统锂离子负极材料如石墨、 硅存在电化学动力学缓慢与高倍率下的安全性等问题, 无法满足目前能源消费终端日益增长的快速充放电性能要求. 因此, 开发有利于锂离子快速嵌入/脱出、 安全性与稳定性优异的负极材料至关重要. 相比于传统的负极材料, 铌基氧化物具有合适的理论容量、 更安全的工作电位、 稳定且快速的离子传输通道等优点. 本文综述了高倍率铌基氧化物负极材料在锂离子储能器件领域的最新研究进展, 重点介绍了典型铌基氧化物的储锂机理与改性手段, 并对铌基氧化物负极材料未来的发展与挑战进行了展望.
中图分类号:
TrendMD:
叶一桦, 巴德良, 刘帅磊, 陈颍霖, 李园园, 刘金平. 高倍率铌基氧化物负极材料的研究进展. 高等学校化学学报, 2021, 42(10): 3005.
YE Yihua, BA Deliang, LIU Shuailei, CHEN Yinglin, LI Yuanyuan, LIU Jinping. Recent Progress on High⁃rate Niobium-based Oxides Anode Materials. Chem. J. Chinese Universities, 2021, 42(10): 3005.
| Material | Modification | Synthetic method | Mass loading/ (mg·cm-2) | Potential range/V | Specific capacity/ (mA·h·g-1) | Rate capability/ (mA·h·g-1) | Cycling stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| Nb2O5 nanobelts | Morphology and microstructure design | Solvothermal | — | 1.0—3.0 | 209.3@0.5C | 108.8@10C | 77.7%(200) @5C | [ |
| Nb2O5 nanoflakes | Morphology and microstructure design | Template | — | 1.0—2.5 | 179@0.2C | 82@20C | 62%(1000) @5C | [ |
| 3DOM?Nb2O5 | Morphology and microstructure design | Template | — | 1.0—3.0 | 205@0.5C | 77@50C | 90%(100) @10C | [ |
Mesoporous T?Nb2O5 nanofibers | Morphology and microstructure design | Electrospinning | 1.5 | 1.0—2.6 | 190@0.05 A/g | 70@5 A/g | 84%(5000) @3 A/g | [ |
HM? Nb2O5 nanospheres | Morphology and microstructure design | Coordination? assisted precipitation | 1.0—1.5 | 1.0—3.0 | 195@1C | 125@50C | 86.2%(2000) @1C | [ |
T?Nb2O5 nanoparticles | Morphology and microstructure design | Sol?gel | — | 1.0—3.0 | 253@0.5C | 130@100C | 93.3%(4000) @25C | [ |
Nb2O5 nanoparticles | Morphology and microstructure design | Hydrothermal | 0.1—2.1 | 0.05—3.0 | 190@0.25C | 43@50C | 98.6%(800) @2.5C | [ |
Nb2O5 microspheres | Morphology and microstructure design | Solvothermal | — | 1.0—3.0 | 164@1C | 98.5@10C | 96.2%(500) @5C | [ |
Carbon?encapsulated T?Nb2O5 nanocrystals | Conductive?phase compositing | Solvothermal | 1.1—1.5 | 1.0—3.0 | 192@0.5C | 90@100C | 88%(1000) @5C | [ |
| Material | Modification | Synthetic method | Mass loading/ (mg·cm-2) | Potential range/V | Specific capacity/ (mA·h·g-1) | Rate capability/ (mA·h·g-1) | Cycling stability | Ref. |
Nb2O5 @C core?shell nanocrystals | Conductive?phase compositing | Template | 0.9—1.1 | 1.1—3.0 | 180@0.05 A/g | 90@5 A/g | ― | [ |
| Ag?1D T?Nb2O5 | Conductive?phase compositing | Electrospinning | 1.5 | 1.0—2.6 | 189.9@0.05 A/g | 103.6@5 A/g | 99.8%(200) @0.5 A/g | [ |
| Nb2O5 ?MWCNT | Conductive?phase compositing | Hydrothermal | 1.8 | 1.0—3.0 | 188.3@0.2C | 75.1@20C | 96.2%(50) @0.2C | [ |
| T?Nb2O5 /NG | Conductive?phase compositing | Hydrothermal | 1.0—3.0 | 1.0—3.0 | 164@0.5C | 90@100C | 70%(20000) @50C | [ |
| T?Nb2O5 /graphene | Conductive?phase compositing | Hydrothermal | — | 1.0—3.0 | 626 C/g@ 1 A/g | 220 C/g@50 A/g | — | [ |
T?Nb2O5 nanorod film | Array/free?standing design | Hydrothermal | 1.5—2.0 | 1.0—3.0 | 220@0.5C | 160@20C | 85%(2500) @10C | [ |
T?Nb2O5/rGO nanohybrids | Conductive?phase compositing | Solvothermal | 1.6 | 1.1—3.0 | 227@0.1C | 80@10C | 72.6%(100) @0.5C | [ |
Ni/T?Nb2O5@Carbon nanofibers | Conductive?phase compositing | Electrospinning | 0.35—0.5 | 0.01—3.0 | 643@0.2 A/g | 227@10 A/g | 76.2%(1400) @10 A/g | [ |
T?Nb2O5/3D Holey GO framework | Conductive?phase compositing | Solvothermal | 1—11 | 1.1—3.0 | 184@1C | 100@100C | 90%(10000) @10C | [ |
Free?standing T?Nb2O5 /Graphene composite papers | Array/free?standing design | Solvothermal | 0.8—1.3 | 0.8—3.0 | 990 F/cm3@5C | 526 F/cm3@100C | 94.8%(1700) @5C | [ |
| Al0.5Nb24.5O62 | Doping | Solvothermal | 1.5 | 0.8—3.0 | 321@0.1C | 192@10C | 90.0%(500) @10C | [ |
FeNb11O29 nanotubes | Doping | Electrospinning | — | 1.0—3.0 | 302@0.1C | 53.36@50C | 71.4%(2000) @10C | [ |
Cr0.5Nb24.5O62 nanowires | Doping | Electrospinning | — | 0.8—3.0 | 344@0.1C | 209@30C | 92.8%(2000) @10C | [ |
CrNb11O29 nanorods | Doping | Hydrothermal | — | 0.8—3.0 | 307@0.5C | 228@10C | 91.1%(400) @10C | [ |
Mg2Nb34O87 porous microspheres | Doping | Solvothermal | — | 0.8—3.0 | 338@0.1C | 230@10C | 93.1%(500) @10C | [ |
ZrNb24O62 nanowires | Doping | Solvothermal | 1.4 | 0.8—3.0 | 320@0.1C | 182@30C | 90.2%(1500) @10C | [ |
GaNb11O29 nanowires | Doping | Electrospinning | 1.8 | 1.0—3.0 | 233@0.1 A/g | 169@0.7 A/g | 97%(500) @ 0.7 A/g | [ |
MoNb12O33 microspheres | Doping | Solvothermal | 1.0 | 0.8—3.0 | 321@0.1C | 200@10C | 95.7%(1000) @10C | [ |
| Nb16W5O55 | Doping | Solid?state reaction | 2.0—3.0 | 1.0—3.0 | 225@0.2C | 70@100C | 95%(750) @20C | [ |
| Nb14W3O44 | Doping | Solution combustion | 1.4—1.7 | 1.0—3.0 | 249@0.5C | 84.4@50C | 96.5%(4000) @10C | [ |
Nb8W9O47 nanofibers | Doping | Electrospinning | — | 1.0—3.0 | 196@1C | 147@5C | 78%(1000) @5C | [ |
Mo3Nb14O44 nanowires | Doping | Electrospinning | — | 0.8—3.0 | 321@0.1C | 174@10C | 75.8%(1000) @5C | [ |
| 3DOM?TiNb2O7 | Morphology and microstructure design | Template | 1.5 | 1.0—3.0 | 251@1C | 99@100C | 82%(1000) @10C | [ |
| Material | Modification | Synthetic method | Mass loading/ (mg·cm-2) | Potential range/V | Specific capacity/ (mA·h·g-1) | Rate capability/ (mA·h·g-1) | Cycling stability | Ref. |
Hollow Ti2Nb10O29 microspheres | Morphology and microstructure design | Hydrothermal | 1.0—1.5 | 1.0—3.0 | 219.8@0.2C | 173.4@10C | 95%(500) @10C | [ |
| TiNb2O7 nanorods | Morphology and microstructure design | Sol?gel | — | 1.0—3.0 | 265@0.1C | 84@50C | 68.4%(500) @10C | [ |
TiNb6O17/C nanoparticles | Conductive?phase compositing | Sol?gel | 1.5 | 1.0—3.0 | 275@1C | 132@30C | 92%(500) @10C | [ |
N?TiNb2O7@C nanoparticles | Conductive?phase compositing | Solid?state reaction | — | 1.0—3.0 | 268@1C | 135@50C | 82.7%(10000)@20C | [ |
Ti2Nb10O29/Ag composite | Conductive?phase compositing | Solid?state reaction and solvothermal | — | 1.0—2.5 | 282@0.2C | 132@20C | 82.1%(500) @10C | [ |
3D TiNb2O7 nanorod | Array/free?standing design | Hydrothermal | 2.0—2.5 | 1.0—3.0 | 240@0.5C | 151@40C | 86.4%(2000) @10C | [ |
Carbon?coated TiNb2O7 nanosheet | Array/free?standing design | Solvothermal | 5.8 | 1.0—3.0 | 250@1C | 96@30C | 87.8%(2000) @5C | [ |
Ti2Nb10O29@TiC/C nanoarray | Array/free?standing design | Chemical vapor deposition and solvothermal | 1.8 | 1.0—2.5 | 318@1C | 202@50C | 85%(10000) @10C | [ |
Ti2Nb10O29@TiC/ C?NC nanoarray | Array/free?standing design | Chemical vapor deposition and electrodeposition | 0.8 | 1.0—2.5 | 320@1C | 165@100C | 65%(10000) @10C | [ |
Ru0.01Ti0.99Nb2O7 micron?particles | Doping | Solid?state reaction | 1.0 | 0.8—3.0 | 295@0.1C | 181@5C | 90.1%(100) @5C | [ |
Cu0.02Ti0.94Nb2.04O7 micron?particles | Doping | Solid?state reaction | 1.0—2.0 | 0.8—3.0 | 315@0.1C | 182@10C | 98.8%(1000) @5C | [ |
| TiNb1.98V0.02O7 | Doping | Solid?state reaction | 2.6—2.7 | 1.0—3.0 | 260@0.3C | 172@10C | 65.6%(30) @5C | [ |
Table 1 Electrochemical performance of niobium-based oxide materials with various modifications
| Material | Modification | Synthetic method | Mass loading/ (mg·cm-2) | Potential range/V | Specific capacity/ (mA·h·g-1) | Rate capability/ (mA·h·g-1) | Cycling stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| Nb2O5 nanobelts | Morphology and microstructure design | Solvothermal | — | 1.0—3.0 | 209.3@0.5C | 108.8@10C | 77.7%(200) @5C | [ |
| Nb2O5 nanoflakes | Morphology and microstructure design | Template | — | 1.0—2.5 | 179@0.2C | 82@20C | 62%(1000) @5C | [ |
| 3DOM?Nb2O5 | Morphology and microstructure design | Template | — | 1.0—3.0 | 205@0.5C | 77@50C | 90%(100) @10C | [ |
Mesoporous T?Nb2O5 nanofibers | Morphology and microstructure design | Electrospinning | 1.5 | 1.0—2.6 | 190@0.05 A/g | 70@5 A/g | 84%(5000) @3 A/g | [ |
HM? Nb2O5 nanospheres | Morphology and microstructure design | Coordination? assisted precipitation | 1.0—1.5 | 1.0—3.0 | 195@1C | 125@50C | 86.2%(2000) @1C | [ |
T?Nb2O5 nanoparticles | Morphology and microstructure design | Sol?gel | — | 1.0—3.0 | 253@0.5C | 130@100C | 93.3%(4000) @25C | [ |
Nb2O5 nanoparticles | Morphology and microstructure design | Hydrothermal | 0.1—2.1 | 0.05—3.0 | 190@0.25C | 43@50C | 98.6%(800) @2.5C | [ |
Nb2O5 microspheres | Morphology and microstructure design | Solvothermal | — | 1.0—3.0 | 164@1C | 98.5@10C | 96.2%(500) @5C | [ |
Carbon?encapsulated T?Nb2O5 nanocrystals | Conductive?phase compositing | Solvothermal | 1.1—1.5 | 1.0—3.0 | 192@0.5C | 90@100C | 88%(1000) @5C | [ |
| Material | Modification | Synthetic method | Mass loading/ (mg·cm-2) | Potential range/V | Specific capacity/ (mA·h·g-1) | Rate capability/ (mA·h·g-1) | Cycling stability | Ref. |
Nb2O5 @C core?shell nanocrystals | Conductive?phase compositing | Template | 0.9—1.1 | 1.1—3.0 | 180@0.05 A/g | 90@5 A/g | ― | [ |
| Ag?1D T?Nb2O5 | Conductive?phase compositing | Electrospinning | 1.5 | 1.0—2.6 | 189.9@0.05 A/g | 103.6@5 A/g | 99.8%(200) @0.5 A/g | [ |
| Nb2O5 ?MWCNT | Conductive?phase compositing | Hydrothermal | 1.8 | 1.0—3.0 | 188.3@0.2C | 75.1@20C | 96.2%(50) @0.2C | [ |
| T?Nb2O5 /NG | Conductive?phase compositing | Hydrothermal | 1.0—3.0 | 1.0—3.0 | 164@0.5C | 90@100C | 70%(20000) @50C | [ |
| T?Nb2O5 /graphene | Conductive?phase compositing | Hydrothermal | — | 1.0—3.0 | 626 C/g@ 1 A/g | 220 C/g@50 A/g | — | [ |
T?Nb2O5 nanorod film | Array/free?standing design | Hydrothermal | 1.5—2.0 | 1.0—3.0 | 220@0.5C | 160@20C | 85%(2500) @10C | [ |
T?Nb2O5/rGO nanohybrids | Conductive?phase compositing | Solvothermal | 1.6 | 1.1—3.0 | 227@0.1C | 80@10C | 72.6%(100) @0.5C | [ |
Ni/T?Nb2O5@Carbon nanofibers | Conductive?phase compositing | Electrospinning | 0.35—0.5 | 0.01—3.0 | 643@0.2 A/g | 227@10 A/g | 76.2%(1400) @10 A/g | [ |
T?Nb2O5/3D Holey GO framework | Conductive?phase compositing | Solvothermal | 1—11 | 1.1—3.0 | 184@1C | 100@100C | 90%(10000) @10C | [ |
Free?standing T?Nb2O5 /Graphene composite papers | Array/free?standing design | Solvothermal | 0.8—1.3 | 0.8—3.0 | 990 F/cm3@5C | 526 F/cm3@100C | 94.8%(1700) @5C | [ |
| Al0.5Nb24.5O62 | Doping | Solvothermal | 1.5 | 0.8—3.0 | 321@0.1C | 192@10C | 90.0%(500) @10C | [ |
FeNb11O29 nanotubes | Doping | Electrospinning | — | 1.0—3.0 | 302@0.1C | 53.36@50C | 71.4%(2000) @10C | [ |
Cr0.5Nb24.5O62 nanowires | Doping | Electrospinning | — | 0.8—3.0 | 344@0.1C | 209@30C | 92.8%(2000) @10C | [ |
CrNb11O29 nanorods | Doping | Hydrothermal | — | 0.8—3.0 | 307@0.5C | 228@10C | 91.1%(400) @10C | [ |
Mg2Nb34O87 porous microspheres | Doping | Solvothermal | — | 0.8—3.0 | 338@0.1C | 230@10C | 93.1%(500) @10C | [ |
ZrNb24O62 nanowires | Doping | Solvothermal | 1.4 | 0.8—3.0 | 320@0.1C | 182@30C | 90.2%(1500) @10C | [ |
GaNb11O29 nanowires | Doping | Electrospinning | 1.8 | 1.0—3.0 | 233@0.1 A/g | 169@0.7 A/g | 97%(500) @ 0.7 A/g | [ |
MoNb12O33 microspheres | Doping | Solvothermal | 1.0 | 0.8—3.0 | 321@0.1C | 200@10C | 95.7%(1000) @10C | [ |
| Nb16W5O55 | Doping | Solid?state reaction | 2.0—3.0 | 1.0—3.0 | 225@0.2C | 70@100C | 95%(750) @20C | [ |
| Nb14W3O44 | Doping | Solution combustion | 1.4—1.7 | 1.0—3.0 | 249@0.5C | 84.4@50C | 96.5%(4000) @10C | [ |
Nb8W9O47 nanofibers | Doping | Electrospinning | — | 1.0—3.0 | 196@1C | 147@5C | 78%(1000) @5C | [ |
Mo3Nb14O44 nanowires | Doping | Electrospinning | — | 0.8—3.0 | 321@0.1C | 174@10C | 75.8%(1000) @5C | [ |
| 3DOM?TiNb2O7 | Morphology and microstructure design | Template | 1.5 | 1.0—3.0 | 251@1C | 99@100C | 82%(1000) @10C | [ |
| Material | Modification | Synthetic method | Mass loading/ (mg·cm-2) | Potential range/V | Specific capacity/ (mA·h·g-1) | Rate capability/ (mA·h·g-1) | Cycling stability | Ref. |
Hollow Ti2Nb10O29 microspheres | Morphology and microstructure design | Hydrothermal | 1.0—1.5 | 1.0—3.0 | 219.8@0.2C | 173.4@10C | 95%(500) @10C | [ |
| TiNb2O7 nanorods | Morphology and microstructure design | Sol?gel | — | 1.0—3.0 | 265@0.1C | 84@50C | 68.4%(500) @10C | [ |
TiNb6O17/C nanoparticles | Conductive?phase compositing | Sol?gel | 1.5 | 1.0—3.0 | 275@1C | 132@30C | 92%(500) @10C | [ |
N?TiNb2O7@C nanoparticles | Conductive?phase compositing | Solid?state reaction | — | 1.0—3.0 | 268@1C | 135@50C | 82.7%(10000)@20C | [ |
Ti2Nb10O29/Ag composite | Conductive?phase compositing | Solid?state reaction and solvothermal | — | 1.0—2.5 | 282@0.2C | 132@20C | 82.1%(500) @10C | [ |
3D TiNb2O7 nanorod | Array/free?standing design | Hydrothermal | 2.0—2.5 | 1.0—3.0 | 240@0.5C | 151@40C | 86.4%(2000) @10C | [ |
Carbon?coated TiNb2O7 nanosheet | Array/free?standing design | Solvothermal | 5.8 | 1.0—3.0 | 250@1C | 96@30C | 87.8%(2000) @5C | [ |
Ti2Nb10O29@TiC/C nanoarray | Array/free?standing design | Chemical vapor deposition and solvothermal | 1.8 | 1.0—2.5 | 318@1C | 202@50C | 85%(10000) @10C | [ |
Ti2Nb10O29@TiC/ C?NC nanoarray | Array/free?standing design | Chemical vapor deposition and electrodeposition | 0.8 | 1.0—2.5 | 320@1C | 165@100C | 65%(10000) @10C | [ |
Ru0.01Ti0.99Nb2O7 micron?particles | Doping | Solid?state reaction | 1.0 | 0.8—3.0 | 295@0.1C | 181@5C | 90.1%(100) @5C | [ |
Cu0.02Ti0.94Nb2.04O7 micron?particles | Doping | Solid?state reaction | 1.0—2.0 | 0.8—3.0 | 315@0.1C | 182@10C | 98.8%(1000) @5C | [ |
| TiNb1.98V0.02O7 | Doping | Solid?state reaction | 2.6—2.7 | 1.0—3.0 | 260@0.3C | 172@10C | 65.6%(30) @5C | [ |
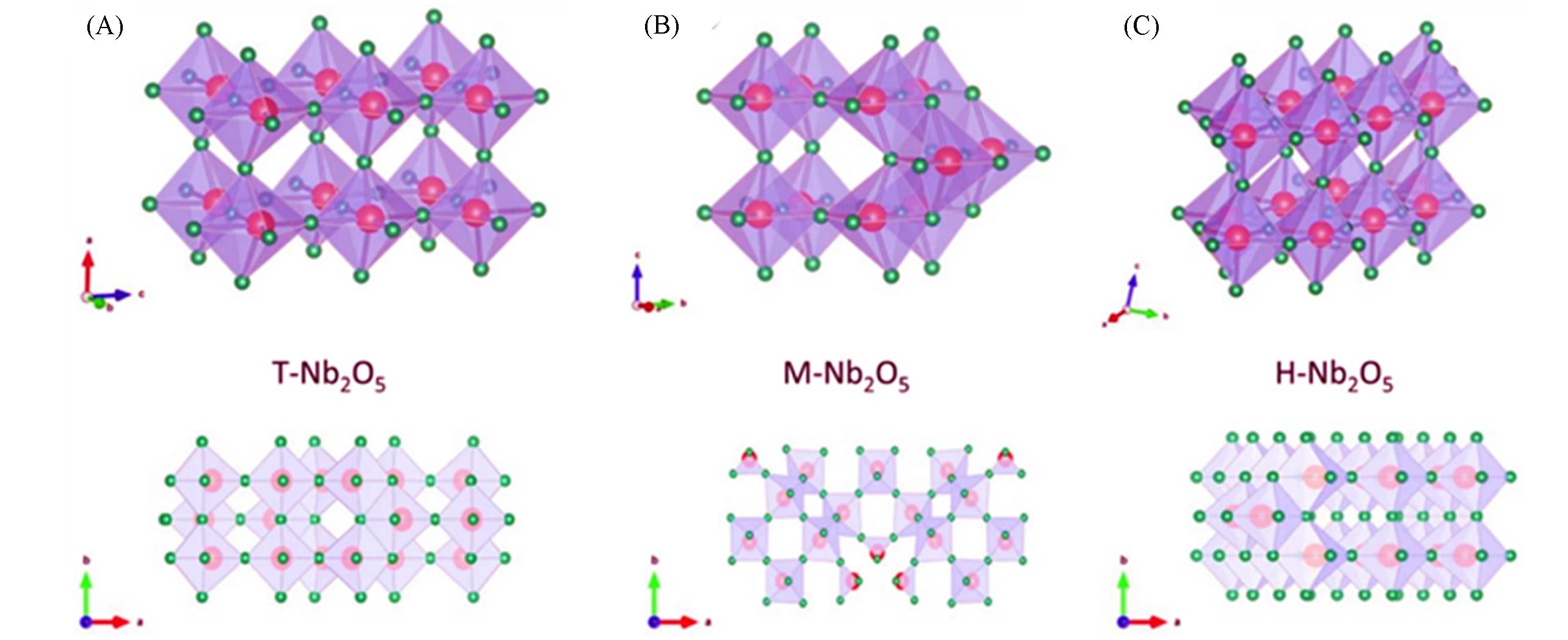
Fig.1 Crystal phase diagrams and the corresponding crystallographic plan views along the c?axis[64](A) T?Nb2O5; (B) M?Nb2O5; (C) H?Nb2O5. The red and green balls represent Nb atoms and O atoms, respectively.Copyright 2020, Springer.
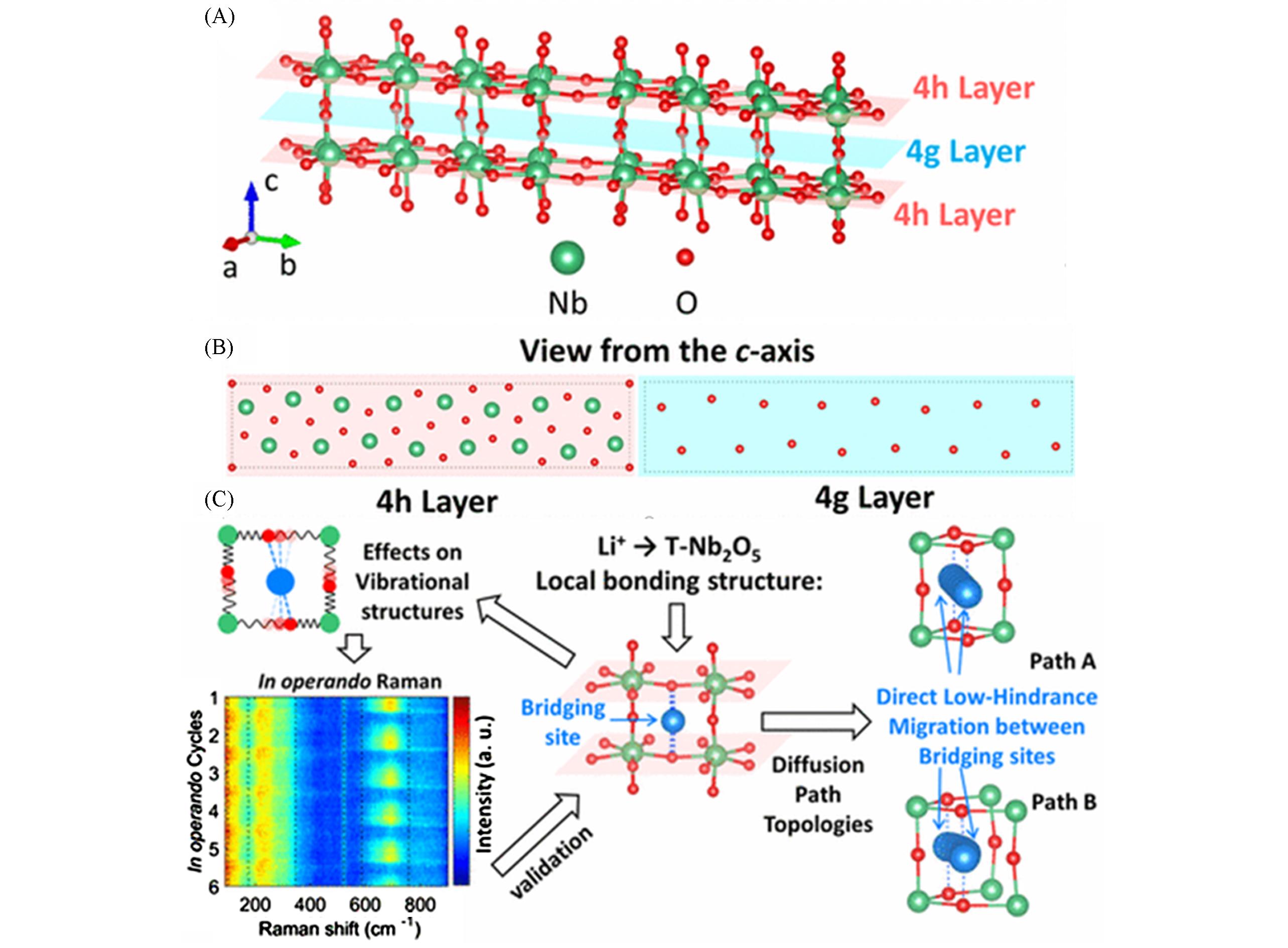
Fig.2 Lithium storage mechanism diagram of T?Nb2O5[71](A) Structure of the unlithiated model T?Nb2O5 with highlighted 4h layers and 4g layer; (B) atomic arrangements of the 4h layer and the 4g layer when viewed from the c?axis; (C) scheme of Li+ storage location and transmission path in T?Nb2O5 structure. Copyright 2017, American Chemical Society.
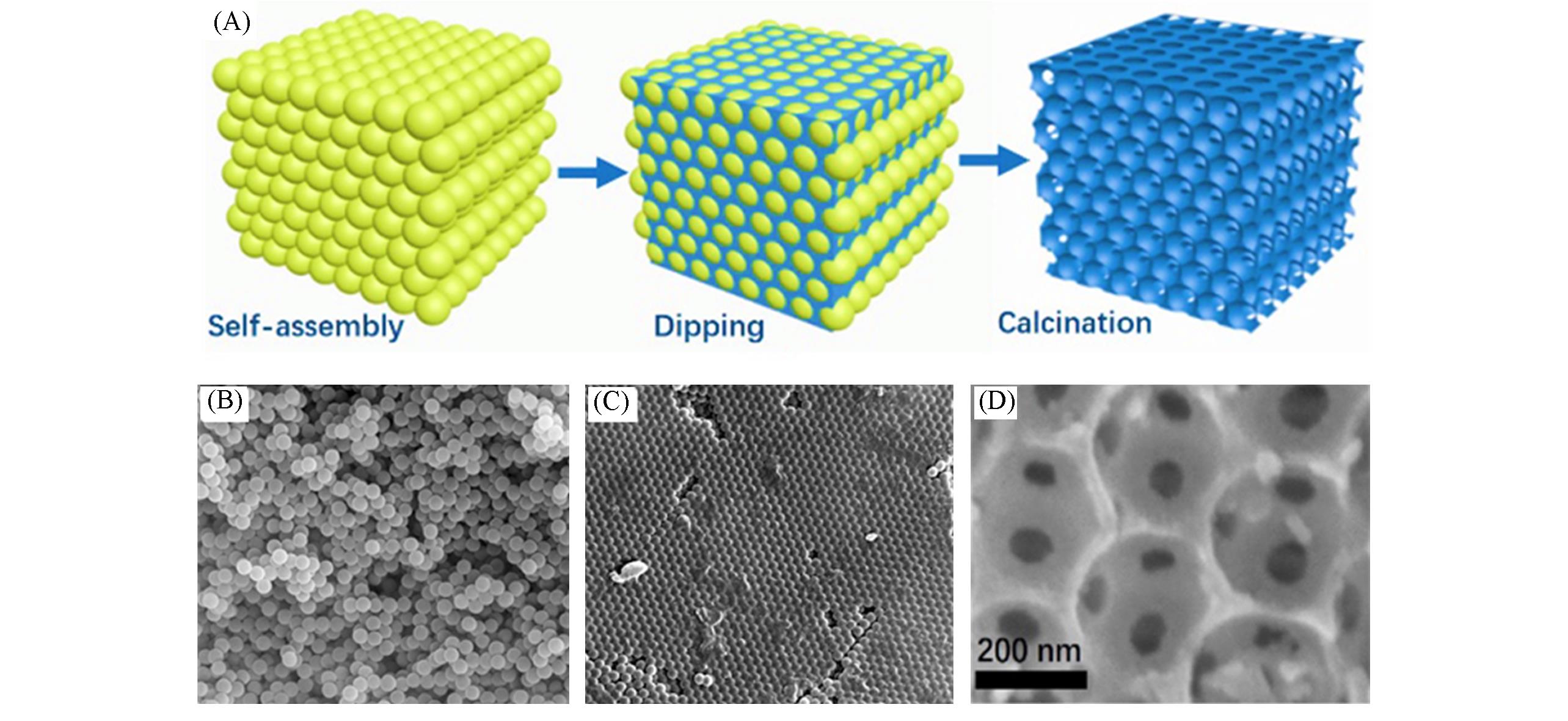
Fig.3 Synthesis route and morphology analysis of 3DOM T?Nb2O5[18](A) Synthesis route and structural analyses of 3DOM T?Nb2O5; (B) SEM image of monodispersed polystyrene microspheres; (C) SEM image of hexagonal close?packing microspheres after self?assembly process; (D) SEM image of as?prepared 3DOM T?Nb2O5. Copyright 2017, Elsevier.
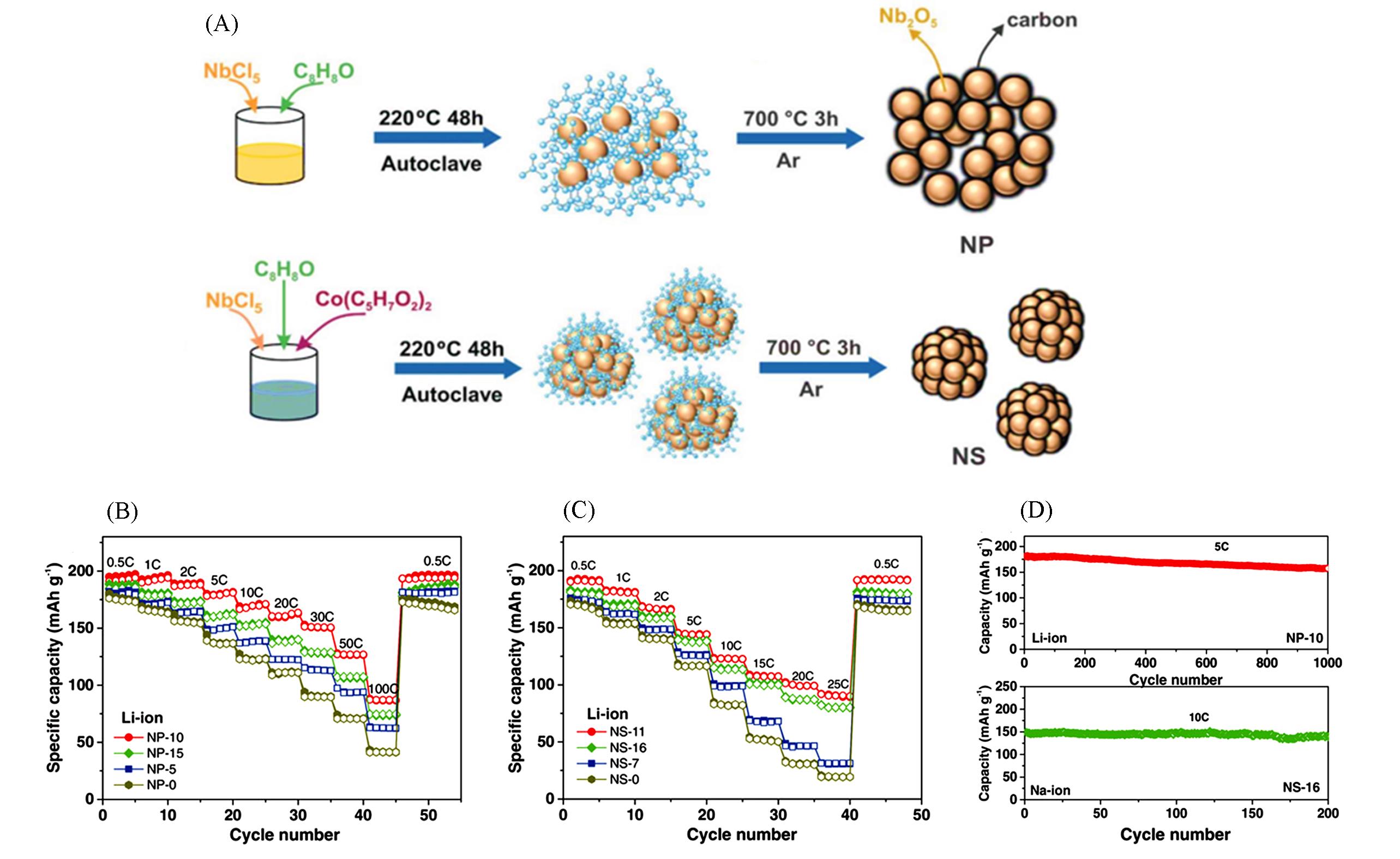
Fig.4 Synthesis diagram and electrochemical performance of NP and NS[24](A) Schematic illustration of the synthesis of the NP and NS Nb2O5 carbon composites; (B) electrochemical performance of the NP Li?ion half cells at various C rates; (C) electrochemical performance of the NS Li?ion half cells at various C rates; (D) cycling stability of NP?10 at 5C(Li?ion) and NS?16 at 10C(Na?ion). Copyright 2019, Wiley?VCH.
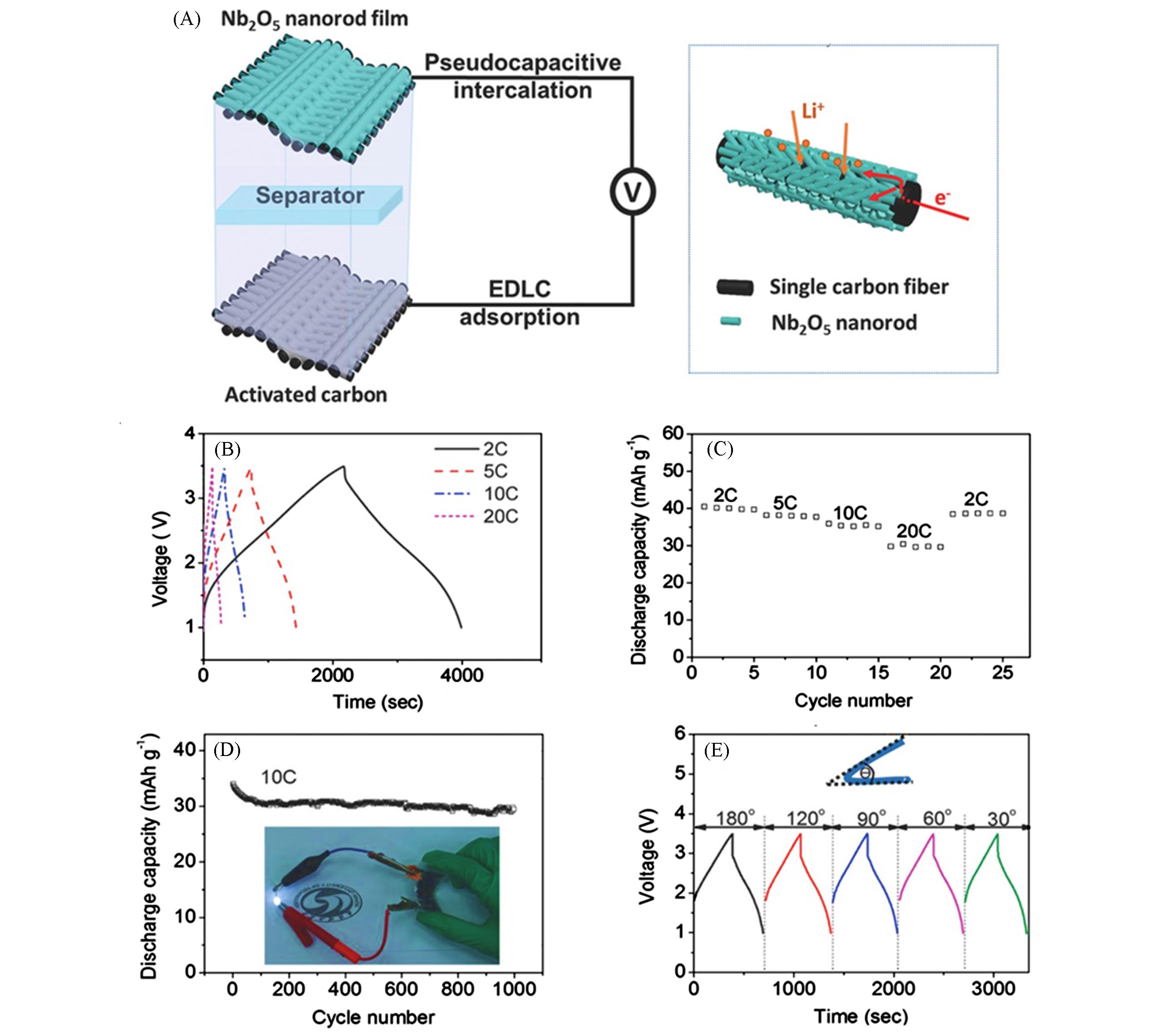
Fig.5 Schematic illustration of device configuration and electrochemical performance of Nb2O5 nanorod films[30](A) Schematic illustration of Li?ion hybrid capacitor device configuration and the advantages of electrochemical kinetics of the Nb2O5 nanorod films; (B) typical charge/discharge profiles of the hybrid Li?ion hybrid capacitor; (C) rate performance; (D) cycling performance at 10C. Inset shows the optical image of our flexible device that powering a white LED indicator; (E) charge/discharge curves at different bending angles. Copyright 2018, Wiley?VCH.
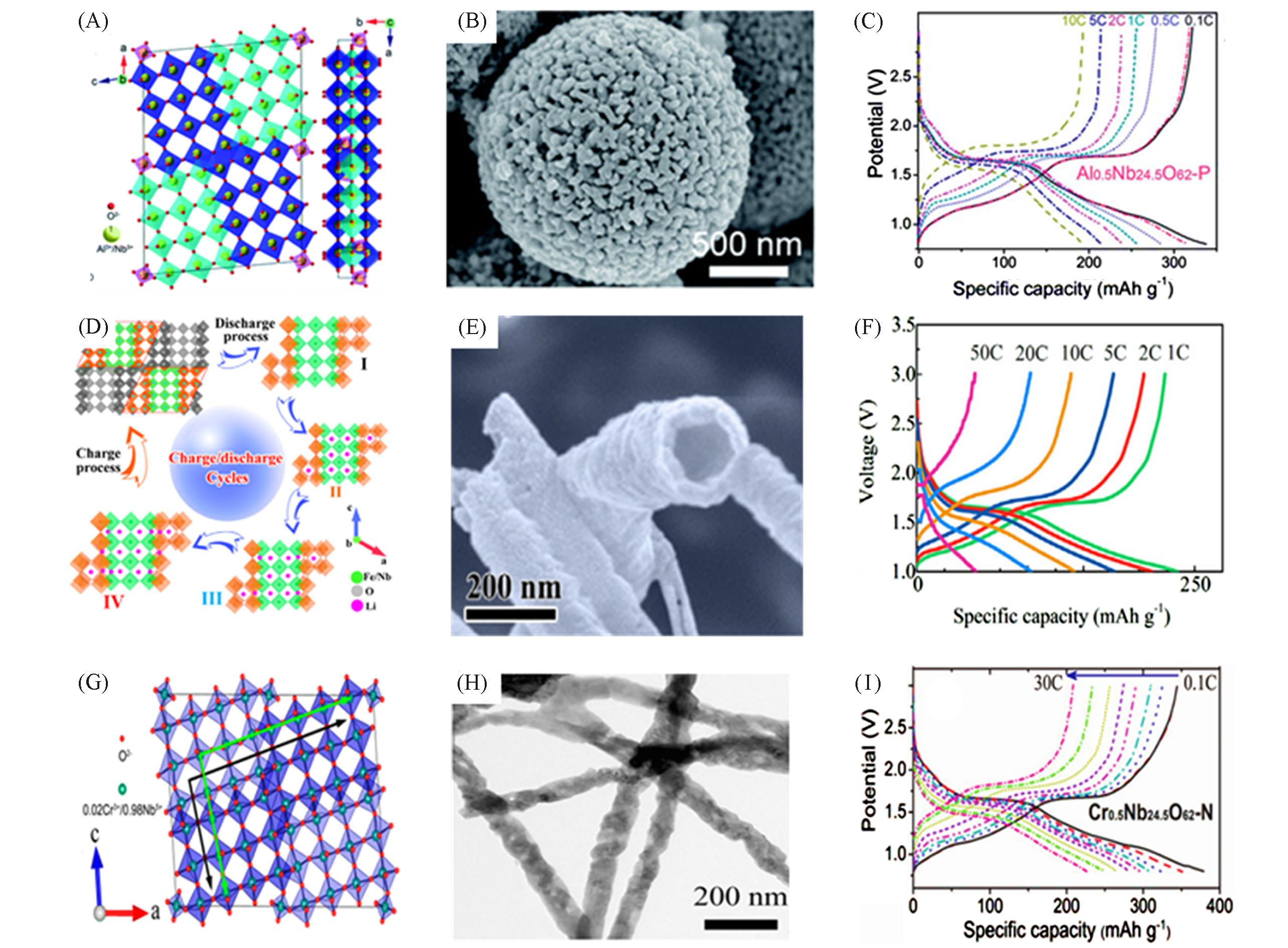
Fig.6 Crystal structure, SEM image and rate performance of various M?Nb?O(A) Crystal structure; (B) FESEM image; (C) discharging/charging curves of Al0.5Nb24.5O62[35]. Copyright 2019, Royal Society of Chemistry. (D) The lithiation model of FeNb11O29 at different lithiation states; (E) TEM image; (F) discharging/charging curves of FeNb11O29[36]. Copyright 2019, Elsevier.(G) Crystal structure; (H) TEM images; (I) discharging/charging curves of Cr0.5Nb24.5O62[37]. Copyright 2017, American Chemical Society.
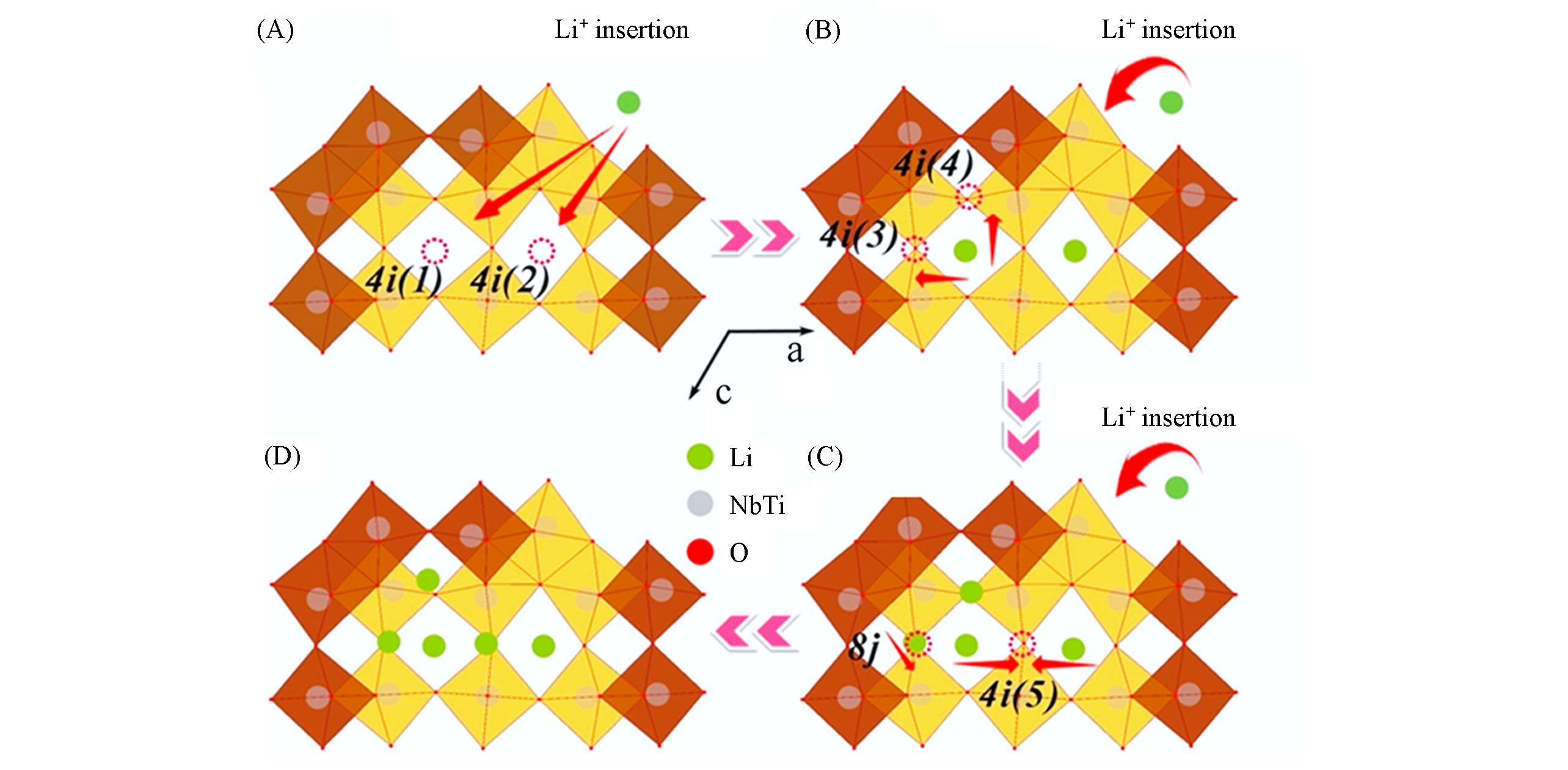
Fig.7 Lithiation process of TiNb2O7 during discharging[91](A) TiNb2O7; (B) Li0.88TiNb2O7; (C) Li2.67TiNb2O7; (D) Li4TiNb2O7. Copyright 2017, Elsevier.
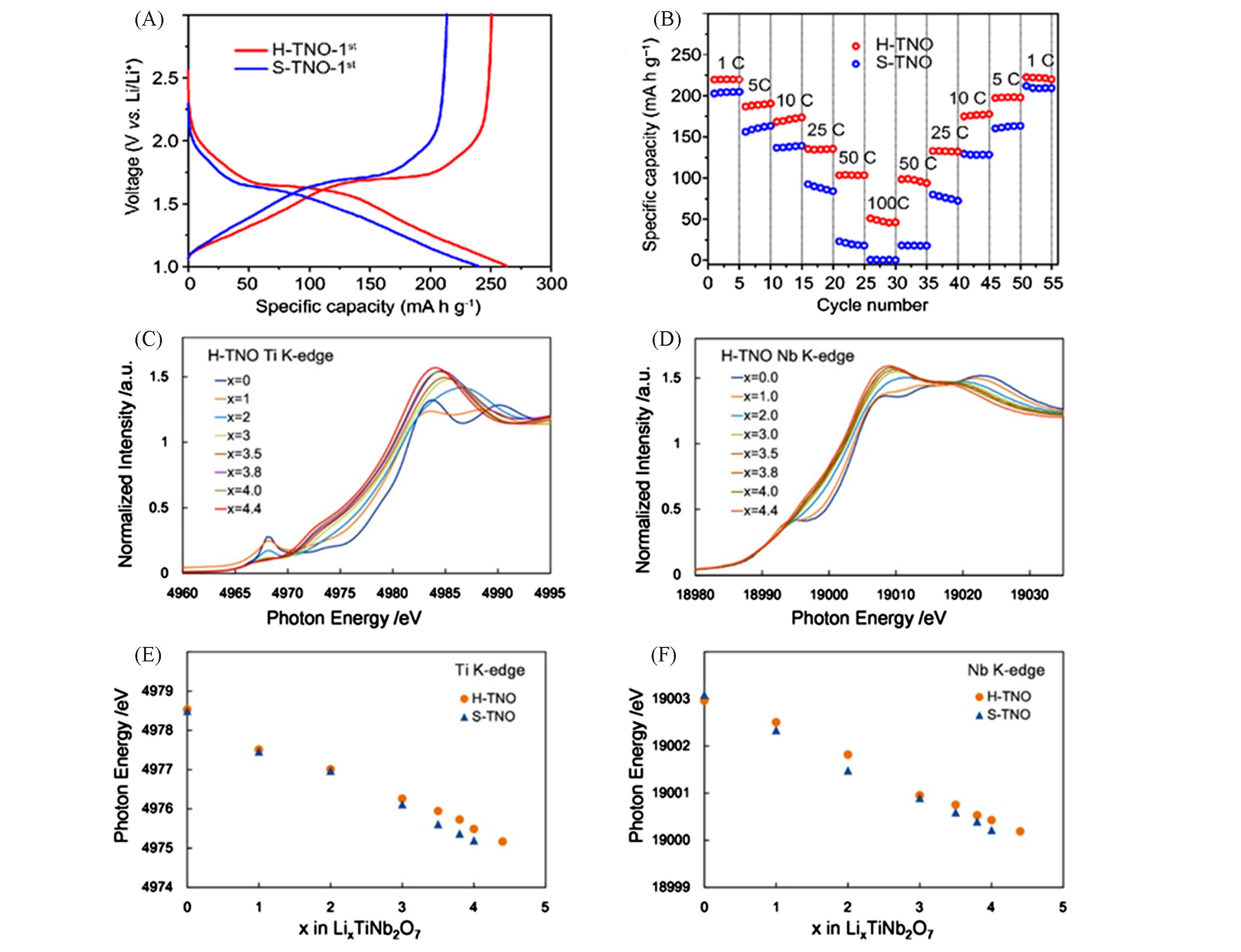
Fig.8 Electrochemical performance and XANES spectra of H?TNO and S?HTO[92](A) Discharging/charging curves of H?TNO and S?HTO; (B) rate performance of H?TNO and S?HTO; (C)―(F) Ti K?edge and Nb K?edge XANES spectra of lithium?inserted H?TNO and S?TNO at various x values in LixTiNb2O7. Copyright 2018, Elsevier.
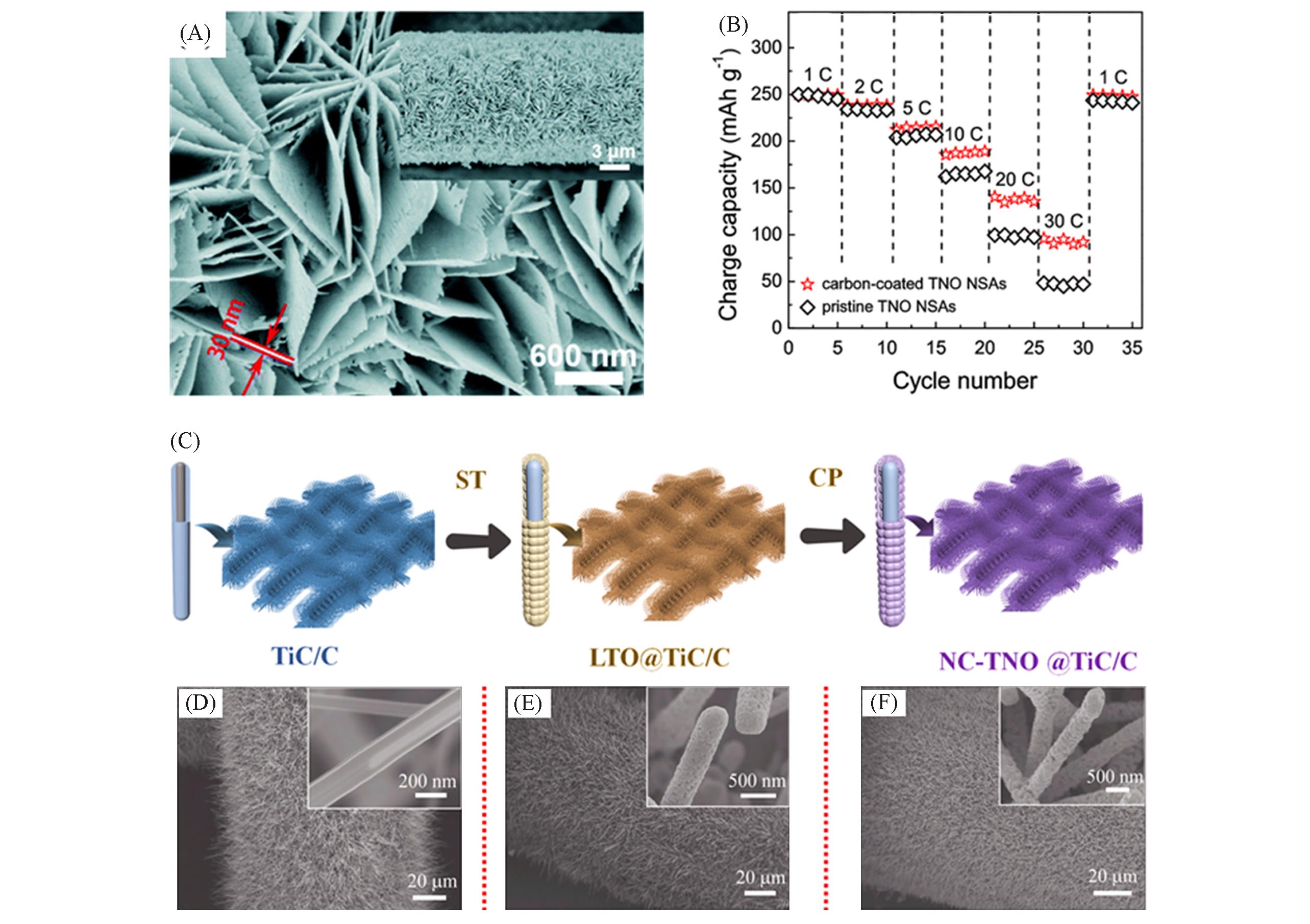
Fig.9 SEM image and rate performance of TNO arrays, synthesis route and morphology analysis of NC?TNO@TiC/C(A) SEM image of TNO arrays; (B) rate performance of TNO arrays[54]. Copyright 2021, the Royal Society of Chemistry.(C) Synthesis route of NC?TNO@TiC/C; (D) SEM image of TiC/C; (E) SEM image of LTO@TiC/C; (F) SEM image of NC?TNO@TiC/C[55]. Copyright 2018, Elsevier.
| 36 | Zheng R., Qian S., Cheng X., Yu H., Peng N., Liu T., Zhang J., Xia M., Zhu H., Shu J., Nano Energy, 2019, 58, 399—409 |
| 37 | Yang C., Yu S., Lin C., Lv F., Wu S., Yang Y., Wang W., Zhu Z. Z., Li J., Wang N., Guo S., ACS Nano, 2017, 11(4), 4217—4224 |
| 38 | Fu Q., Liu X., Hou J., Pu Y., Lin C., Yang L., Zhu X., Hu L., Lin S., Luo L., Chen Y., J. Power Sources, 2018, 397, 231—239 |
| 39 | Zhu X., Fu Q., Tang L., Lin C., Xu J., Liang G., Li R., Luo L., Chen Y., ACS Appl. Mater. Inter., 2018, 10(28), 23711—23720 |
| 40 | Yang C., Zhang Y., Lv F., Lin C., Liu Y., Wang K., Feng J., Wang X., Chen Y., Li J., Guo S., J. Mater. Chem. A, 2017, 5(42), 22297—22304 |
| 41 | Wang Z., Zheng R., Li Y., Yu H., Liu T., Peng N., Zhang J., Shui M., Shu J., Ceram. Int., 2020, 46(12), 20537—20544 |
| 42 | Zhu X., Xu J., Luo Y., Fu Q., Liang G., Luo L., Chen Y., Lin C., Zhao X. S., J. Mater. Chem. A, 2019, 7(11), 6522—6532 |
| 43 | Griffith K. J., Wiaderek K. M., Cibin G., Marbella L. E., Grey C. P., Nature, 2018, 559(7715), 556—563 |
| 44 | Yang Y., Zhu H., Xiao J., Geng H., Zhang Y., Zhao J., Li G., Wang X. L., Li C. C., Liu Q., Adv. Mater., 2020, 32(12), 1905295 |
| 45 | Yan L., Cheng X., Yu H., Zhu H., Liu T., Zheng R., Zhang R., Shui M., Shu J., Energy Storage Mater., 2018, 14, 159—168 |
| 46 | Li Z. H., Feng X. M., Mi L. W., Zheng J. Y., Chen X. Y., Chen W. H., Nano Res., 2018, 11(8), 4038—4048 |
| 47 | Lou S., Cheng X., Zhao Y., Lushington A., Gao J., Li Q., Zuo P., Wang B., Gao Y., Ma Y., Du C., Yin G., Sun X., Nano Energy, 2017, 34, 15—25 |
| 48 | Sun Y. G., Sun T. Q., Lin X. J., Tao X. S., Zhang D., Zeng C., Cao A. M., Wan L. J., Sci. China Chem., 2018, 61(6), 670—676 |
| 49 | Li Z. H., Feng X. M., Mi L. W., Zheng J. Y., Chen X. Y., Chen W. H., Nano Res., 2018, 11(8), 4038—4048 |
| 50 | Sun R., Tao Y., Sun H., Chen W., Liu G., Yue Y., Hu M., Liu M., J. Mater. Sci., 2019, 54(24), 14825—14833 |
| 51 | Gao J., Cheng X., Lou S., Wu X., Ding F., Zuo P., Ma Y., Du C., Gao Y., Yin G., J. Alloy. Compd., 2020, 835, 155241 |
| 52 | Mao W., Liu K., Guo G., Liu G., Bao K., Guo J., Hu M., Wang W., Li B., Zhang K., Qian Y., Electrochim. Acta, 2017, 253, 396—402 |
| 53 | Deng B., Dong H., Lei T., Yue N., Xiao L., Liu J., Sci. China Mater., 2019, 63(4), 492—504 |
| 54 | Zhou J., Dong H., Chen Y., Ye Y., Xiao L., Deng B., Liu J., Chem. Commun., 2021, 57(14), 1822—1825 |
| 55 | Yao Z., Xia X., Zhang Y., Xie D., Ai C., Lin S., Wang Y., Deng S., Shen S., Wang X., Yu Y., Tu J., Nano Energy, 2018, 54, 304—312 |
| 56 | Yao Z., Xia X., Zhang S., Zhou C., Pan G., Xiong Q., Wang Y., Wang X., Tu J., Energy Storage Mater., 2020, 25, 555—562 |
| 57 | Lin C., Yu S., Wu S., Lin S., Zhu Z. Z., Li J., Lu L., J. Mater. Chem. A, 2015, 3(16), 8627—8635 |
| 58 | Wen X., Ma C., Du C., Liu J., Zhang X., Qu D., Tang Z., Electrochim. Acta, 2015, 186, 58—63 |
| 59 | Yang C., Lin C., Lin S., Chen Y., Li J., J. Power Sources, 2016, 328, 336—344 |
| 60 | Zhao J. L., Yang M., Yang N. L., Wang J. Y., Wang D., Chem. Res. Chinese Universities, 2020, 36(3), 313—319 |
| 61 | Barkhordarian G., Klassen T., Bormann R., Scr. Mater., 2003, 49(3), 213—217 |
| 62 | Nico C., Monteiro T., Graca M. P. F., Prog. Mater. Sci., 2016, 80, 1—37 |
| 63 | Wang J., Y., Cui Y., Wang D., Nanoscale Horiz., 2020, 5(9), 1287—1292 |
| 64 | Shen P., Zhang B., Wang Y., Liu X., Yu C., Xu T., Mofarah S. S., Yu Y., Liu Y., Sun H., Arandiyan H., J. Nanostructure Chem., 2020, 11(1), 33—68 |
| 65 | Zhang C., Maloney R., Lukatskaya M. R., Beidaghi M., Dyatkin B., Perre E., Long D., Qiao W., Dunn B., Gogotsi Y., J. Power Sources, 2015, 274, 121—129 |
| 66 | Nowak I., Ziolek M., Chem. Rev., 1999, 99(12), 3603—3624 |
| 67 | Kong L., Cao X., Wang J., Qiao W., Ling L., Long D., J. Power Sources, 2016, 309, 42—49 |
| 68 | Song Z., Li H., Liu W., Zhang H., Yan J., Tang Y., Huang J., Zhang H., Li X., Adv. Mater., 2020, 32(22), 2001001 |
| 69 | Griffith K. J., Forse A. C., Griffin J. M., Grey C. P., J. Am. Chem. Soc., 2016, 138(28), 8888—8899 |
| 70 | Augustyn V., Simon P., Dunn B., Energy Environ. Sci., 2014, 7(5), 1597—1614 |
| 71 | Chen D., Wang J. H., Chou T. F., Zhao B., El⁃Sayed M. A., Liu M., J. Am. Chem. Soc., 2017, 139(20), 7071—7081 |
| 72 | Augustyn V., Come J., Lowe M. A., Kim J. W., Taberna P. L., Tolbert S. H., Abruna H. D., Simon P., Dunn B., Nat. Mater., 2013, 12(6), 518—522 |
| 73 | Jiang Y., Liu J., Energy Environ. Mater., 2019, 2(1), 30—37 |
| 74 | Shi C., Xiang K., Zhu Y., Zhou W., Chen X., Chen H., Ceram. Int., 2017,43, 12388—12395 |
| 75 | Liu M., Yan C., Zhang Y., Sci. Rep., 2015, 5, 8326 |
| 76 | Yang H., Xu H., Wang L., Zhang L., Huang Y., Hu X., Chem. Eur. J., 2017, 23(17), 4203—4209 |
| 77 | Dien L. X., Truong Q. D., Murayama T., Chinh H. D., Taketoshi A., Honma I., Haruta M., Ishida T., Appl. Catal. A: Gen., 2020, 603, 117747 |
| 78 | Chen Y., Dong H. Y., Li Y. Y., Liu J. P., Acta Phys. Chim. Sin., doi:10.3866/PKU.WHXB202007075(陈瑶, 董浩洋, 李园园, 刘金平. 物理化学学报, doi:10.3866/PKU.WHXB202007075) |
| 79 | Liu W. Y., Li L. P., Gui Q. Y., Deng B. H., Li Y. Y., Liu J. P., Acta Phys. Chim. Sin., 2020, 36(2), 1904049(刘文燚, 栗林坡, 桂秋月, 邓伯华, 李园园, 刘金平. 物理化学学报, 2020, 36(2), 1904049) |
| 80 | Lou X., Lin C., Luo Q., Zhao J., Wang B., Shao Q., Guo X., Wang N., Guo Z., ChemElectroChem, 2017, 4(12), 3171—3180 |
| 81 | Ette P. M., Babu D. B., Roy M. L., Ramesha K., J. Power Sources, 2019, 436, 226850 |
| 82 | Lee S. Y., Lim A. S., Kwon Y. M., Cho K. Y., Yoon S., Inorg. Chem. Front., 2020, 7(17), 3176—3183 |
| 83 | Qian S., Yu H., Cheng X., Zheng R., Zhu H., Liu T., Shui M., Xie Y., Shu J., J. Mater. Chem. A, 2018, 6(36), 17389—17400 |
| 84 | Kocer C. P., Griffith K. J., Grey C. P., Morris A. J., J. Am. Chem. Soc., 2019, 141(38), 15121—15134 |
| 85 | Chen Z., Cheng X., Long N., Zhu H., Yu H., Ye W., Zheng R., Shui M., Shu J., Ceram. Int., 2018, 44(4), 4080—4087 |
| 86 | Yan L., Lan H., Yu H., Qian S., Cheng X., Long N., Zhang R., Shui M., Shu J., J. Mater. Chem. A, 2017, 5(19), 8972—8980 |
| 87 | Ye W., Yu H., Cheng X., Zhu H., Zheng R., Liu T., Long N., Shui M., Shu J., Electrochim. Acta, 2018, 292, 331—338 |
| 88 | Tian Q., Ye W., Yu H., Cheng X., Zhu H., Long N., Shui M., Shu J., Ceram. Int., 2019, 45(2), 1893—1899 |
| 89 | Cava R. J., Murphy D. W., Zahurak S. M., J. Electrochem. Soc., 1983, 130(12), 2345 |
| 90 | Han J. T., Huang Y. H., Goodenough J. B., Chem. Mater., 2011, 23(8), 2027—2029 |
| 91 | Yu H., Lan H., Yan L., Qian S., Cheng X., Zhu H., Nano Energy, 2017, 38, 109—117 |
| 92 | Ise K., Morimoto S., Harada Y., Takami N., Solid State Ionics, 2018, 320, 7—15 |
| 93 | Zhao L., Wang S., Dong Y., Quan W., Han F., Huang Y., Li Y., Liu X., Li M., Zhang Z., Zhang J., Tang Z., Li J., Energy Storage Mater., 2021, 34, 574—581 |
| 94 | Takami N., Inagaki H., Kishi T., Hoshina K., J. Electrochem. Soc., 2009, 156(2), A128 |
| 95 | Harada Y., Hoshina K., Inagaki H., Takami N., Electrochim. Acta, 2013, 112, 310—317 |
| 96 | Takami N., Ise K., Harada Y., Iwasaki T., Kishi T., Hoshina K., J. Power Sources, 2018, 396, 429—436 |
| 97 | Dong S., Wang Y., Chen C., Shen L., Zhang X., Nanomicro Lett., 2020, 12(1), 1—11 |
| 98 | Lakhnot A. S., Gupta T., Singh Y., Hundekar P., Jain R., Han F., Koratkar N., Energy Storage Mater., 2020, 27, 506—513 |
| 1 | Yoshino A., Angew. Chem. Int. Ed., 2012, 51(24), 5798—5800 |
| 2 | Li H., Han C., Huang Y., Huang Y., Zhu M., Pei Z., Xue Q., Wang Z., Liu Z., Tang Z., Wang Y., Kang F., Li B., Zhi C., Energy Environ. Sci., 2018, 11(4), 941—951 |
| 99 | Buannic L., Colin J. F., Chapuis M., Chakir M., Patoux S., J. Mater. Chem. A, 2016, 4(29), 11531—11541 |
| 3 | Wang Z. Q., Sun Y. L., Qian Z. F., Wang R. H., Chem. J. Chinese Universities, 2021, 42(4), 1017—1030(王增强, 孙一翎, 钱正芳, 王任衡. 高等学校化学学报, 2021, 42(4), 1017—1030) |
| 4 | Zeng X., Li M., Abd El⁃Hady D., Alshitari W., Al⁃Bogami A. S., Lu J., Amine K., Adv. Energy Mater., 2019, 9(27), 1900161 |
| 5 | Liu D., Zhao Y., Tan R., Tian L. L., Liu Y., Chen H., Pan F., Nano Energy, 2017, 36, 206—212 |
| 6 | Bi R. Y., Mao D., Wang J. Y., Yu R. B., Wang D., Acta Chim. Sinica, 2021, 78(11), 1200—1212(毕如一, 毛丹, 王江艳, 于然波, 王丹. 化学学报, 2021, 78(11), 1200—1212) |
| 7 | Bi R. Y., Xu N., Ren H., Yang N. L., Sun Y. Y., Can A. M., Yu R. B., Wang D., Angew. Chem. Int. Ed., 2012, 132(12), 4895—4898 |
| 8 | Wang C., Wang J. Y., Hu W. P., Wang D., Chem. Res. Chinese Universities, 2020, 36(1), 68—73 |
| 9 | Yi T. F., Yang S. Y., Xie Y., J. Mater. Chem. A, 2015, 3(11), 5750—5777 |
| 10 | Deng Q., Fu Y., Zhu C., Yu Y., Small, 2019, 15(32), 1804884 |
| 11 | Yan L., Rui X., Chen G., Xu W., Zou G., Luo H., Nanoscale, 2016, 8(16), 8443—8465 |
| 12 | Ding H., Song Z., Zhang H., Zhang H., Li X., MT Nano, 2020, 11, 100082 |
| 13 | Griffith K. J., Harada Y., Egusa S., Ribas R. M., Monteiro R. S., Von Dreele R. B., Cheetham A. K., Cava R. J., Grey C. P., Goodenough J. B., Chem. Mater., 2020, 33(1), 4—18 |
| 14 | Hu L., Luo L., Tang L., Lin C., Li R., Chen Y., J. Mater. Chem. A, 2018, 6(21), 9799—9815 |
| 15 | Li Z. H., Feng X. M., Mi L. W., Zheng J. Y., Chen X. Y., Chen W. H., Nano Res., 2018, 11(8), 4038—4048 |
| 16 | Liu X., Liu G., Chen H., Ma J., Zhang R., J. Phys. Chem. Solids, 2017, 111, 8—11 |
| 17 | Shen S., Zhang S., Cao X., Deng S., Pan G., Liu Q., Wang X., Xia X., Tu J., Energy Storage Mater., 2020, 25, 695—701 |
| 18 | Lou S., Cheng X., Wang L., Gao J., Li Q., Ma Y., Gao Y., Zuo P., Du C., Yin G., J. Power Sources, 2017, 361, 80—86 |
| 19 | Cheong J. Y., Jung J. W., Youn D. Y., Kim C., Yu S., Cho S. H., Yoon K. R., Kim I. D., J. Power Sources, 2017, 360, 434—442 |
| 20 | Sun Y. G., Piao J. Y., Hu L. L., Bin D. S., Lin X. J., Duan S. Y., Cao A. M., Wan L. J., J. Am. Chem. Soc., 2018, 140(29), 9070—9073 |
| 21 | Liu Z., Dong W., Wang J., Dong C., Lin Y., Chen I. W., Huang F., iScience, 2020, 23(1), 100767 |
| 22 | Lübke M., Sumboja A., Johnson I. D., Brett D. J. L., Shearing P. R., Liu Z., Darr J. A., Electrochim. Acta, 2016, 192, 363—369 |
| 23 | Liu X., Liu G., Liu Y., Sun R., Ma J., Guo J., Hu M., Dalton Trans., 2017, 46(33), 10935—10940 |
| 24 | Han X., Russo P. A., Goubard⁃Bretesché N., Patanè S., Santangelo S., Zhang R., Pinna N., Adv. Energy Mater., 2019, 9(42), 1902813 |
| 25 | Lim E., Jo C., Kim H., Kang K., Yoon S., Lee J., ACS Nano, 2015, 9(7), 7497—7505 |
| 26 | Cheong J. Y., Youn D. Y., Kim C., Jung J. W., Ogata A. F., Bae J. G., Kim I. D., Electrochim. Acta, 2018, 269, 388—396 |
| 27 | Kong F., Tao S., Qian B., Gao L., Ceram. Int., 2018, 44(18), 23226—23231 |
| 28 | Deng Q., Li M., Wang J., Jiang K., Hu Z., Chu J., Nanotechnology, 2018, 29(18), 185401 |
| 29 | Kong L., Zhang C., Zhang S., Wang J., Cai R., Lv C., Qiao W., Ling L., Long D., J. Mater. Chem. A, 2014, 2(42), 17962—17970 |
| 30 | Deng B., Lei T., Zhu W., Xiao L., Liu J., Adv. Funct. Mater., 2018, 28(1), 1704330 |
| 31 | Jiao Y. Z., Zhang H. T., Zhang H. L., Liu A., Liu Y. X., Zhang S. J., Nano Res., 2018, 11(9), 4673—4685 |
| 32 | He S. R., Zou J. P., Chen L. B., Chen Y. J., Rare Met., 2021, 40, 374—382 |
| 33 | Sun H. T., Mei L., Liang J. F., Zhao Z. P., Lee C., Fei H. L., Ding M., Lau J., Li M. F., Wang C., Xu X., Hao G. L., Papandrea B., Shakir I., Dunn B., Huang Y., Duan X. F., Science, 2017, 356(6338), 599—604 |
| 34 | Kong L., Zhang C., Wang J., Qiao W., Ling L., Long D., ACS Nano, 2015, 9(11), 11200—11208 |
| 35 | Fu Q., Li R., Zhu X., Liang G., Luo L., Chen Y., Lin C., Zhao X. S., J. Mater. Chem. A, 2019, 7(34), 19862—19871 |
| [1] | 贾洋刚, 邵霞, 程婕, 王朋朋, 冒爱琴. 赝电容控制型钙钛矿高熵氧化物La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3负极材料的制备及储锂性能[J]. 高等学校化学学报, 2022, 43(8): 20220157. |
| [2] | 田润赛, 卢芊, 张洪滨, 张渤, 冯源源, 魏金香, 冯季军. 氮杂碳原位包覆Cu2O/Co3O4@C异质结构复合材料的设计构筑及高效储锂性能[J]. 高等学校化学学报, 2021, 42(8): 2592. |
| [3] | 吴卓彦, 李至, 赵旭东, 王倩, 陈顺鹏, 常兴华, 刘志亮. 一步法高效制备纳米Si/C复合材料及其在高性能锂离子电池中的应用[J]. 高等学校化学学报, 2021, 42(8): 2500. |
| [4] | 韩慕瑶, 赵丽娜, 孙洁. 硅及硅基负极材料的研究进展[J]. 高等学校化学学报, 2021, 42(12): 3547. |
| [5] | 荣华, 王春刚, 周明. 用作锂离子电池负极的FeS2微球的制备及性能[J]. 高等学校化学学报, 2020, 41(3): 447. |
| [6] | 方亮, 丁晓丽, 宋云, 柳东明, 李永涛, 张庆安. 钙钛矿型LaCoO3电化学储锂的形貌调制效应[J]. 高等学校化学学报, 2019, 40(7): 1456. |
| [7] | 林伟国, 孙伟航, 曲宗凯, 冯晓磊, 荣峻峰, 陈旭, 杨文胜. 锂离子电池负极材料纳米多孔硅/石墨/碳复合微球的制备与性能[J]. 高等学校化学学报, 2019, 40(6): 1216. |
| [8] | 王秋娴, 李凯, 杨贝宁, 岳红云, 杨书廷. 海藻酸钠引导合成纳微复合结构ZnFe2O4及其在锂离子电池中的应用[J]. 高等学校化学学报, 2018, 39(9): 2039. |
| [9] | 李常青, 杨东杰, 席跃宾, 秦延林, 邱学青. 二氧化硅/木质素多孔碳复合材料的制备及作为锂离子电池负极材料的性能[J]. 高等学校化学学报, 2018, 39(12): 2725. |
| [10] | 许笑目, 张兴帅, 陈志柠, 王静, 郭玉忠, 黄瑞安, 王剑华. 无序介孔硅复合纳米结构的制备与慢活化行为[J]. 高等学校化学学报, 2017, 38(5): 713. |
| [11] | 史楠楠, 姜雪, 张莹, 程魁, 叶克, 王贵领, 曹殿学. N掺杂C包覆Li4Ti5O12锂离子电池负极材料的制备与性能[J]. 高等学校化学学报, 2015, 36(5): 981. |
| [12] | 岳红云, 王秋娴, 张雪, 华双, 马华, 岳东媛, 杨书廷. 微纳复合结构MFe2O4负极材料的可控合成与性能[J]. 高等学校化学学报, 2015, 36(4): 745. |
| [13] | 尹红, 周丹, 丛丽娜, 谢海明, 仇永清. MoO2/C共包覆Si/石墨复合锂离子电池负极材料的研究[J]. 高等学校化学学报, 2015, 36(10): 1990. |
| [14] | 石慧敏, 王惠, 尹金维, 朱青云, 吴平, 唐亚文, 周益明, 陆天虹. MWCNT@SiO2纳米同轴电缆的制备及储锂性能[J]. 高等学校化学学报, 2015, 36(1): 175. |
| [15] | 吴平, 杜宁, 张辉, 杨德仁, 陆天虹. 锂离子电池用α-Fe2O3-Ag复合纳米棒负极材料的制备及储锂性能[J]. 高等学校化学学报, 2013, 34(3): 668. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||