

高等学校化学学报 ›› 2014, Vol. 35 ›› Issue (11): 2447.doi: 10.7503/cjcu20140195
收稿日期:2014-03-10
出版日期:2014-11-10
发布日期:2014-10-09
作者简介:联系人简介: 黄耀东, 男, 博士, 副教授, 主要从事不对称催化和有机功能化合物研究. E-mail:
FAN Dongli, ZHAI Yan, ZHANG Yan, TU Wei, HUANG Yaodong*( )
)
Received:2014-03-10
Online:2014-11-10
Published:2014-10-09
Contact:
HUANG Yaodong
E-mail:huangyaodong@tju.edu.cn
摘要:
合成了3个系列各6类的偶氮苯衍生物1a~6a, 1b~6b和1c~6c. 凝胶性能测试结果表明, 这些化合物均能在多种极性或非极性有机溶剂中形成凝胶. 运用扫描电子显微镜和核磁共振波谱仪对代表性化合物4b形成的凝胶结构和成胶驱动力进行了分析. 化合物4a~4c形成的凝胶在紫外光和可见光照射下, 能够发生凝胶-溶胶的相互转化. 计算了溶剂和凝胶因子的梯氏参数, 利用梯氏三角图分析了凝胶测试结果, 发现凝胶因子在溶剂中的4种行为(溶液、 半凝胶、 凝胶和沉淀)分别分布在三角图的不同区域; 在凝胶区域, 溶剂与凝胶因子之间的距离反映了凝胶的热稳定性, 距离越远表示凝胶的热稳定性越好.
中图分类号:
TrendMD:
樊冬丽, 翟岩, 张妍, 涂伟, 黄耀东. 光响应偶氮苯类小分子凝胶因子的合成及性能. 高等学校化学学报, 2014, 35(11): 2447.
FAN Dongli, ZHAI Yan, ZHANG Yan, TU Wei, HUANG Yaodong. Synthesis and Properties of Photoresponsive Organogels Based on Azobenzene Derivatives. Chem. J. Chinese Universities, 2014, 35(11): 2447.
| Compd. | 1H NMR(300 MHz, CDCl3), δ | m.p./ ℃ | Yield (%) | GC-MS [M+H]+, m/z |
|---|---|---|---|---|
| 1a | 7.90(d, J=9.1 Hz, 2H, HAr), 7.83(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.06(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.89(m,8H,CH2), 0.9[t, J=6.6 Hz, 3H,—O(CH2)5CH3] | 113—115 | 92 | 409.1 |
| 1b | 7.91(d, J=9.1 Hz, 2H, H—Ar), 7.84(d, J=8.7 Hz, 2H, H—Ar), 7.62(d, J=8.7 Hz, 2H, H—Ar), 6.98(d, J=9.1 Hz, 2H, H—Ar), 4.02(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.89(m, 20H, CH2), 0.9[t, J=6.6 Hz, 3H,—O(CH2)11CH3] | 107—109 | 95 | 493.2 |
| 1c | 7.89(d, J=9.1 Hz, 2H, H—Ar), 7.82(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.99(d, J=9.1 Hz, 2H, H—Ar), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.89(m, 28H, CH2), 0.9[t, J=6.6 Hz, 3H,—O(CH2)15CH3] | 109—110 | 94 | 549.3 |
| 2a | 7.92(d, J=9.1 Hz, 2H, H—Ar), 7.84(d, J=8.4 Hz, 2H, H—Ar), 7.63(d, J=8.7 Hz, 2H, H—Ar), 6.94(d, J=9.1 Hz, 2H, H—Ar), 3.99(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.89(m, 8H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)5CH3], 0.083(s, 9H, CH3) | 100—102 | 81 | 379.1 |
| 2b | 7.91(d, J=9.1Hz, 2H, H—Ar), 7.82(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.95(d, J=9.1 Hz, 2H, H—Ar), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.89(m, 20H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)11CH3], 0.084(s, 9H, CH3) | 105—106 | 80 | 463.2 |
| 2c | 7.89(d, J=9.1 Hz, 2H, H—Ar), 7.81(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.94(d, J=9.1 Hz, 2H, H—Ar), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.89(m, 28H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)15CH3], 0.083(s, 9H, CH3) | 86—88 | 78 | 519.3 |
| Compd. | 1H NMR(300 MHz,CDCl3), δ | m.p./ ℃ | Yield (%) | GC-MS [M+H]+, m/z |
| 3a | 7.89(d, J=9.1 Hz, 2H, H—Ar), 7.82(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.94(d, J=9.1 Hz, 2H, H—Ar), 4.01(t, J=6.6 Hz, 2H, —OCH2), 3.1(s, 1H), 1.32—1.89(m, 8H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)5CH3] | 89—91 | 72 | 307.1 |
| 3b | 7.89(d, J=9.1 Hz, 2H, H—Ar), 7.82(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.94(d, J=9.1 Hz, 2H, H—Ar), 4.01(t, J=6.6 Hz, 2H, —OCH2), 3.1(s, 1H), 1.32—1.89(m, 20H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)11CH3] | 90—92 | 75 | 391.2 |
| 3c | 7.89(d, J=9.1 Hz, 2H, H—Ar), 7.82(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.94(d, J=9.1 Hz, 2H, H—Ar), 4.01(t, J=6.6 Hz, 2H, —OCH2), 3.1(s, 1H), 1.32—1.89(m, 28H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)15CH3] | 99—101 | 74 | 447.3 |
| 4a | 7.91(d, J=9.0 Hz, 2H, H—Ar), 7.87(d, J=8.7 Hz, 2H, H—Ar), 7.52(d, J=8.2 Hz, 2H, H—Ar), 6.99(d, J=9.0 Hz, 2H, H—Ar), 4.77(s, 2H, —CH2OH), 4.04(t, J=6.6 Hz, 2H, —OCH2), 1.75—1.86(m, 2H,CH2), 1.68(s, 1H, OH), 1.32—1.51(m, 6H, CH2), 0.9(t, J=6.6 Hz, 3H, —O(CH2)15CH3) | 118—120 | 94 | 313.2 |
| 4b | 7.91(d, J=9.1 Hz, 2H, H—Ar), 7.87(d, J=8.4 Hz, 2H, H—Ar), 7.55(d, J=8.4 Hz, 2H, H—Ar), 6.99(d, J=8.9 Hz, 2H, H—Ar), 4.77(s, 2H, —CH2OH), 4.04(t, J=6.6 Hz, 2H, —OCH2), 1.75—1.86(m, 2H, CH2), 1.68(s, 1H, OH), 1.32—1.51(m, 18H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)15CH3] | 104—106 | 95 | 397.3 |
| 4c | 7.91(d, J=9.1 Hz, 2H, H—Ar), 7.87(d, J=8.4 Hz, 2H, H—Ar), 7.55(d, J=8.4 Hz, 2H, H—Ar), 6.99(d, J=8.9 Hz, 2H, H—Ar), 4.77(s, 2H, —CH2OH), 4.04(t, J=6.6 Hz, 2H, —OCH2), 1.75—1.86(m, 2H, CH2), 1.68(s, 1H, OH), 1.32—1.51(m, 26H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)15CH3] | 118—120 | 95 | 453.4 |
| 5a | 7.91(d, J=9.1 Hz, 2H, H—Ar), 7.87(d, J=8.4 Hz, 2H, H—Ar), 7.53(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.64(s, 2H, —CH2Cl), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.87(m, 8H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)5CH3] | 72—74 | 83 | 331.1 |
| 5b | 7.91(d, J=9.1 Hz, 2H, H—Ar), 7.87(d, J=8.4 Hz, 2H, H—Ar), 7.53(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.64(s, 2H, —CH2Cl), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.87(m, 20H, CH2), 0.9[t, J=6.6 Hz, 3H,—O(CH2)11CH3] | 88—89 | 85 | 415.2 |
| 5c | 7.91(d, J=9.1 Hz, 2H, H—Ar), 7.87(d, J=8.4 Hz, 2H, H—Ar), 7.53(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.64(s, 2H, —CH2Cl), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.87(m, 28H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)15CH3] | 93—95 | 84 | 471.2 |
| 6a | 7.82(d, J=9.1 Hz, 2H, H—Ar), 7.79(d, J=8.4 Hz, 2H, H—Ar), 7.53(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.40(s,2H, —CH2N3), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.87(m, 8H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)5CH3] | 61—63 | 82 | 331.9 |
| 6b | 7.81(d, J=9.1 Hz, 2H, H—Ar), 7.79(d, J=8.4 Hz, 2H, H—Ar), 7.53(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.40(s, 2H, —CH2N3), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.87(m, 20H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)11CH3] | 78—80 | 80 | 422.6 |
| 6c | 7.81(d, J=9.1 Hz, 2H, H—Ar), 7.79(d, J=8.4 Hz, 2H, H—Ar), 7.53(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.40(s, 2H, —CH2N3), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.87(m, 28H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)15CH3] | 89—91 | 79 | 472.1 |
Table 1 1H NMR, melting points, yields and GC-MS data for compounds 1—6
| Compd. | 1H NMR(300 MHz, CDCl3), δ | m.p./ ℃ | Yield (%) | GC-MS [M+H]+, m/z |
|---|---|---|---|---|
| 1a | 7.90(d, J=9.1 Hz, 2H, HAr), 7.83(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.06(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.89(m,8H,CH2), 0.9[t, J=6.6 Hz, 3H,—O(CH2)5CH3] | 113—115 | 92 | 409.1 |
| 1b | 7.91(d, J=9.1 Hz, 2H, H—Ar), 7.84(d, J=8.7 Hz, 2H, H—Ar), 7.62(d, J=8.7 Hz, 2H, H—Ar), 6.98(d, J=9.1 Hz, 2H, H—Ar), 4.02(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.89(m, 20H, CH2), 0.9[t, J=6.6 Hz, 3H,—O(CH2)11CH3] | 107—109 | 95 | 493.2 |
| 1c | 7.89(d, J=9.1 Hz, 2H, H—Ar), 7.82(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.99(d, J=9.1 Hz, 2H, H—Ar), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.89(m, 28H, CH2), 0.9[t, J=6.6 Hz, 3H,—O(CH2)15CH3] | 109—110 | 94 | 549.3 |
| 2a | 7.92(d, J=9.1 Hz, 2H, H—Ar), 7.84(d, J=8.4 Hz, 2H, H—Ar), 7.63(d, J=8.7 Hz, 2H, H—Ar), 6.94(d, J=9.1 Hz, 2H, H—Ar), 3.99(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.89(m, 8H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)5CH3], 0.083(s, 9H, CH3) | 100—102 | 81 | 379.1 |
| 2b | 7.91(d, J=9.1Hz, 2H, H—Ar), 7.82(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.95(d, J=9.1 Hz, 2H, H—Ar), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.89(m, 20H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)11CH3], 0.084(s, 9H, CH3) | 105—106 | 80 | 463.2 |
| 2c | 7.89(d, J=9.1 Hz, 2H, H—Ar), 7.81(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.94(d, J=9.1 Hz, 2H, H—Ar), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.89(m, 28H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)15CH3], 0.083(s, 9H, CH3) | 86—88 | 78 | 519.3 |
| Compd. | 1H NMR(300 MHz,CDCl3), δ | m.p./ ℃ | Yield (%) | GC-MS [M+H]+, m/z |
| 3a | 7.89(d, J=9.1 Hz, 2H, H—Ar), 7.82(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.94(d, J=9.1 Hz, 2H, H—Ar), 4.01(t, J=6.6 Hz, 2H, —OCH2), 3.1(s, 1H), 1.32—1.89(m, 8H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)5CH3] | 89—91 | 72 | 307.1 |
| 3b | 7.89(d, J=9.1 Hz, 2H, H—Ar), 7.82(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.94(d, J=9.1 Hz, 2H, H—Ar), 4.01(t, J=6.6 Hz, 2H, —OCH2), 3.1(s, 1H), 1.32—1.89(m, 20H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)11CH3] | 90—92 | 75 | 391.2 |
| 3c | 7.89(d, J=9.1 Hz, 2H, H—Ar), 7.82(d, J=8.4 Hz, 2H, H—Ar), 7.61(d, J=8.7 Hz, 2H, H—Ar), 6.94(d, J=9.1 Hz, 2H, H—Ar), 4.01(t, J=6.6 Hz, 2H, —OCH2), 3.1(s, 1H), 1.32—1.89(m, 28H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)15CH3] | 99—101 | 74 | 447.3 |
| 4a | 7.91(d, J=9.0 Hz, 2H, H—Ar), 7.87(d, J=8.7 Hz, 2H, H—Ar), 7.52(d, J=8.2 Hz, 2H, H—Ar), 6.99(d, J=9.0 Hz, 2H, H—Ar), 4.77(s, 2H, —CH2OH), 4.04(t, J=6.6 Hz, 2H, —OCH2), 1.75—1.86(m, 2H,CH2), 1.68(s, 1H, OH), 1.32—1.51(m, 6H, CH2), 0.9(t, J=6.6 Hz, 3H, —O(CH2)15CH3) | 118—120 | 94 | 313.2 |
| 4b | 7.91(d, J=9.1 Hz, 2H, H—Ar), 7.87(d, J=8.4 Hz, 2H, H—Ar), 7.55(d, J=8.4 Hz, 2H, H—Ar), 6.99(d, J=8.9 Hz, 2H, H—Ar), 4.77(s, 2H, —CH2OH), 4.04(t, J=6.6 Hz, 2H, —OCH2), 1.75—1.86(m, 2H, CH2), 1.68(s, 1H, OH), 1.32—1.51(m, 18H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)15CH3] | 104—106 | 95 | 397.3 |
| 4c | 7.91(d, J=9.1 Hz, 2H, H—Ar), 7.87(d, J=8.4 Hz, 2H, H—Ar), 7.55(d, J=8.4 Hz, 2H, H—Ar), 6.99(d, J=8.9 Hz, 2H, H—Ar), 4.77(s, 2H, —CH2OH), 4.04(t, J=6.6 Hz, 2H, —OCH2), 1.75—1.86(m, 2H, CH2), 1.68(s, 1H, OH), 1.32—1.51(m, 26H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)15CH3] | 118—120 | 95 | 453.4 |
| 5a | 7.91(d, J=9.1 Hz, 2H, H—Ar), 7.87(d, J=8.4 Hz, 2H, H—Ar), 7.53(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.64(s, 2H, —CH2Cl), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.87(m, 8H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)5CH3] | 72—74 | 83 | 331.1 |
| 5b | 7.91(d, J=9.1 Hz, 2H, H—Ar), 7.87(d, J=8.4 Hz, 2H, H—Ar), 7.53(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.64(s, 2H, —CH2Cl), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.87(m, 20H, CH2), 0.9[t, J=6.6 Hz, 3H,—O(CH2)11CH3] | 88—89 | 85 | 415.2 |
| 5c | 7.91(d, J=9.1 Hz, 2H, H—Ar), 7.87(d, J=8.4 Hz, 2H, H—Ar), 7.53(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.64(s, 2H, —CH2Cl), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.87(m, 28H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)15CH3] | 93—95 | 84 | 471.2 |
| 6a | 7.82(d, J=9.1 Hz, 2H, H—Ar), 7.79(d, J=8.4 Hz, 2H, H—Ar), 7.53(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.40(s,2H, —CH2N3), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.87(m, 8H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)5CH3] | 61—63 | 82 | 331.9 |
| 6b | 7.81(d, J=9.1 Hz, 2H, H—Ar), 7.79(d, J=8.4 Hz, 2H, H—Ar), 7.53(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.40(s, 2H, —CH2N3), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.87(m, 20H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)11CH3] | 78—80 | 80 | 422.6 |
| 6c | 7.81(d, J=9.1 Hz, 2H, H—Ar), 7.79(d, J=8.4 Hz, 2H, H—Ar), 7.53(d, J=8.7 Hz, 2H, H—Ar), 6.97(d, J=9.1 Hz, 2H, H—Ar), 4.40(s, 2H, —CH2N3), 4.01(t, J=6.6 Hz, 2H, —OCH2), 1.32—1.87(m, 28H, CH2), 0.9[t, J=6.6 Hz, 3H, —O(CH2)15CH3] | 89—91 | 79 | 472.1 |
| Solvent | Minimum gel concentration/(mg·mL-1) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 1b | 1c | 2a | 2b | 2c | 3a | 3b | 3c | 4a | 4b | 4c | 5a | 5b | 5c | 6a | 6b | 6c | |
| PE | 100 | P | P | 100 | 100 | 55 | 100 | P | P | Ins | Ins | 44 | PG | 20 | P | PG | 67 | P |
| Cyclohexane | PG | P | P | PG | PG | P | PG | P | P | 27 | P | 53 | PG | 40 | 56 | PG | PG | P |
| Hexane | 100 | P | P | PG | 80 | P | 67 | 80 | P | Ins | P | 40 | PG | 23 | P | PG | 50 | P |
| DCM | PG | 40 | 50 | PG | 100 | S | PG | 40 | 100 | 57 | 20 | 10 | PG | 100 | S | PG | PG | S |
| DCE | PG | 33 | 20 | P | P | P | P | P | P | 26 | 20 | 26 | P | P | P | P | P | P |
| Chloroform | PG | 100 | PG | S | PG | S | S | PG | S | 80 | 80 | PG | S | PG | S | S | PG | S |
| CTC | 20 | P | PG | S | PG | PG | PG | P | P | 36 | P | PG | S | PG | P | S | PG | PG |
| Ethyl acetate | PG | 80 | PG | PG | 100 | PG | PG | 80 | P | 80 | 57 | P | S | 50 | PG | S | PG | 55 |
| THF | S | PG | S | S | PG | S | S | PG | P | S | 20 | PG | S | PG | S | S | PG | PG |
| Methanol | PG | 68 | PG | 83 | P | PG | 83 | P | 35 | 33 | 50 | 53 | 56 | 100 | P | PG | 80 | 37 |
| Ethanol | 67 | 71 | 35 | 83 | 48 | 35 | 100 | 40 | PG | 40 | 20 | 53 | 100 | 49 | P | PG | 36 | P |
| Acetonitrile | 67 | 68 | 18 | 43 | 32 | 55 | 77 | 28 | 15 | 57 | 19 | 26 | PG | 24 | 37 | 67 | 22 | 27 |
| Isopropanol | 50 | 100 | 35 | 59 | 100 | PG | 59 | PG | P | 33 | 100 | PG | 100 | 60 | P | 67 | 40 | P |
| Butanol | 56 | 50 | 20 | 83 | 75 | 30 | 56 | 60 | 24 | 40 | 30 | 18 | PG | 40 | 24 | 62 | 40 | 21 |
| Acetone | PG | 100 | 27 | PG | PG | 50 | PG | PG | P | PG | 100 | 29 | S | PG | 56 | S | 80 | 37 |
| Ether | PG | 100 | Ins | PG | PG | Ins | PG | P | Ins | PG | 100 | Ins | PG | 80 | Ins | PG | 80 | Ins |
| Benzene | PG | S | 55 | S | PG | S | S | PG | P | 80 | P | 53 | S | S | P | S | PG | P |
| Toluene | S | PG | P | S | PG | S | S | PG | S | PG | PG | P | S | P | PG | S | PG | S |
| Dioxane | PG | S | 55 | S | PG | PG | S | PG | P | 50 | S | PG | S | 80 | PG | S | PG | 55 |
| Triethylamine | PG | PG | PG | PG | PG | P | PG | PG | P | S | P | PG | PG | PG | 30 | PG | PG | P |
| Pyridine | S | PG | 37 | S | PG | S | S | PG | P | S | S | PG | S | S | 37 | S | 80 | S |
Table 2 Gelation properties of compounds 1—6*
| Solvent | Minimum gel concentration/(mg·mL-1) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 1b | 1c | 2a | 2b | 2c | 3a | 3b | 3c | 4a | 4b | 4c | 5a | 5b | 5c | 6a | 6b | 6c | |
| PE | 100 | P | P | 100 | 100 | 55 | 100 | P | P | Ins | Ins | 44 | PG | 20 | P | PG | 67 | P |
| Cyclohexane | PG | P | P | PG | PG | P | PG | P | P | 27 | P | 53 | PG | 40 | 56 | PG | PG | P |
| Hexane | 100 | P | P | PG | 80 | P | 67 | 80 | P | Ins | P | 40 | PG | 23 | P | PG | 50 | P |
| DCM | PG | 40 | 50 | PG | 100 | S | PG | 40 | 100 | 57 | 20 | 10 | PG | 100 | S | PG | PG | S |
| DCE | PG | 33 | 20 | P | P | P | P | P | P | 26 | 20 | 26 | P | P | P | P | P | P |
| Chloroform | PG | 100 | PG | S | PG | S | S | PG | S | 80 | 80 | PG | S | PG | S | S | PG | S |
| CTC | 20 | P | PG | S | PG | PG | PG | P | P | 36 | P | PG | S | PG | P | S | PG | PG |
| Ethyl acetate | PG | 80 | PG | PG | 100 | PG | PG | 80 | P | 80 | 57 | P | S | 50 | PG | S | PG | 55 |
| THF | S | PG | S | S | PG | S | S | PG | P | S | 20 | PG | S | PG | S | S | PG | PG |
| Methanol | PG | 68 | PG | 83 | P | PG | 83 | P | 35 | 33 | 50 | 53 | 56 | 100 | P | PG | 80 | 37 |
| Ethanol | 67 | 71 | 35 | 83 | 48 | 35 | 100 | 40 | PG | 40 | 20 | 53 | 100 | 49 | P | PG | 36 | P |
| Acetonitrile | 67 | 68 | 18 | 43 | 32 | 55 | 77 | 28 | 15 | 57 | 19 | 26 | PG | 24 | 37 | 67 | 22 | 27 |
| Isopropanol | 50 | 100 | 35 | 59 | 100 | PG | 59 | PG | P | 33 | 100 | PG | 100 | 60 | P | 67 | 40 | P |
| Butanol | 56 | 50 | 20 | 83 | 75 | 30 | 56 | 60 | 24 | 40 | 30 | 18 | PG | 40 | 24 | 62 | 40 | 21 |
| Acetone | PG | 100 | 27 | PG | PG | 50 | PG | PG | P | PG | 100 | 29 | S | PG | 56 | S | 80 | 37 |
| Ether | PG | 100 | Ins | PG | PG | Ins | PG | P | Ins | PG | 100 | Ins | PG | 80 | Ins | PG | 80 | Ins |
| Benzene | PG | S | 55 | S | PG | S | S | PG | P | 80 | P | 53 | S | S | P | S | PG | P |
| Toluene | S | PG | P | S | PG | S | S | PG | S | PG | PG | P | S | P | PG | S | PG | S |
| Dioxane | PG | S | 55 | S | PG | PG | S | PG | P | 50 | S | PG | S | 80 | PG | S | PG | 55 |
| Triethylamine | PG | PG | PG | PG | PG | P | PG | PG | P | S | P | PG | PG | PG | 30 | PG | PG | P |
| Pyridine | S | PG | 37 | S | PG | S | S | PG | P | S | S | PG | S | S | 37 | S | 80 | S |
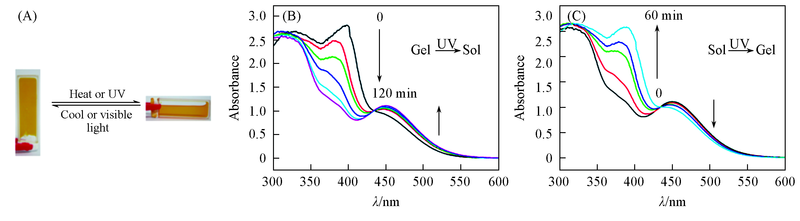
Fig.2 Pictures(A) and UV-Vis spectra(B, C) of the gel-sol transitions of gel 4c (A) Heated/cooled or irradiated by UV/visible light; (B) UV irradiation(0.1 cm path length); (C) visible irradiation.
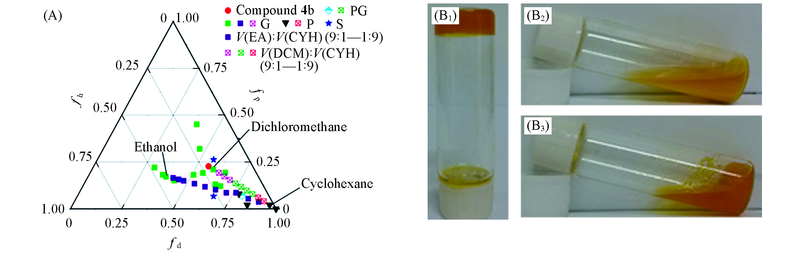
Fig.3 Teas plot of solubility parameters(A) and pictures of gelation behaviors of compound 4b(100 mg/mL) at mixed solvents(B1—B3) (A) Compound 4b and the single/mixed solvents. EAC: ethanol; CYH: cyclohexane; DCM: dichloromethane. (B1—B2) Gelation behavior and DCM:CYH(volume ratios): (B1) gel, 9:1—7:3; (B2) precipitation, 2:8, 1:9; (B3) partial gel, 6:4—3:7.
| Solvent volume ratio | 9:1 | 8:2 | 7:3 | 6:4 | 5:5 | 4:6 | 3:7 | 2:8 | 1:9 |
|---|---|---|---|---|---|---|---|---|---|
| EAC:CYH | 25 | 30 | 50 | 100 | 50 | 30 | 50 | 30 | 50 |
| DCM:CYH | 50 | 50 | 100 | PG | PG | PG | PG | P | P |
Table 3 Gelation test of compound 4b in the mixed solvents(mg/mL)*
| Solvent volume ratio | 9:1 | 8:2 | 7:3 | 6:4 | 5:5 | 4:6 | 3:7 | 2:8 | 1:9 |
|---|---|---|---|---|---|---|---|---|---|
| EAC:CYH | 25 | 30 | 50 | 100 | 50 | 30 | 50 | 30 | 50 |
| DCM:CYH | 50 | 50 | 100 | PG | PG | PG | PG | P | P |
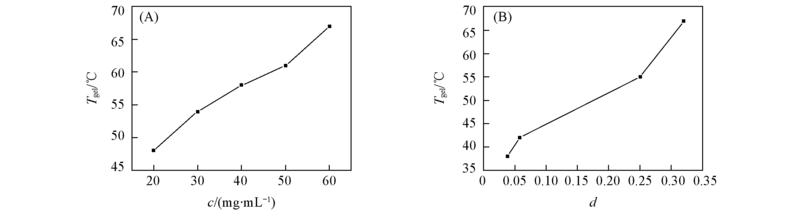
Fig.4 Plots of Tgel against the influence factors of the thermal stability of the gel (A) Concentration of compound 4b in ethanol; (B) distance between compound 4b and solvents on the Teas plot(dichloromethane, THF, butano and ethanol).
| [1] | Terech P., Weiss R. G., Chem. Rev., 1997, 97(8), 3133—3159 |
| [2] | Raeburn J., Zamith Cardoso A., Adams D. J., Chem. Soc. Rev., 2013, 42(12), 5143—5156 |
| [3] | Gawlitza K., Wu C. Z., Georgieva R., Wang D. Y., Ansorge-Schumacher M. B., von Klitzing R., Phys. Chem. Chem. Phys., 2012, 14(27), 9594—9600 |
| [4] | Jiang H. L., Zhu Y. H., Chen C., Shen J. H., Bao H., Peng L. M., Yang X. L., Li C. Z., New J. Chem., 2012, 36(4), 1051—1056 |
| [5] | Sugiyasu K., Fujita N., Shinkai S., Angew. Chem. Int. Ed., 2004, 43(10), 1229—1233 |
| [6] | Zhu S. J., Liu F. Q., Wang J. C., Su F., Li S. M., Chem. J. Chinese Universities,2014, 35(4), 863—868 |
| (朱寿进, 刘法谦, 王璟朝, 宿烽, 李速明. 高等学校化学学报,2014, 35(4), 863—868) | |
| [7] | Kubo W., Murakoshi K., Kitamura T., Yoshida S., Haruki M., Hanabusa K., Shirai H., Wada Y., Yanagida S., J. Phys. Chem., 2001, 105(51), 12809—12815 |
| [8] | Liu Z. X., Feng Y., Yan Z. C., He Y. M., Liu C. Y., Fan Q. H., Chem. Mater., 2012, 24(19), 3751—3757 |
| [9] | Wu Y. P., Wu S., Zou G., Zhang Q. J., Soft Matter,2011, 7(19), 9177—9183 |
| [10] | Deindorfer P., Davis R., Zentel R., Soft Matter,2007, 3(10), 1308—1311 |
| [11] | Edwards W., Lagadec C. A., Smith D. K., Soft Matter,2011, 7(1), 110—117 |
| [12] | Löfman M. L., Koivukorpi J., Noponen V., Salo H., Sieüanen E., Colloid Interface Sci., 2011, 360(2), 633—644 |
| [13] | Zhu G. Y., Dordick J. S., Chem. Mater., 2006, 18(25), 5988—5995 |
| [14] | Bielejewski M., Lapinski A., Luboradzki R., Tritt-Goc J., Langmuir,2009, 25(14), 8274—8279 |
| [15] | Tong C. Q., Fan K. Q., Niu L. B., Li J. J., Guan X. D., Tao N. M., Shen H. H., Song J., Soft Matter,2014, 10(5), 767—772 |
| [16] | Matthieu R., Laurent B., Chem. Commun., 2011, 47(29), 8271—8273 |
| [17] | Xu H., Zeng X. W., Bioorg. Med. Chem. Lett., 2010, 20(14), 4193—4195 |
| [18] | Huang Y. D., Dong X. L., Zhang L. L., Chai W., Chang J. Y., J. Mol. Struct., 2013, 1031(1), 43—48 |
| [19] | Austin W. B., Bilow N., Kelleghan W. J., Lau K. S. Y., J. Org. Chem., 1981, 46(11), 2280—2286 |
| [20] | Jiao T. F., Wang Y. J., Gao F. Q., Zhou J. X., Gao F. M., Prog. Nat. Sci. Mater. Int., 2012, 22(1), 64—70 |
| [21] | Wu S., Zhang Q. J., Bubeck C., Macromolecules,2010, 43(14), 6142—6151 |
| [22] | Barton A. F. M., Chem. Rev., 1975, 75(6), 731—753 |
| [23] | Stefanis E., Panayiotou C., Int. J. Thermophys,2008, 29(2), 568—585 |
| [1] | 刘苏毓, 丁飞, 李茜, 樊春海, 冯景. 偶氮苯类DNA纳米机器[J]. 高等学校化学学报, 2022, 43(8): 20220122. |
| [2] | 王学斌, 薛源, 茆华女, 项艳鑫, 包春燕. 光/还原双重响应水凝胶微球的制备及在细胞三维(3D)培养中的应用[J]. 高等学校化学学报, 2022, 43(8): 20220116. |
| [3] | 高健, 冯奕钰, 方文宇, 王慧, 葛婧, 封伟. 基于低温热释放的烷基接枝相变偶氮苯材料[J]. 高等学校化学学报, 2022, 43(8): 20220146. |
| [4] | 蒋小康, 周琦, 周恒为. Gd2ZnTiO6∶Dy3+, Eu3+单基质白光荧光粉的制备与发光性能[J]. 高等学校化学学报, 2022, 43(6): 20220029. |
| [5] | 闫嘉森, 韩现英, 党兆涵, 李建刚, 何向明. 石蜡/膨胀石墨/石墨烯复合相变储热材料的制备及性能[J]. 高等学校化学学报, 2022, 43(6): 20220054. |
| [6] | 黄益, 吕玲玲, 潘小鹏, 孙广东, 李永强, 姚菊明, 邵建中. 光交联自支撑丝素蛋白水凝胶的三维快速成型[J]. 高等学校化学学报, 2022, 43(4): 20210841. |
| [7] | 周永辉, 李尧, 吴雨轩, 田晶, 徐龙权, 费旭. 一种新型光致发光自愈合水凝胶的合成[J]. 高等学校化学学报, 2022, 43(2): 20210606. |
| [8] | 周宁, 唐小华, 曹红, 查飞, 李春, 谢春燕, 徐明平, 孙艺格. 石榴状凝胶微球固定化漆酶的制备、 表征及降解双酚A[J]. 高等学校化学学报, 2022, 43(2): 20210705. |
| [9] | 张洁, 银波, 刘玮欣, 刘兴平, 连文贤, 唐韶坤. 勃姆石纤维增强二氧化硅气凝胶的制备及性能[J]. 高等学校化学学报, 2022, 43(11): 20220483. |
| [10] | 颜舒婷, 姚远, 陶鑫峰, 林绍梁. 含硫正离子聚类肽水凝胶的合成与性能[J]. 高等学校化学学报, 2022, 43(11): 20220381. |
| [11] | 高慧玲, 曹珍珍, 顾芳, 王海军. 氢键型水凝胶自修复行为的Monte Carlo模拟[J]. 高等学校化学学报, 2022, 43(11): 20220482. |
| [12] | 李占峰, 刘本学, 刘晓婵, 王新强, 张晶, 于诗摩, 赵新富, 张新恩, 伊希斌. 氧化锆湿凝胶中乙酰丙酮配体的脱除机理及气凝胶复合材料的制备[J]. 高等学校化学学报, 2021, 42(9): 2904. |
| [13] | 蔡雅倩, 张家怀, 刘方哲, 李海潮, 石建平, 关爽. Hofmeister效应辅助的蛋白质基水凝胶应变传感器[J]. 高等学校化学学报, 2021, 42(8): 2609. |
| [14] | 李义山, 郭亮, 彭思凡, 张庆茂, 张瑜皓, 徐诗淇. 钴掺杂锰酸镧光催化剂的第一性原理与可见光响应光催化性能研究[J]. 高等学校化学学报, 2021, 42(6): 1881. |
| [15] | 雒春辉, 赵宇斐. 强韧抗溶胀水凝胶的简单构筑及性能[J]. 高等学校化学学报, 2021, 42(6): 2024. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||