

高等学校化学学报 ›› 2018, Vol. 39 ›› Issue (4): 749.doi: 10.7503/cjcu20170463
田琳飞1, 张春华2, 曲宁1, 毕艳婷1, 张红星3( ), 潘清江1(
), 潘清江1( )
)
收稿日期:2017-07-14
出版日期:2018-04-10
发布日期:2018-03-16
作者简介:联系人简介: 张红星, 男, 博士, 教授, 博士生导师, 主要从事理论与应用量子化学方面的研究. E-mail:基金资助:
TIAN Linfei1, ZHANG Chunhua2, QU Ning1, BI Yanting1, ZHANG Hongxing3,*( ), PAN Qingjiang1,*(
), PAN Qingjiang1,*( )
)
Received:2017-07-14
Online:2018-04-10
Published:2018-03-16
Contact:
ZHANG Hongxing,PAN Qingjiang
E-mail:zhanghx@jlu.edu.cn;panqjitc@163.com
Supported by:摘要:
为探索四聚吡咯配体和低价铀离子相互作用, 以实验合成单层三明治结构配合物PcUⅥPc(Pc=酞菁)为基础, 设计双层三明治型PzUmPzUmPz(m=Ⅲ, Ⅳ, Pz=氮杂卟啉), 采用相对论密度泛函理论考察了其几何结构、 异构体相对稳定性以及成键和轨道性质. 得到se(staggered-eclipsed)和es(eclipsed-staggered) 2种类型稳定空间异构体, 并进一步优化其所有可能的电子自旋态异构体. 计算结果表明, 这些低价铀配合物均具有五重态基态. 分子中的原子量子理论(quantum theory of atoms in molecule, QTAIM)在U-N键临界点处的电子/能量密度拓扑分析显示U-N键为弱极性共价键. 四价配合物拥有4个U(5f)性质高能占据轨道, 与2个U4+的5f单电子数相一致; 而三价配合物有很大配体参与作用. 2个铀原子和中间Pz配体质心近似成线性, 这与配合物具有稳定的σ(U-U)成键轨道密切相关.
中图分类号:
TrendMD:
田琳飞, 张春华, 曲宁, 毕艳婷, 张红星, 潘清江. 双层三明治四聚吡咯铀配合物的结构设计和稳定性理论计算. 高等学校化学学报, 2018, 39(4): 749.
TIAN Linfei, ZHANG Chunhua, QU Ning, BI Yanting, ZHANG Hongxing, PAN Qingjiang. Theoretical Studies of Structural Design and Stability of Double-layered Sandwich-like Tetrapyrrolic Uranium Complexes†. Chem. J. Chinese Universities, 2018, 39(4): 749.
| Complex | Config. | ESS | ΔEa/(kJ·mol-1) | Δ | ΔGa/(kJ·mol-1) | ΔHa/(kJ·mol-1) |
|---|---|---|---|---|---|---|
| se | Singlet | 86.6 | 82.2 | 89.5 | 81.4 | |
| Triplet | 11.7 | 11.1 | 12.5 | 11.2 | ||
| Quintet | 0 | 0 | 0 | 0 | ||
| es | Singlet | 92.9 | 91.5 | 99.2 | 90.6 | |
| Tripletb | 23.6 | 16.9 | 21.8 | 14.2 | ||
| Quintet | 0.2 | 2.4 | 6.2 | 1.9 | ||
| se | Singlet | 77.8 | 70.9 | 70.2 | 71.7 | |
| Triplet | 7.9 | 3.5 | 5.4 | 1.5 | ||
| Quintet | 2.9 | -0.2 | 3.2 | -2.0 | ||
| Septet | 10.0 | 9.1 | 5.0 | 9.6 | ||
| es | Singlet | 82.0 | 76.7 | 77.3 | 77.1 | |
| Triplet | 6.7 | 5.0 | 3.9 | 5.4 | ||
| Quintet | 0 | 0 | 0 | 0 | ||
| Septet | 10.0 | 9.2 | 12.0 | 7.1 |
Table 1 Relative energy of Um2Pz3(m=Ⅳ, Ⅲ) complexes in various configurations(staggered-eclipsed and eclipsed-staggered) and electron-spin states(ESS)
| Complex | Config. | ESS | ΔEa/(kJ·mol-1) | Δ | ΔGa/(kJ·mol-1) | ΔHa/(kJ·mol-1) |
|---|---|---|---|---|---|---|
| se | Singlet | 86.6 | 82.2 | 89.5 | 81.4 | |
| Triplet | 11.7 | 11.1 | 12.5 | 11.2 | ||
| Quintet | 0 | 0 | 0 | 0 | ||
| es | Singlet | 92.9 | 91.5 | 99.2 | 90.6 | |
| Tripletb | 23.6 | 16.9 | 21.8 | 14.2 | ||
| Quintet | 0.2 | 2.4 | 6.2 | 1.9 | ||
| se | Singlet | 77.8 | 70.9 | 70.2 | 71.7 | |
| Triplet | 7.9 | 3.5 | 5.4 | 1.5 | ||
| Quintet | 2.9 | -0.2 | 3.2 | -2.0 | ||
| Septet | 10.0 | 9.1 | 5.0 | 9.6 | ||
| es | Singlet | 82.0 | 76.7 | 77.3 | 77.1 | |
| Triplet | 6.7 | 5.0 | 3.9 | 5.4 | ||
| Quintet | 0 | 0 | 0 | 0 | ||
| Septet | 10.0 | 9.2 | 12.0 | 7.1 |
| Species | se | es | Expt.h | |||
|---|---|---|---|---|---|---|
| Singlet | Triplet | Quintet | Singlet | Quintet | ||
| U1-U2/nm | 0.3513 | 0.3568 | 0.3611 | 0.3499 | 0.3607 | 0.331 |
| U1-N(Pz1 | 0.2326 | 0.2342 | 0.2346 | 0.2348 | 0.2355 | |
| U1-N(Pz2 | 0.2588 | 0.2604 | 0.2619 | 0.2568 | 0.2633 | 0.242-0.248 |
| (U1-N | 0.2457 | 0.2473 | 0.2482 | 0.2458 | 0.2494 | |
| U2-N(Pz2 | 0.2588 | 0.2603 | 0.2619 | 0.2585 | 0.2607 | 0.243-0.253 |
| U2-N(Pz3 | 0.2326 | 0.2342 | 0.2346 | 0.2341 | 0.2350 | |
| (U2-N | 0.2457 | 0.2472 | 0.2482 | 0.2463 | 0.2479 | |
| U1-N4(Pz1)c/nm | 0.1262 | 0.1290 | 0.1291 | 0.1303 | 0.1304 | |
| U1-N4(Pz2)c/nm | 0.1752 | 0.1785 | 0.1806 | 0.1735 | 0.1823 | |
| U1-N | 0.1507 | 0.1538 | 0.1548 | 0.1519 | 0.1563 | |
| U2-N4(Pz2)c/nm | 0.1752 | 0.1784 | 0.1806 | 0.1761 | 0.1785 | |
| U2-N4(Pz3)c/nm | 0.1262 | 0.1290 | 0.1291 | 0.1289 | 0.1298 | |
| U2-N | 0.1507 | 0.1537 | 0.1548 | 0.1525 | 0.1541 | |
| U1-(Pz2)cent. -U2e/(°) | 180.0 | 180.0 | 180.0 | 179.3 | 180.0 | |
| h1f/(°) | 44.9 | 52.7 | 48.9 | 19.0g | -12.8g | |
| h2f/(°) | 0.8 | 0.2 | 7.7 | 42.5 | 30.8 | |
Table 2 Optimized geometry parameters of U2ⅣPz3
| Species | se | es | Expt.h | |||
|---|---|---|---|---|---|---|
| Singlet | Triplet | Quintet | Singlet | Quintet | ||
| U1-U2/nm | 0.3513 | 0.3568 | 0.3611 | 0.3499 | 0.3607 | 0.331 |
| U1-N(Pz1 | 0.2326 | 0.2342 | 0.2346 | 0.2348 | 0.2355 | |
| U1-N(Pz2 | 0.2588 | 0.2604 | 0.2619 | 0.2568 | 0.2633 | 0.242-0.248 |
| (U1-N | 0.2457 | 0.2473 | 0.2482 | 0.2458 | 0.2494 | |
| U2-N(Pz2 | 0.2588 | 0.2603 | 0.2619 | 0.2585 | 0.2607 | 0.243-0.253 |
| U2-N(Pz3 | 0.2326 | 0.2342 | 0.2346 | 0.2341 | 0.2350 | |
| (U2-N | 0.2457 | 0.2472 | 0.2482 | 0.2463 | 0.2479 | |
| U1-N4(Pz1)c/nm | 0.1262 | 0.1290 | 0.1291 | 0.1303 | 0.1304 | |
| U1-N4(Pz2)c/nm | 0.1752 | 0.1785 | 0.1806 | 0.1735 | 0.1823 | |
| U1-N | 0.1507 | 0.1538 | 0.1548 | 0.1519 | 0.1563 | |
| U2-N4(Pz2)c/nm | 0.1752 | 0.1784 | 0.1806 | 0.1761 | 0.1785 | |
| U2-N4(Pz3)c/nm | 0.1262 | 0.1290 | 0.1291 | 0.1289 | 0.1298 | |
| U2-N | 0.1507 | 0.1537 | 0.1548 | 0.1525 | 0.1541 | |
| U1-(Pz2)cent. -U2e/(°) | 180.0 | 180.0 | 180.0 | 179.3 | 180.0 | |
| h1f/(°) | 44.9 | 52.7 | 48.9 | 19.0g | -12.8g | |
| h2f/(°) | 0.8 | 0.2 | 7.7 | 42.5 | 30.8 | |
| Species | se | es | ||||||
|---|---|---|---|---|---|---|---|---|
| Singlet | Triplet | Quintet | Septet | Singlet | Triplet | Quintet | Septet | |
| U1-U2/nm | 0.3400 | 0.3521 | 0.3548 | 0.3548 | 0.3391 | 0.3497 | 0.3504 | 0.3557 |
| U1-N(Pz1 | 0.2338 | 0.2346 | 0.2352 | 0.2378 | 0.2356 | 0.2372 | 0.2374 | 0.2382 |
| U1-N(Pz2 | 0.2554 | 0.2588 | 0.2598 | 0.2603 | 0.2547 | 0.2575 | 0.2573 | 0.2597 |
| (U1-N | 0.2446 | 0.2467 | 0.2475 | 0.2490 | 0.2452 | 0.2474 | 0.2473 | 0.2490 |
| U2-N(Pz2 | 0.2554 | 0.2589 | 0.2598 | 0.2605 | 0.2548 | 0.2593 | 0.2602 | 0.2613 |
| U2-N(Pz3 | 0.2338 | 0.2346 | 0.2352 | 0.2359 | 0.2334 | 0.2351 | 0.2354 | 0.2357 |
| (U2-N | 0.2446 | 0.2467 | 0.2475 | 0.2482 | 0.2441 | 0.2472 | 0.2478 | 0.2485 |
| U1-N4(Pz1)c/nm | 0.1279 | 0.1291 | 0.1298 | 0.1335 | 0.1307 | 0.1328 | 0.1329 | 0.1340 |
| U1-N4(Pz2)c/nm | 0.1700 | 0.1760 | 0.1774 | 0.1773 | 0.1695 | 0.1736 | 0.1731 | 0.1767 |
| U1-N | 0.1490 | 0.1526 | 0.1536 | 0.1554 | 0.1501 | 0.1532 | 0.1530 | 0.1554 |
| U2-N4(Pz2)c/nm | 0.1700 | 0.1761 | 0.1774 | 0.1775 | 0.1696 | 0.1761 | 0.1773 | 0.1790 |
| U2-N4(Pz3)c/nm | 0.1279 | 0.1291 | 0.1297 | 0.1308 | 0.1325 | 0.1298 | 0.1302 | 0.1301 |
| U2-N | 0.1490 | 0.1526 | 0.1536 | 0.1541 | 0.1510 | 0.1530 | 0.1537 | 0.1546 |
| U1-(Pz2)cent.-U2e/(°) | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 |
| h1f/(°) | 45.1 | 45.2 | 49.1 | 50.9 | -0.1 | 0.2 | -0.2 | 0.2 |
| h2f/(°) | 0.8 | -0.7 | 7.4 | 30.5g | 44.8 | 38.8 | 36.9 | 44.2 |
Table 3 Optimized geometry parameters of U2ⅢPz3
| Species | se | es | ||||||
|---|---|---|---|---|---|---|---|---|
| Singlet | Triplet | Quintet | Septet | Singlet | Triplet | Quintet | Septet | |
| U1-U2/nm | 0.3400 | 0.3521 | 0.3548 | 0.3548 | 0.3391 | 0.3497 | 0.3504 | 0.3557 |
| U1-N(Pz1 | 0.2338 | 0.2346 | 0.2352 | 0.2378 | 0.2356 | 0.2372 | 0.2374 | 0.2382 |
| U1-N(Pz2 | 0.2554 | 0.2588 | 0.2598 | 0.2603 | 0.2547 | 0.2575 | 0.2573 | 0.2597 |
| (U1-N | 0.2446 | 0.2467 | 0.2475 | 0.2490 | 0.2452 | 0.2474 | 0.2473 | 0.2490 |
| U2-N(Pz2 | 0.2554 | 0.2589 | 0.2598 | 0.2605 | 0.2548 | 0.2593 | 0.2602 | 0.2613 |
| U2-N(Pz3 | 0.2338 | 0.2346 | 0.2352 | 0.2359 | 0.2334 | 0.2351 | 0.2354 | 0.2357 |
| (U2-N | 0.2446 | 0.2467 | 0.2475 | 0.2482 | 0.2441 | 0.2472 | 0.2478 | 0.2485 |
| U1-N4(Pz1)c/nm | 0.1279 | 0.1291 | 0.1298 | 0.1335 | 0.1307 | 0.1328 | 0.1329 | 0.1340 |
| U1-N4(Pz2)c/nm | 0.1700 | 0.1760 | 0.1774 | 0.1773 | 0.1695 | 0.1736 | 0.1731 | 0.1767 |
| U1-N | 0.1490 | 0.1526 | 0.1536 | 0.1554 | 0.1501 | 0.1532 | 0.1530 | 0.1554 |
| U2-N4(Pz2)c/nm | 0.1700 | 0.1761 | 0.1774 | 0.1775 | 0.1696 | 0.1761 | 0.1773 | 0.1790 |
| U2-N4(Pz3)c/nm | 0.1279 | 0.1291 | 0.1297 | 0.1308 | 0.1325 | 0.1298 | 0.1302 | 0.1301 |
| U2-N | 0.1490 | 0.1526 | 0.1536 | 0.1541 | 0.1510 | 0.1530 | 0.1537 | 0.1546 |
| U1-(Pz2)cent.-U2e/(°) | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 |
| h1f/(°) | 45.1 | 45.2 | 49.1 | 50.9 | -0.1 | 0.2 | -0.2 | 0.2 |
| h2f/(°) | 0.8 | -0.7 | 7.4 | 30.5g | 44.8 | 38.8 | 36.9 | 44.2 |
| Complex | Config. | ESS | QU1/e | QU2/e | SU1/a.u. | SU2/a.u. |
|---|---|---|---|---|---|---|
| se | Singlet | 2.057 | 2.058 | 0 | 0 | |
| Triplet | 2.048 | 2.048 | 1.627 | 1.627 | ||
| Quintet | 2.035 | 2.035 | 1.877 | 1.877 | ||
| es | Singlet | 2.030 | 2.035 | 0 | 0 | |
| Triplet* | 2.043 | 2.031 | 1.470 | 1.741 | ||
| Quintet | 2.028 | 2.007 | 1.879 | 1.995 | ||
| se | Singlet | 2.027 | 2.027 | 0 | 0 | |
| Triplet | 2.024 | 2.024 | 1.747 | 1.749 | ||
| Quintet | 2.013 | 2.013 | 1.924 | 1.926 | ||
| Septet | 1.968 | 1.996 | 2.171 | 2.047 | ||
| es | Singlet | 2.025 | 2.002 | 0 | 0 | |
| Triplet | 2.012 | 1.985 | 1.777 | 1.958 | ||
| Quintet | 2.004 | 1.979 | 1.918 | 2.013 | ||
| Septet | 1.992 | 1.963 | 2.059 | 2.158 |
Table 4 Calculated electron-spin density(SU) and charge(QU) of the uranium atom of Um2Pz3(m=Ⅳ, Ⅲ) complexes
| Complex | Config. | ESS | QU1/e | QU2/e | SU1/a.u. | SU2/a.u. |
|---|---|---|---|---|---|---|
| se | Singlet | 2.057 | 2.058 | 0 | 0 | |
| Triplet | 2.048 | 2.048 | 1.627 | 1.627 | ||
| Quintet | 2.035 | 2.035 | 1.877 | 1.877 | ||
| es | Singlet | 2.030 | 2.035 | 0 | 0 | |
| Triplet* | 2.043 | 2.031 | 1.470 | 1.741 | ||
| Quintet | 2.028 | 2.007 | 1.879 | 1.995 | ||
| se | Singlet | 2.027 | 2.027 | 0 | 0 | |
| Triplet | 2.024 | 2.024 | 1.747 | 1.749 | ||
| Quintet | 2.013 | 2.013 | 1.924 | 1.926 | ||
| Septet | 1.968 | 1.996 | 2.171 | 2.047 | ||
| es | Singlet | 2.025 | 2.002 | 0 | 0 | |
| Triplet | 2.012 | 1.985 | 1.777 | 1.958 | ||
| Quintet | 2.004 | 1.979 | 1.918 | 2.013 | ||
| Septet | 1.992 | 1.963 | 2.059 | 2.158 |
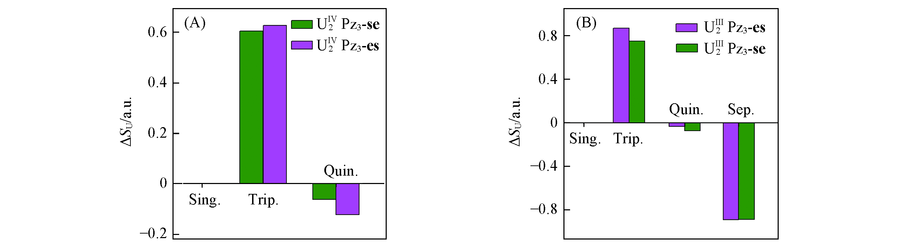
Fig.3 Difference of spin density of uranium atom(ΔSU) of se- and es-configurationUm2Pz3[(m=Ⅳ(A), Ⅲ(B)] in various electron-spin states from the respective expected formal value,i.e., 0, 1, 2 and 3 for the singlet, triplet, quintet and septet states, respectively.
| BCP | Parameter* | ||||
|---|---|---|---|---|---|
| se | es | se | es | ||
| U1-N(Pz1) | ρ(r)/a.u. | 0.0861 | 0.0853 | 0.0841 | 0.0839 |
| ?2ρ(r)/a.u. | 0.2083 | 0.2047 | 0.2109 | 0.2080 | |
| H(r)/a.u. | -0.0203 | -0.0198 | -0.0190 | -0.0189 | |
| U1-N(Pz2) | ρ(r)/a.u. | 0.0467 | 0.0478 | 0.0499 | 0.0477 |
| ?2ρ(r)/a.u. | 0.1299 | 0.1387 | 0.1324 | 0.1638 | |
| H(r)/a.u. | -0.0030 | -0.0032 | -0.0041 | -0.0027 | |
| U2-N(Pz2) | ρ(r)/a.u. | 0.0468 | 0.0451 | 0.0477 | 0.0497 |
| ?2ρ(r)/a.u. | 0.1301 | 0.1268 | 0.1496 | 0.1301 | |
| H(r)/a.u. | -0.0030 | -0.0029 | -0.0028 | -0.0042 | |
| U2-N(Pz3) | ρ(r)/a.u. | 0.0861 | 0.0831 | 0.0799 | 0.0740 |
| ?2ρ(r)/a.u. | 0.2088 | 0.2055 | 0.2391 | 0.2349 | |
| H(r)/a.u. | -0.0203 | -0.0189 | -0.0162 | -0.0133 | |
Table 5 QTAIM parameters for the U-N bond of ground-state Um2Pz3(m=Ⅳ, Ⅲ) in various steric configurations, including electron density[ρ(r)], Laplacian[?2ρ(r)] and energy density[H(r)] at BCPs
| BCP | Parameter* | ||||
|---|---|---|---|---|---|
| se | es | se | es | ||
| U1-N(Pz1) | ρ(r)/a.u. | 0.0861 | 0.0853 | 0.0841 | 0.0839 |
| ?2ρ(r)/a.u. | 0.2083 | 0.2047 | 0.2109 | 0.2080 | |
| H(r)/a.u. | -0.0203 | -0.0198 | -0.0190 | -0.0189 | |
| U1-N(Pz2) | ρ(r)/a.u. | 0.0467 | 0.0478 | 0.0499 | 0.0477 |
| ?2ρ(r)/a.u. | 0.1299 | 0.1387 | 0.1324 | 0.1638 | |
| H(r)/a.u. | -0.0030 | -0.0032 | -0.0041 | -0.0027 | |
| U2-N(Pz2) | ρ(r)/a.u. | 0.0468 | 0.0451 | 0.0477 | 0.0497 |
| ?2ρ(r)/a.u. | 0.1301 | 0.1268 | 0.1496 | 0.1301 | |
| H(r)/a.u. | -0.0030 | -0.0029 | -0.0028 | -0.0042 | |
| U2-N(Pz3) | ρ(r)/a.u. | 0.0861 | 0.0831 | 0.0799 | 0.0740 |
| ?2ρ(r)/a.u. | 0.2088 | 0.2055 | 0.2391 | 0.2349 | |
| H(r)/a.u. | -0.0203 | -0.0189 | -0.0162 | -0.0133 | |
| Orbital | Energy/eV | U1(%) | U2(%) | Ligand(%) | Assignment(%) | ||
|---|---|---|---|---|---|---|---|
| d | f | d | f | ||||
| L+2 | -5.878 | 1.57 | 43.89 | 1.58 | 43.83 | 5f | |
| L+1 | -5.883 | 1.43 | 44.03 | 1.44 | 43.56 | 5f | |
| LUMO | -5.897 | 37.08 | 37.27 | 5f | |||
| HOMO | -5.912 | 43.58 | 43.68 | 5f | |||
| H-1 | -5.942 | 35.41 | 33.91 | 5f | |||
| H-2 | -5.967 | 22.03 | 22.10 | 26.31 | 5f+L | ||
| H-3 | -6.183 | 2.73 | 44.38 | 2.73 | 44.28 | 5fσ | |
| H-4 | -6.634 | 72.88 | L | ||||
| H-5 | -6.919 | 86.28 | L | ||||
Table 6 Contributions of α-spin orbitals of U2ⅣPz3-se in the quintet(ground) state
| Orbital | Energy/eV | U1(%) | U2(%) | Ligand(%) | Assignment(%) | ||
|---|---|---|---|---|---|---|---|
| d | f | d | f | ||||
| L+2 | -5.878 | 1.57 | 43.89 | 1.58 | 43.83 | 5f | |
| L+1 | -5.883 | 1.43 | 44.03 | 1.44 | 43.56 | 5f | |
| LUMO | -5.897 | 37.08 | 37.27 | 5f | |||
| HOMO | -5.912 | 43.58 | 43.68 | 5f | |||
| H-1 | -5.942 | 35.41 | 33.91 | 5f | |||
| H-2 | -5.967 | 22.03 | 22.10 | 26.31 | 5f+L | ||
| H-3 | -6.183 | 2.73 | 44.38 | 2.73 | 44.28 | 5fσ | |
| H-4 | -6.634 | 72.88 | L | ||||
| H-5 | -6.919 | 86.28 | L | ||||
| [1] | Lu J., Deng Y., Zhang X., Kobayashi N., Jiang J., Inorg. Chem., 2011, 50, 2562-2567 |
| [2] | Gao F., Li Y. Y., Liu C. M., Li Y. Z., Zuo J. L., Dalton Trans., 2013, 42, 11043-11046 |
| [3] | Zhang X., Chen Y., Inorg. Chem. Commun., 2014, 39, 79-82 |
| [4] | Birin K. P., Gorbunova Y. G., Tsivadze A. Y., Dalton Trans., 2012, 41, 9672-9681 |
| [5] | Lu J., Zhang D., Wang H., Jiang J., Zhang X., Inorg. Chem. Commun., 2010, 13, 1144-1147 |
| [6] | Moussavi M., De Cian A., Fischer J., Weiss R., Inorg. Chem., 1986, 25, 2107-2108 |
| [7] | Sun X., Li R., Wang D., Dou J., Zhu P., Lu F., Ma C., Choi C. F., Cheng D. Y. Y., Ng D. K. P., Kobayashi N., Jiang J., Eur. J. Inorg. Chem., 2004, 2004, 3806-3813 |
| [8] | Zhu P., Zhang X., Wang H., Zhang Y., Bian Y., Jiang J., Inorg. Chem., 2012, 51, 5651-5659 |
| [9] | Padmaja K., Youngblood W. J., Wei L., Bocian D. F., Lindsey J. S., Inorg. Chem., 2006, 45, 5479-5492 |
| [10] | Le Borgne T., Lance M., Nierlich M., Ephritikhine M., J. Organomet. Chem., 2000, 598, 313-317 |
| [11] | Evans W. J., Nyce G. W., Ziller J. W., Angew. Chem. Int. Ed., 2000, 39, 240-242 |
| [12] | Zong M. R., He H. C., Dong F. Q., He P., Sun S. Y., Liu M. X., Nie X. Q., Chem. J. Chinese Universities, 2016, 37(9), 1701-1709 |
| (宗美荣, 何辉超, 董发勤, 何平, 孙仕勇, 刘明学, 聂小琴. 高等学校化学学报, 2016, 37(9), 1701-1709.) | |
| [13] | Yue G. Z., Gao R., Zhao P. X., Chu M. F., Shuai M. B., Acta Chim. Sinica, 2016, 74, 657-663 |
| (岳国宗, 高瑞, 赵鹏翔, 褚明福, 帅茂兵. 化学学报, 2016, 74, 657-663) | |
| [14] | Laikov D. N., Ustynyuk Y. A., Russ. Chem. Bull., 2005, 54, 820-826 |
| [15] | Laikov D. N., J. Comput. Chem., 2007, 28, 698-702 |
| [16] | Perdew J. P., Burke K., Ernzerhof M., Phys. Rev. Lett., 1996, 77, 3865-3868 |
| [17] | Bader R. F. W., J. Phys. Chem. A, 1998, 102, 7314-7323 |
| [18] | Bader R. F. W., Chem. Rev., 1991, 91, 893-928 |
| [19] | Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam N. J., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas Ö., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision A. 1, Gaussian Inc., Wallingford CT, 2009 |
| [20] | Cao X., Dolg M., Stoll H., J. Chem. Phys., 2003, 118, 487-496 |
| [21] | Lu T., Chen F., J. Comput. Chem., 2012, 33, 580-592 |
| [22] | Baerends E. J., Ziegler T., Autschbach J., Bashford D., Bérces A., Bickelhaupt F. M., Bo C., Boerrigter P. M., Cavallo L., Chong D. P., Deng L., Dickson R. M., Ellis D. E., van Faassen M., Fan L., Fischer T. H., Fonseca G. C., Franchini M., Ghysels A., Giammona A., van Gisbergen S. J. A., Götz A. W., Groeneveld J. A., Gritsenko O. V., Grüning M., Gusarov S., Harris F. E., van den Hoek P., Jacob C. R., Jacobsen H., Jensen L., Kaminski J. W., van Kessel G., Kootstra F., Kovalenko A., Krykunov M. V., van Lenthe E., McCormack D. A., Michalak A., Mitoraj M., Morton S. M., Neugebauer J., Nicu V. P., Noodleman L., Osinga V. P., Patchkovskii S., Pavanello M., Philipsen P. H. T., Post D., Pye C. C., Ravenek W., Rodríguez J. I., Ros P., Schipper P. R. T., van Schoot H., Schreckenbach G., Seldenthuis J. S., Seth M., Snijders J. G., SolàM., Swart M., Swerhone D., te Velde G., Vernooijs P., Versluis L., Visscher L., Visser O., Wang F., Wesolowski T. A., van Wezenbeek E. M., Wiesenekker G., Wolff S. K., Woo T. K., Yakovlev A. L., ADF2012, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, 2012 |
| [23] | Van Lenthe E., Ehlers A., Baerends E. J., J. Chem. Phys., 1999, 110, 8943-8953 |
| [24] | Klamt A., Jonas V., Burger T., Lohrenz J. C. W., J. Phys. Chem. A, 1998, 102, 5074-5085 |
| [25] | Bao Z., Zhao H. B., Qu N., Schreckenbach G., Pan Q. J., Dalton Trans., 2016, 45, 15970-15982 |
| [26] | Yu L. A., Hu B., Luo M. B., Zhang X., Chen H. W., Chem. J. Chinese Universities, 2009, 30(12), 2460-2463 |
| (于立安, 胡斌, 罗明标, 张燮, 陈焕文. 高等学校化学学报, 2009, 30(12), 2460-2463) | |
| [27] | Wang D., van Gunsteren W. F., Chai Z., Chem. Soc. Rev., 2012, 41, 5836-5865 |
| [28] | Kaltsoyannis N., Inorg. Chem., 2013, 52, 3407-3413 |
| [29] | Huang Q. R., Kingham J. R., Kaltsoyannis N., Dalton Trans., 2015, 44, 2554-2566 |
| [30] | Yao J., Zheng X. J., Pan Q. J., Schreckenbach G., Inorg. Chem., 2015, 54, 5438-5449 |
| [31] | Lewis F. W., Harwood L. M., Hudson M. J., Drew M. G. B., Modolo G., Sypula M., Desreux J. F., Bouslimani N., Vidick G., Dalton Trans., 2010, 39, 5172-5182 |
| [1] | 夏天, 万家炜, 于然波. 异原子配位结构碳基单原子电催化剂结构与性能相关性的研究进展[J]. 高等学校化学学报, 2022, 43(5): 20220162. |
| [2] | 王祖民, 孟程, 于然波. 过渡金属磷化物析氢催化剂的掺杂调控[J]. 高等学校化学学报, 2022, 43(11): 20220544. |
| [3] | 卓增庆, 潘锋. 基于软X射线光谱的锂电池材料的电子结构与演变的研究进展[J]. 高等学校化学学报, 2021, 42(8): 2332. |
| [4] | 史海涵,吴香萍,彭辛哲,余国静,董朝阳,纪瑶瑶,杨思文,陈俊林,王锦,冉雪芹,杨磊,解令海,黄维. 一种基于风车格结构的有效降低内重组能的咔唑类格子化分子[J]. 高等学校化学学报, 2020, 41(7): 1670. |
| [5] | 孙国栋, 王雪, 江国亮, 徐之勇, 刘洪梅. 二维金属-六亚氨基苯框架材料的气体吸附效应[J]. 高等学校化学学报, 2019, 40(5): 995. |
| [6] | 周和根, 金华, 郭辉瑞, 林晶, 章永凡. 黄铜矿型铜基硫属半导体材料的电子结构和光学性质[J]. 高等学校化学学报, 2019, 40(3): 518. |
| [7] | 张兆燕,陈宏善. Al6ONa2组装Zintl相晶体的理论研究[J]. 高等学校化学学报, 2019, 40(11): 2354. |
| [8] | 李坦, 张小超, 王凯, 李瑞, 樊彩梅. α,β,γ,δ,ε,η-Bi2O3电子结构和光学性质的第一性原理研究[J]. 高等学校化学学报, 2016, 37(5): 920. |
| [9] | 仓玉萍, 陈东, 杨帆, 杨慧明. 氮化锗多形体的四方、 单斜和正交畸变的理论研究[J]. 高等学校化学学报, 2016, 37(4): 674. |
| [10] | 周冰, 刘传林, 杨继萍, 陈功, 黄鹏程. 结构对称齐聚(3-甲基噻吩)的电子结构和分子堆积[J]. 高等学校化学学报, 2014, 35(12): 2593. |
| [11] | 王艳, 张小超, 赵丽军, 赵晓霞, 史宝萍, 樊彩梅. 采用第一性原理研究非金属掺杂BiOCl的电子结构和光吸收性质[J]. 高等学校化学学报, 2014, 35(12): 2624. |
| [12] | 仇毅翔, 王曙光. 膦配体对金团簇[Au@Au8(PR3)8]3+(R=Me,OMe,H,F,Cl,CN)稳定化作用的理论研究[J]. 高等学校化学学报, 2012, 33(11): 2549. |
| [13] | 孟素慈, 殷秀莲, 马晶, 谢吉民. 有机π共轭配体溶剂化效应与分子间相互作用的理论研究[J]. 高等学校化学学报, 2012, 33(11): 2492. |
| [14] | 洪波, 金东日, 李蕴, 马亚娟, 卢敏, 李霞, 张好好, 孙钰. Ge@C82结构及性质的理论研究[J]. 高等学校化学学报, 2012, 33(06): 1259. |
| [15] | 贾金乾, 解学佳, 梁镇海, 张小超, 樊彩梅, 韩培德. Ti掺杂SnO2 半导体固溶体的第一性原理研究[J]. 高等学校化学学报, 2012, 33(05): 1050. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||