

高等学校化学学报 ›› 2025, Vol. 46 ›› Issue (8): 20250031.doi: 10.7503/cjcu20250031
张琦璘1,2,3, 史衍常2,3, 高堃2,3, 王林松2,3, 宋亚东2,3, 王郁森2,3, 李鹏程2,3, 刘松2,3, 邢荣娥2,3, 宋琳1( ), 秦玉坤2,3(
), 秦玉坤2,3( )
)
收稿日期:2025-01-28
出版日期:2025-08-10
发布日期:2025-03-29
通讯作者:
秦玉坤
E-mail:03231@qust.edu.cn;ykqin@qdio.ac.cn
作者简介:宋 琳, 女, 博士, 讲师, 主要从事天然活性物质和海洋分子生物学方面的研究. E-mail: 03231@qust.edu.cn
基金资助:
ZHANG Qilin1,2,3, SHI Yanchang2,3, GAO Kun2,3, WANG Linsong2,3, SONG Yadong2,3, WANG Yusen2,3, LI Pengcheng2,3, LIU Song2,3, XING Ronge2,3, SONG Lin1( ), QIN Yukun2,3(
), QIN Yukun2,3( )
)
Received:2025-01-28
Online:2025-08-10
Published:2025-03-29
Contact:
QIN Yukun
E-mail:03231@qust.edu.cn;ykqin@qdio.ac.cn
Supported by:摘要:
通过一锅法三组分反应, 合成了一系列氨基烷基萘酚类化合物, 并对其抗植物病原真菌和杀线虫活性进行了评估. 结果表明, 在200~25 μg/mL浓度范围内, 化合物8对链格孢菌的抑制活性约为50%, 且抑制效果与浓度无明显相关性. 活体抑菌实验表明, 化合物8在50 μg/mL浓度下具有与阳性对照嘧菌酯相当的抑菌作用. 此外, 在温室试管实验中, 合成的化合物对南方根结线虫表现出显著的抑制活性, 其中化合物10在 10 μg/mL浓度下能显著减少根结数量, 抑制率高达90.91%, 与阳性对照氟吡菌酰胺相当. 综上, 氨基烷基萘酚类化合物作为一种新型农药活性物质, 具有潜在的抑菌和杀线虫双重功效, 研究结果为开发环境友好型多靶点农药提供了新思路.
中图分类号:
TrendMD:
张琦璘, 史衍常, 高堃, 王林松, 宋亚东, 王郁森, 李鹏程, 刘松, 邢荣娥, 宋琳, 秦玉坤. 氨基烷基萘酚类化合物的合成及抑菌和杀线虫活性. 高等学校化学学报, 2025, 46(8): 20250031.
ZHANG Qilin, SHI Yanchang, GAO Kun, WANG Linsong, SONG Yadong, WANG Yusen, LI Pengcheng, LIU Song, XING Ronge, SONG Lin, QIN Yukun. Synthesis of Aminoalkyl Naphthol Analogues and Their Anti-fungal and Nematicidal Activities. Chem. J. Chinese Universities, 2025, 46(8): 20250031.
| Compd. | Appearance | m. p./℃ | LC⁃MS | ||
|---|---|---|---|---|---|
| Formula | Calcd. | Found | |||
| 1 | White solid | 218.5—220.7 | C19H17NO2 | 291.35 | 291.1 |
| 2 | White solid | 224.9—227.4 | C20H19NO2 | 305.37 | 305.8 |
| 3 | White solid | 194.7—195.5 | C20H19NO3 | 322.37 | 322.1 |
| 4 | White solid | 232.4—234.6 | C19H16FNO2 | 310.34 | 310.8 |
| 5 | White solid | 214.7—215.7 | C21H21NO2 | 320.40 | 320.7 |
| 6 | Brown solid | 256.2—258.3 | C21H17NO2 | 316.37 | 316.2 |
| 7 | White solid | 173.6—176.5 | C18H16N2O2 | 293.33 | 293.0 |
| 8 | White solid | 180.4—183.9 | C19H18N2O2 | 307.36 | 307.1 |
| 9 | White solid | 220.2—221.8 | C20H20N2O2 | 321.39 | 321.2 |
| 10 | White solid | 233.6—235.7 | C20H16N2O2 | 317.36 | 317.1 |
| 11 | Yellow solid | 206.9—210.3 | C17H15NO3 | 282.31 | 282.9 |
| 12 | White solid | 205.9—208.7 | C18H17NO3 | 296.33 | 296.0 |
| 13 | Yellow liquid | — | C17H14BrNO3 | 361.20 | 361.9 |
| 14 | White solid | 171.2—173.4 | C16H14N2O3 | 283.29 | 283.0 |
| 15 | Yellow solid | 169.6—173.8 | C17H16N2O3 | 297.32 | 297.8 |
| 16 | Yellow liquid | — | C17H16N2O4 | 313.32 | 313.9 |
Table 1 Appearances, melting points(m. p.) and LC-MS data of compounds 1—16
| Compd. | Appearance | m. p./℃ | LC⁃MS | ||
|---|---|---|---|---|---|
| Formula | Calcd. | Found | |||
| 1 | White solid | 218.5—220.7 | C19H17NO2 | 291.35 | 291.1 |
| 2 | White solid | 224.9—227.4 | C20H19NO2 | 305.37 | 305.8 |
| 3 | White solid | 194.7—195.5 | C20H19NO3 | 322.37 | 322.1 |
| 4 | White solid | 232.4—234.6 | C19H16FNO2 | 310.34 | 310.8 |
| 5 | White solid | 214.7—215.7 | C21H21NO2 | 320.40 | 320.7 |
| 6 | Brown solid | 256.2—258.3 | C21H17NO2 | 316.37 | 316.2 |
| 7 | White solid | 173.6—176.5 | C18H16N2O2 | 293.33 | 293.0 |
| 8 | White solid | 180.4—183.9 | C19H18N2O2 | 307.36 | 307.1 |
| 9 | White solid | 220.2—221.8 | C20H20N2O2 | 321.39 | 321.2 |
| 10 | White solid | 233.6—235.7 | C20H16N2O2 | 317.36 | 317.1 |
| 11 | Yellow solid | 206.9—210.3 | C17H15NO3 | 282.31 | 282.9 |
| 12 | White solid | 205.9—208.7 | C18H17NO3 | 296.33 | 296.0 |
| 13 | Yellow liquid | — | C17H14BrNO3 | 361.20 | 361.9 |
| 14 | White solid | 171.2—173.4 | C16H14N2O3 | 283.29 | 283.0 |
| 15 | Yellow solid | 169.6—173.8 | C17H16N2O3 | 297.32 | 297.8 |
| 16 | Yellow liquid | — | C17H16N2O4 | 313.32 | 313.9 |
| Compd. | 1H NMR(600 MHz, DMSO⁃d6) | 13C NMR(150 MHz, DMSO⁃d6) |
|---|---|---|
| 1 | 8.40(d, J=8.4 Hz, 1H, —NH), 7.8—37.67(m, 3H, Ar—H), 7.34—7.24(m, 1H, Ar—H), 7.24—7.14(m, 4H, Ar—H), 7.09(dd, J=14.0, 7.8 Hz, 4H, Ar—H, —C3H), 1.92(s, 3H, —CH3) | 169.80, 153.69, 143.14, 132.85, 129.78, 129.07, 128.52, 126.57, 122.92, 119.36, 48.32, 23.19 |
| 2 | 9.93(s, 1H, Ar—H), 8.38(d, J=8.4 Hz, 1H, —NH), 7.84—7.74(m, 2H, Ar—H), 7.72(d, J=8.8 Hz, 1H, Ar—H), 7.32(t, J=7.8 Hz, 1H, Ar—H), 7.22 (t, J=7.5 Hz, 1H, Ar—H), 7.18(d, J=8.8 Hz, 1H, Ar—H), 7.06(d, J=8.4 Hz, 1H, —C3H), 7.01(s, 4H, Ar—H), 2.20(s, 3H, —CH3), 1.94(s, 3H, —CH3) | 169.62, 153.55, 140.04, 135.48, 132.78, 129.60, 129.01, 126.71, 122.82, 119.46, 118.94, 48.07, 23.15, 21.02 |
| 3 | 9.63(s, 1H, Ar—H), 8.08(d, J=8.5 Hz, 1H, —NH), 7.45(dd, J=8.2, 1.4 Hz, 1H, Ar—H), 7.40(d, J=8.8 Hz, 1H, Ar—H), 6.91(t, J=7.4 Hz, 1H, Ar—H), 6.87(d, J=8.8 Hz, 1H, Ar—H), 6.75—6.70(m, 3H, Ar—H), 6.46 (d, J=8.8 Hz, 2H, Ar—H, —C3H), 3.03(d, J=1.5 Hz, 3H, —CH3), 1.62(s, 3H, —CH3) | 169.56, 158.14, 153.52, 134.87, 132.75, 129.57, 128.99, 127.72, 126.71, 122.83, 119.44, 118.96, 113.88, 55.46, 47.90, 23.17 |
| 4 | 10.56(s, 1H, Ar—H), 8.99(d, J=8.3 Hz, 1H, —NH), 8.37—8.26(m, 2H, Ar—H), 7.90(t, J=7.7 Hz, 1H, Ar—H), 7.83—7.77(m, 1H, Ar—H), 7.74(d, J=8.8 Hz, 2H, Ar—H), 7.69(dd, J=8.6, 5.5 Hz, 2H, Ar—H), 7.66—7.55(m, 2H, Ar—H, —C3H), 3.02(p, J=1.8 Hz, 3H, —CH3) | 169.77, 161.97, 160.37, 153.61, 139.16, 132.68, 129.84, 129.05, 128.34, 126.88, 118.92, 115.07, 47.81, 23.10 |
| 5 | 9.98(d, J=2.4 Hz, 1H, Ar—H), 8.43(d, J=8.3 Hz, 1H, —NH), 7.81(dd, J=8.2, 3.2 Hz, 1H, Ar—H), 7.76(dd, J=8.9, 3.0 Hz, 1H, Ar—H), 7.24 (ddd, J=25.1, 8.5, 3.6 Hz, 3H, Ar—H), 7.09(dd, J=12.1, 5.7 Hz, 5H, Ar—H), 2.51(dd, J=4.1, 2.2 Hz, 2H, —CH2), 1.98(d, J=2.9 Hz, 3H, —CH3), 1.13(td, J=7.8, 3.5 Hz, 3H, —CH3) | 169.57, 153.55, 141.96, 140.27, 132.81, 129.59, 128.95(d, J=12.0 Hz), 127.84, 126.75, 126.58, 122.82, 119.43, 118.96, 48.16, 28.19, 23.15, 16.18 |
| 6 | 9.99(s, 1H, Ar—H), 8.42(d, J=8.1 Hz, 1H, —NH), 7.78—7.68(m, 2H, Ar—H), 7.35—7.28(m, 2H, Ar—H), 7.21(t, J=7.4 Hz, 1H, Ar—H), 7.17(d, J=8.7 Hz, 1H, Ar—H), 7.09(dd, J=19.6, 8.3 Hz, 4H, Ar—H, —C3H), 4.04(d, J=1.3 Hz, 1H, —CH), 1.94(d, J=1.3 Hz, 3H, —CH3) | 169.92, 153.68, 144.33, 132.71, 131.89, 129.95, 129.06, 126.75, 122.93, 119.84, 118.86, 84.00, 80.77, 48.14, 23.07 |
| Compd. | 1H NMR(600 MHz, DMSO⁃d6) | 13C NMR(150 MHz, DMSO⁃d6) |
| 7 | 8.81(d, J=3.1 Hz, 1H, —NH), 7.89(d, J=8.9 Hz, 1H, Ar—H), 7.86—7.82(m, 1H, Ar—H), 7.74—7.71(m, 1H, Ar—H), 7.38(ddd, J=8.4, 6.9, 1.5 Hz, 1H, Ar—H), 7.34(ddd, J=8.0, 6.8, 1.2 Hz, 1H, Ar—H), 7.30(d, J=8.9 Hz, 1H, Ar—H), 7.26—7.20(m, 4H, Ar—H), 7.17—7.13(m, 1H, Ar—H), 6.12 (d, J=3.1 Hz, 1H, —C3H) | 154.58, 152.66, 148.10, 135.64, 135.46, 134.17, 134.12, 133.85, 133.24, 132.58, 132.20, 130.29, 128.32, 122.09, 119.27, 59.04 |
| 8 | 8.78(d, J=3.1 Hz, 1H, —NH), 7.92(d, J=8.9 Hz, 1H, Ar—H), 7.88(d, J=8.0 Hz, 1H, Ar—H), 7.74(d, J=8.3 Hz, 1H, Ar—H), 7.44—7.35(m, 2H, Ar—H), 7.32(d, J=8.9 Hz, 1H, Ar—H), 7.14(d, J=7.9 Hz, 2H, Ar—H), 7.06(d, J=7.9 Hz, 2H, Ar—H), 6.09(d, J=3.1 Hz, 1H, —C3H), 2.15(s, 3H, —CH3) | 149.82, 147.84, 140.47, 137.78, 130.87, 130.60, 129.90, 129.37, 129.07, 127.77, 127.34, 125.50, 123.59, 117.32, 114.65, 54.01, 21.08 |
| 9 | 8.79(d, J=3.1 Hz, 1H, —NH), 7.96(d, J=8.9 Hz, 1H, Ar—H), 7.92(d, J=8.0 Hz, 1H, Ar—H), 7.79(d, J=8.3 Hz, 1H, Ar—H), 7.46(ddt, J=8.3, 6.8, 1.2 Hz, 1H, Ar—H), 7.42(ddd, J=8.0, 6.9, 1.2 Hz, 1H, Ar—H), 7.36(d, J=8.9 Hz, 1H, Ar—H), 7.20(d, J=8.0 Hz, 2H, Ar—H), 7.13(d, J=8.0 Hz, 2H, Ar—H), 6.13(d, J=3.1 Hz, 1H, —C3H), 2.53—2.46(m, 2H, —CH2), 1.08(td, J=7.6, 1.0 Hz, 3H, —CH3) | 149.83, 147.83, 144.09, 140.74, 130.87, 130.59, 129.36, 129.07, 128.76, 127.80, 127.39, 125.52, 123.60, 117.33, 114.69, 53.99, 28.23, 15.96 |
| 10 | 8.86(d, J=3.2 Hz, 1H, .—NH), 7.93(d, J=9.0 Hz, 1H, Ar—H), 7.88(d, J=8.0 Hz, 1H, Ar—H), 7.73(d, J=8.4 Hz, 1H, Ar—H), 7.40(dd, J=17.4, 8.1 Hz, 4H, Ar—H), 7.32(d, J=8.9 Hz, 1H, Ar—H), 7.27(d, J=7.9 Hz, 2H, Ar—H), 6.18(d, J=3.1 Hz, 1H, —C3H), 4.11(s, 1H, —CH) | 149.68, 147.95, 143.93, 132.81, 130.89, 129.29, 129.14, 127.87(d, J=16.3 Hz), 125.63, 123.49, 121.88, 117.36, 114.01, 83.44, 81.71, 53.85 |
| 11 | 9.95(s, 1H, Ar—H), 8.50(d, J=8.2 Hz, 1H, —NH), 7.91(d, J=8.6 Hz, 1H, Ar—H), 7.71(d, J=8.1 Hz, 1H, Ar—H), 7.67(d, J=8.8 Hz, 1H, Ar—H), 7.39(s, 1H, Ar—H), 7.31(t, J=7.7 Hz, 1H, Ar—H), 7.19(t, J=7.4 Hz, 1H, Ar—H), 7.13(d, J=8.8 Hz, 1H, Ar—H), 7.03(d, J=8.2 Hz, 1H, Ar—H), 6.26(t, J=2.4 Hz, 1H, Ar—H), 6.02(s, 1H, —C3H), 1.85 (s, 3H, —CH3) | 168.88, 154.82, 153.28, 141.58, 132.37, 129.36, 128.43, 126.20, 122.98, 122.33, 118.39, 116.57, 110.30, 106.01, 43.48, 22.52 |
| 12 | 9.92(s, 1H, Ar—H), 8.47(d, J=8.3 Hz, 1H, —NH), 7.94(d, J=8.7 Hz, 1H, Ar—H), 7.72—7.68(m, 1H, Ar—H), 7.65(d, J=8.9 Hz, 1H, Ar—H), 7.33—7.28(m, 1H, Ar—H), 7.20—7.16(m, 1H, Ar—H), 7.11(d, J=8.8 Hz, 1H, Ar—H), 6.95(d, J=8.2 Hz, 1H), 5.84(dd, J=3.2, 1.3 Hz, 1H, Ar—H), 5.81(dd, J=3.0, 1.3 Hz, 1H, —C3H), 2.06(s, 3H, —CH3), 1.81(d, J=2.7 Hz, 3H, —CH3) | 169.26, 153.78, 153.37, 150.80, 132.92, 129.82, 128.88, 126.68, 122.87, 118.97, 117.27, 107.64, 106.85, 44.00, 23.11, 13.90 |
| 13 | 10.09(s, 1H, Ar—H), 8.61(d, J=8.1 Hz, 1H, —NH), 7.97(d, J=8.6 Hz, 1H, Ar—H), 7.79(dd, J=17.1, 8.5 Hz, 2H, Ar—H), 7.41(t, J=7.8 Hz, 1H, Ar—H), 7.28(t, J=7.4 Hz, 1H, Ar—H), 7.20(d, J=8.8 Hz, 1H, Ar—H), 7.09—7.01(m, 1H, Ar—H), 6.44(d, J=3.3 Hz, 1H, Ar—H), 6.13(dd, J=3.4, 1.4 Hz, 1H, —C3H), 1.17(t, J=7.1 Hz, 3H, —CH3) | 169.43, 157.51, 153.91, 132.76, 130.17, 129.03, 128.77, 126.89, 123.12 (d, J = 45.8 Hz), 119.69, 118.84, 116.09, 112.79, 109.65, 43.85, 22.96 |
| 14 | 8.83(d, J=3.1 Hz, 1H, —NH), 7.99—7.95(m, 2H, Ar—H), 7.95—7.91 (m, 2H, Ar—H), 7.55— 7.52(m, 1H, Ar—H), 7.51(t, J=2.6 Hz, 1H, Ar—H), 7.45(t, J=7.5 Hz, 1H, Ar—H), 7.31(d, J=8.9 Hz, 1H, Ar—H), 6.43 (d, J=3.3 Hz, 1H, Ar—H), 6.34(dd, J=3.3, 1.8 Hz, 1H, Ar—H), 6.31(d, J=3.1 Hz, 1H, —C3H) | 154.19, 150.33, 148.01, 143.60, 130.76(d, J=7.6 Hz), 129.32, 129.06, 127.87, 125.60, 123.28, 117.28, 112.46, 111.00, 107.71, 47.71 |
| 15 | 9.64(d, J=3.1 Hz, 1H, Ar—H), 8.90—8.73(m, 3H, Ar—H), 8.53—8.36 (m, 1H, —NH), 8.30(t, J=7.5 Hz, 1H, Ar—H), 8.16(d, J=8.9 Hz, 1H, Ar—H), 7.10(dd, J=17.7, 3.1 Hz, 2H, Ar—H), 6.79(d, J=3.1 Hz, 1H, —C3H), 4.19(s, 2H, —NH2), 2.95(s, 3H, —CH3) | 152.55, 152.35, 150.38, 148.07, 130.78, 129.44, 129.11, 127.88, 125.63, 123.38, 117.39, 112.55, 108.66, 107.12, 47.89, 13.86 |
| 16 | 8.86—8.78(m, 1H, —NH), 7.99—7.87(m, 3H, Ar—H), 7.52(t, J=7.8 Hz, 1H, Ar—H), 7.44(t, J=7.5 Hz, 1H, Ar—H), 7.30(d, J=8.9 Hz, 1H, Ar—H), 6.29(dd, J=25.1, 3.1 Hz, 2H, Ar—H), 6.15(d, J=3.1 Hz, 1H, Ar—H), 5.72 (d, J=1.5 Hz, 1H, —C3H), 5.12(t, J=5.7 Hz, 1H, -OH), 4.21(d, J=5.5 Hz, 2H, —CH2) | 156.12, 153.39, 150.23, 148.04, 130.76, 129.38, 129.06, 127.84, 125.58, 123.29, 117.34, 112.33, 108.46, 108.22, 55.98, 47.89 |
Table 2 1H NMR and 13C NMR data of compounds 1—16
| Compd. | 1H NMR(600 MHz, DMSO⁃d6) | 13C NMR(150 MHz, DMSO⁃d6) |
|---|---|---|
| 1 | 8.40(d, J=8.4 Hz, 1H, —NH), 7.8—37.67(m, 3H, Ar—H), 7.34—7.24(m, 1H, Ar—H), 7.24—7.14(m, 4H, Ar—H), 7.09(dd, J=14.0, 7.8 Hz, 4H, Ar—H, —C3H), 1.92(s, 3H, —CH3) | 169.80, 153.69, 143.14, 132.85, 129.78, 129.07, 128.52, 126.57, 122.92, 119.36, 48.32, 23.19 |
| 2 | 9.93(s, 1H, Ar—H), 8.38(d, J=8.4 Hz, 1H, —NH), 7.84—7.74(m, 2H, Ar—H), 7.72(d, J=8.8 Hz, 1H, Ar—H), 7.32(t, J=7.8 Hz, 1H, Ar—H), 7.22 (t, J=7.5 Hz, 1H, Ar—H), 7.18(d, J=8.8 Hz, 1H, Ar—H), 7.06(d, J=8.4 Hz, 1H, —C3H), 7.01(s, 4H, Ar—H), 2.20(s, 3H, —CH3), 1.94(s, 3H, —CH3) | 169.62, 153.55, 140.04, 135.48, 132.78, 129.60, 129.01, 126.71, 122.82, 119.46, 118.94, 48.07, 23.15, 21.02 |
| 3 | 9.63(s, 1H, Ar—H), 8.08(d, J=8.5 Hz, 1H, —NH), 7.45(dd, J=8.2, 1.4 Hz, 1H, Ar—H), 7.40(d, J=8.8 Hz, 1H, Ar—H), 6.91(t, J=7.4 Hz, 1H, Ar—H), 6.87(d, J=8.8 Hz, 1H, Ar—H), 6.75—6.70(m, 3H, Ar—H), 6.46 (d, J=8.8 Hz, 2H, Ar—H, —C3H), 3.03(d, J=1.5 Hz, 3H, —CH3), 1.62(s, 3H, —CH3) | 169.56, 158.14, 153.52, 134.87, 132.75, 129.57, 128.99, 127.72, 126.71, 122.83, 119.44, 118.96, 113.88, 55.46, 47.90, 23.17 |
| 4 | 10.56(s, 1H, Ar—H), 8.99(d, J=8.3 Hz, 1H, —NH), 8.37—8.26(m, 2H, Ar—H), 7.90(t, J=7.7 Hz, 1H, Ar—H), 7.83—7.77(m, 1H, Ar—H), 7.74(d, J=8.8 Hz, 2H, Ar—H), 7.69(dd, J=8.6, 5.5 Hz, 2H, Ar—H), 7.66—7.55(m, 2H, Ar—H, —C3H), 3.02(p, J=1.8 Hz, 3H, —CH3) | 169.77, 161.97, 160.37, 153.61, 139.16, 132.68, 129.84, 129.05, 128.34, 126.88, 118.92, 115.07, 47.81, 23.10 |
| 5 | 9.98(d, J=2.4 Hz, 1H, Ar—H), 8.43(d, J=8.3 Hz, 1H, —NH), 7.81(dd, J=8.2, 3.2 Hz, 1H, Ar—H), 7.76(dd, J=8.9, 3.0 Hz, 1H, Ar—H), 7.24 (ddd, J=25.1, 8.5, 3.6 Hz, 3H, Ar—H), 7.09(dd, J=12.1, 5.7 Hz, 5H, Ar—H), 2.51(dd, J=4.1, 2.2 Hz, 2H, —CH2), 1.98(d, J=2.9 Hz, 3H, —CH3), 1.13(td, J=7.8, 3.5 Hz, 3H, —CH3) | 169.57, 153.55, 141.96, 140.27, 132.81, 129.59, 128.95(d, J=12.0 Hz), 127.84, 126.75, 126.58, 122.82, 119.43, 118.96, 48.16, 28.19, 23.15, 16.18 |
| 6 | 9.99(s, 1H, Ar—H), 8.42(d, J=8.1 Hz, 1H, —NH), 7.78—7.68(m, 2H, Ar—H), 7.35—7.28(m, 2H, Ar—H), 7.21(t, J=7.4 Hz, 1H, Ar—H), 7.17(d, J=8.7 Hz, 1H, Ar—H), 7.09(dd, J=19.6, 8.3 Hz, 4H, Ar—H, —C3H), 4.04(d, J=1.3 Hz, 1H, —CH), 1.94(d, J=1.3 Hz, 3H, —CH3) | 169.92, 153.68, 144.33, 132.71, 131.89, 129.95, 129.06, 126.75, 122.93, 119.84, 118.86, 84.00, 80.77, 48.14, 23.07 |
| Compd. | 1H NMR(600 MHz, DMSO⁃d6) | 13C NMR(150 MHz, DMSO⁃d6) |
| 7 | 8.81(d, J=3.1 Hz, 1H, —NH), 7.89(d, J=8.9 Hz, 1H, Ar—H), 7.86—7.82(m, 1H, Ar—H), 7.74—7.71(m, 1H, Ar—H), 7.38(ddd, J=8.4, 6.9, 1.5 Hz, 1H, Ar—H), 7.34(ddd, J=8.0, 6.8, 1.2 Hz, 1H, Ar—H), 7.30(d, J=8.9 Hz, 1H, Ar—H), 7.26—7.20(m, 4H, Ar—H), 7.17—7.13(m, 1H, Ar—H), 6.12 (d, J=3.1 Hz, 1H, —C3H) | 154.58, 152.66, 148.10, 135.64, 135.46, 134.17, 134.12, 133.85, 133.24, 132.58, 132.20, 130.29, 128.32, 122.09, 119.27, 59.04 |
| 8 | 8.78(d, J=3.1 Hz, 1H, —NH), 7.92(d, J=8.9 Hz, 1H, Ar—H), 7.88(d, J=8.0 Hz, 1H, Ar—H), 7.74(d, J=8.3 Hz, 1H, Ar—H), 7.44—7.35(m, 2H, Ar—H), 7.32(d, J=8.9 Hz, 1H, Ar—H), 7.14(d, J=7.9 Hz, 2H, Ar—H), 7.06(d, J=7.9 Hz, 2H, Ar—H), 6.09(d, J=3.1 Hz, 1H, —C3H), 2.15(s, 3H, —CH3) | 149.82, 147.84, 140.47, 137.78, 130.87, 130.60, 129.90, 129.37, 129.07, 127.77, 127.34, 125.50, 123.59, 117.32, 114.65, 54.01, 21.08 |
| 9 | 8.79(d, J=3.1 Hz, 1H, —NH), 7.96(d, J=8.9 Hz, 1H, Ar—H), 7.92(d, J=8.0 Hz, 1H, Ar—H), 7.79(d, J=8.3 Hz, 1H, Ar—H), 7.46(ddt, J=8.3, 6.8, 1.2 Hz, 1H, Ar—H), 7.42(ddd, J=8.0, 6.9, 1.2 Hz, 1H, Ar—H), 7.36(d, J=8.9 Hz, 1H, Ar—H), 7.20(d, J=8.0 Hz, 2H, Ar—H), 7.13(d, J=8.0 Hz, 2H, Ar—H), 6.13(d, J=3.1 Hz, 1H, —C3H), 2.53—2.46(m, 2H, —CH2), 1.08(td, J=7.6, 1.0 Hz, 3H, —CH3) | 149.83, 147.83, 144.09, 140.74, 130.87, 130.59, 129.36, 129.07, 128.76, 127.80, 127.39, 125.52, 123.60, 117.33, 114.69, 53.99, 28.23, 15.96 |
| 10 | 8.86(d, J=3.2 Hz, 1H, .—NH), 7.93(d, J=9.0 Hz, 1H, Ar—H), 7.88(d, J=8.0 Hz, 1H, Ar—H), 7.73(d, J=8.4 Hz, 1H, Ar—H), 7.40(dd, J=17.4, 8.1 Hz, 4H, Ar—H), 7.32(d, J=8.9 Hz, 1H, Ar—H), 7.27(d, J=7.9 Hz, 2H, Ar—H), 6.18(d, J=3.1 Hz, 1H, —C3H), 4.11(s, 1H, —CH) | 149.68, 147.95, 143.93, 132.81, 130.89, 129.29, 129.14, 127.87(d, J=16.3 Hz), 125.63, 123.49, 121.88, 117.36, 114.01, 83.44, 81.71, 53.85 |
| 11 | 9.95(s, 1H, Ar—H), 8.50(d, J=8.2 Hz, 1H, —NH), 7.91(d, J=8.6 Hz, 1H, Ar—H), 7.71(d, J=8.1 Hz, 1H, Ar—H), 7.67(d, J=8.8 Hz, 1H, Ar—H), 7.39(s, 1H, Ar—H), 7.31(t, J=7.7 Hz, 1H, Ar—H), 7.19(t, J=7.4 Hz, 1H, Ar—H), 7.13(d, J=8.8 Hz, 1H, Ar—H), 7.03(d, J=8.2 Hz, 1H, Ar—H), 6.26(t, J=2.4 Hz, 1H, Ar—H), 6.02(s, 1H, —C3H), 1.85 (s, 3H, —CH3) | 168.88, 154.82, 153.28, 141.58, 132.37, 129.36, 128.43, 126.20, 122.98, 122.33, 118.39, 116.57, 110.30, 106.01, 43.48, 22.52 |
| 12 | 9.92(s, 1H, Ar—H), 8.47(d, J=8.3 Hz, 1H, —NH), 7.94(d, J=8.7 Hz, 1H, Ar—H), 7.72—7.68(m, 1H, Ar—H), 7.65(d, J=8.9 Hz, 1H, Ar—H), 7.33—7.28(m, 1H, Ar—H), 7.20—7.16(m, 1H, Ar—H), 7.11(d, J=8.8 Hz, 1H, Ar—H), 6.95(d, J=8.2 Hz, 1H), 5.84(dd, J=3.2, 1.3 Hz, 1H, Ar—H), 5.81(dd, J=3.0, 1.3 Hz, 1H, —C3H), 2.06(s, 3H, —CH3), 1.81(d, J=2.7 Hz, 3H, —CH3) | 169.26, 153.78, 153.37, 150.80, 132.92, 129.82, 128.88, 126.68, 122.87, 118.97, 117.27, 107.64, 106.85, 44.00, 23.11, 13.90 |
| 13 | 10.09(s, 1H, Ar—H), 8.61(d, J=8.1 Hz, 1H, —NH), 7.97(d, J=8.6 Hz, 1H, Ar—H), 7.79(dd, J=17.1, 8.5 Hz, 2H, Ar—H), 7.41(t, J=7.8 Hz, 1H, Ar—H), 7.28(t, J=7.4 Hz, 1H, Ar—H), 7.20(d, J=8.8 Hz, 1H, Ar—H), 7.09—7.01(m, 1H, Ar—H), 6.44(d, J=3.3 Hz, 1H, Ar—H), 6.13(dd, J=3.4, 1.4 Hz, 1H, —C3H), 1.17(t, J=7.1 Hz, 3H, —CH3) | 169.43, 157.51, 153.91, 132.76, 130.17, 129.03, 128.77, 126.89, 123.12 (d, J = 45.8 Hz), 119.69, 118.84, 116.09, 112.79, 109.65, 43.85, 22.96 |
| 14 | 8.83(d, J=3.1 Hz, 1H, —NH), 7.99—7.95(m, 2H, Ar—H), 7.95—7.91 (m, 2H, Ar—H), 7.55— 7.52(m, 1H, Ar—H), 7.51(t, J=2.6 Hz, 1H, Ar—H), 7.45(t, J=7.5 Hz, 1H, Ar—H), 7.31(d, J=8.9 Hz, 1H, Ar—H), 6.43 (d, J=3.3 Hz, 1H, Ar—H), 6.34(dd, J=3.3, 1.8 Hz, 1H, Ar—H), 6.31(d, J=3.1 Hz, 1H, —C3H) | 154.19, 150.33, 148.01, 143.60, 130.76(d, J=7.6 Hz), 129.32, 129.06, 127.87, 125.60, 123.28, 117.28, 112.46, 111.00, 107.71, 47.71 |
| 15 | 9.64(d, J=3.1 Hz, 1H, Ar—H), 8.90—8.73(m, 3H, Ar—H), 8.53—8.36 (m, 1H, —NH), 8.30(t, J=7.5 Hz, 1H, Ar—H), 8.16(d, J=8.9 Hz, 1H, Ar—H), 7.10(dd, J=17.7, 3.1 Hz, 2H, Ar—H), 6.79(d, J=3.1 Hz, 1H, —C3H), 4.19(s, 2H, —NH2), 2.95(s, 3H, —CH3) | 152.55, 152.35, 150.38, 148.07, 130.78, 129.44, 129.11, 127.88, 125.63, 123.38, 117.39, 112.55, 108.66, 107.12, 47.89, 13.86 |
| 16 | 8.86—8.78(m, 1H, —NH), 7.99—7.87(m, 3H, Ar—H), 7.52(t, J=7.8 Hz, 1H, Ar—H), 7.44(t, J=7.5 Hz, 1H, Ar—H), 7.30(d, J=8.9 Hz, 1H, Ar—H), 6.29(dd, J=25.1, 3.1 Hz, 2H, Ar—H), 6.15(d, J=3.1 Hz, 1H, Ar—H), 5.72 (d, J=1.5 Hz, 1H, —C3H), 5.12(t, J=5.7 Hz, 1H, -OH), 4.21(d, J=5.5 Hz, 2H, —CH2) | 156.12, 153.39, 150.23, 148.04, 130.76, 129.38, 129.06, 127.84, 125.58, 123.29, 117.34, 112.33, 108.46, 108.22, 55.98, 47.89 |
| Compd. | Inhibition rate±SD(%) | |||
|---|---|---|---|---|
| R. solani | A. alternate | F. rhododendron | B. cinerea | |
| 1 | 13.77±0.05 | 2.04±0 | 14.85±0.05 | 26.32±0.05 |
| 2 | 24.64±0 | 32.65±0.05 | 17.82±0.07 | 49..47±0.06 |
| 3 | 15.22±0 | 17.69±0.04 | 20.79±0.05 | 7.37±0.08 |
| 4 | 26.09±0.05 | 55.10±0 | 50.50±0.08 | 23.16±0.09 |
| 5 | 40.58±0.06 | 49.66±0.05 | 51.49±0.04 | 25.26±0.11 |
| 6 | 37.68±0 | 66.67±0.04 | 43.56±0.05 | 17.89±0.10 |
| 7 | 36.96±0.02 | 39.46±0.04 | 37.62±0.05 | 16.84±0.07 |
| 8 | 54.35±0.01 | 66.67±0.07 | 55.45±0.05 | 56.84±0.17 |
| 9 | 17.39±0 | 3.40±0.05 | 14.85±0.10 | + |
| 10 | 54.81±0.07 | 15.22±0.05 | 16.79±0.08 | 20.35±0.30 |
| 11 | 34.81±0 | 18.12±0.04 | 13.14±0.04 | 12.39±0.24 |
| 12 | 43.70±0.05 | 27.54±0.05 | 25.55±0.06 | 62.83±0.06 |
| 13 | 39.26±0.05 | 42.03±0.05 | 29.2±0.07 | 58.41±0.07 |
| 14 | 48.15±0 | 49.28±0.05 | 62.77±0.05 | 60.18±0.10 |
| 15 | 34.81±0.01 | 22.46±0.04 | 14.60±0.05 | 68.14±0.14 |
| 16 | 30.37±0.04 | 17.39±0.05 | 15.33±0.05 | 50.44±0.22 |
| DMSO | 4.87±0.05 | 2.80±0 | 4.92±1.08 | + |
| Fludioxonil | 100±0 | 100±0 | 61.00±0 | 100±0 |
| Azoxystrobin | 100±0 | 57.82±0.05 | 100±0 | 41.05±0.08 |
Table 3 In vitro antifungal activities of 1-amidoalkyl-2-naphthol derivatives against four phytopathogenic fungi at 100 μg/mL*
| Compd. | Inhibition rate±SD(%) | |||
|---|---|---|---|---|
| R. solani | A. alternate | F. rhododendron | B. cinerea | |
| 1 | 13.77±0.05 | 2.04±0 | 14.85±0.05 | 26.32±0.05 |
| 2 | 24.64±0 | 32.65±0.05 | 17.82±0.07 | 49..47±0.06 |
| 3 | 15.22±0 | 17.69±0.04 | 20.79±0.05 | 7.37±0.08 |
| 4 | 26.09±0.05 | 55.10±0 | 50.50±0.08 | 23.16±0.09 |
| 5 | 40.58±0.06 | 49.66±0.05 | 51.49±0.04 | 25.26±0.11 |
| 6 | 37.68±0 | 66.67±0.04 | 43.56±0.05 | 17.89±0.10 |
| 7 | 36.96±0.02 | 39.46±0.04 | 37.62±0.05 | 16.84±0.07 |
| 8 | 54.35±0.01 | 66.67±0.07 | 55.45±0.05 | 56.84±0.17 |
| 9 | 17.39±0 | 3.40±0.05 | 14.85±0.10 | + |
| 10 | 54.81±0.07 | 15.22±0.05 | 16.79±0.08 | 20.35±0.30 |
| 11 | 34.81±0 | 18.12±0.04 | 13.14±0.04 | 12.39±0.24 |
| 12 | 43.70±0.05 | 27.54±0.05 | 25.55±0.06 | 62.83±0.06 |
| 13 | 39.26±0.05 | 42.03±0.05 | 29.2±0.07 | 58.41±0.07 |
| 14 | 48.15±0 | 49.28±0.05 | 62.77±0.05 | 60.18±0.10 |
| 15 | 34.81±0.01 | 22.46±0.04 | 14.60±0.05 | 68.14±0.14 |
| 16 | 30.37±0.04 | 17.39±0.05 | 15.33±0.05 | 50.44±0.22 |
| DMSO | 4.87±0.05 | 2.80±0 | 4.92±1.08 | + |
| Fludioxonil | 100±0 | 100±0 | 61.00±0 | 100±0 |
| Azoxystrobin | 100±0 | 57.82±0.05 | 100±0 | 41.05±0.08 |
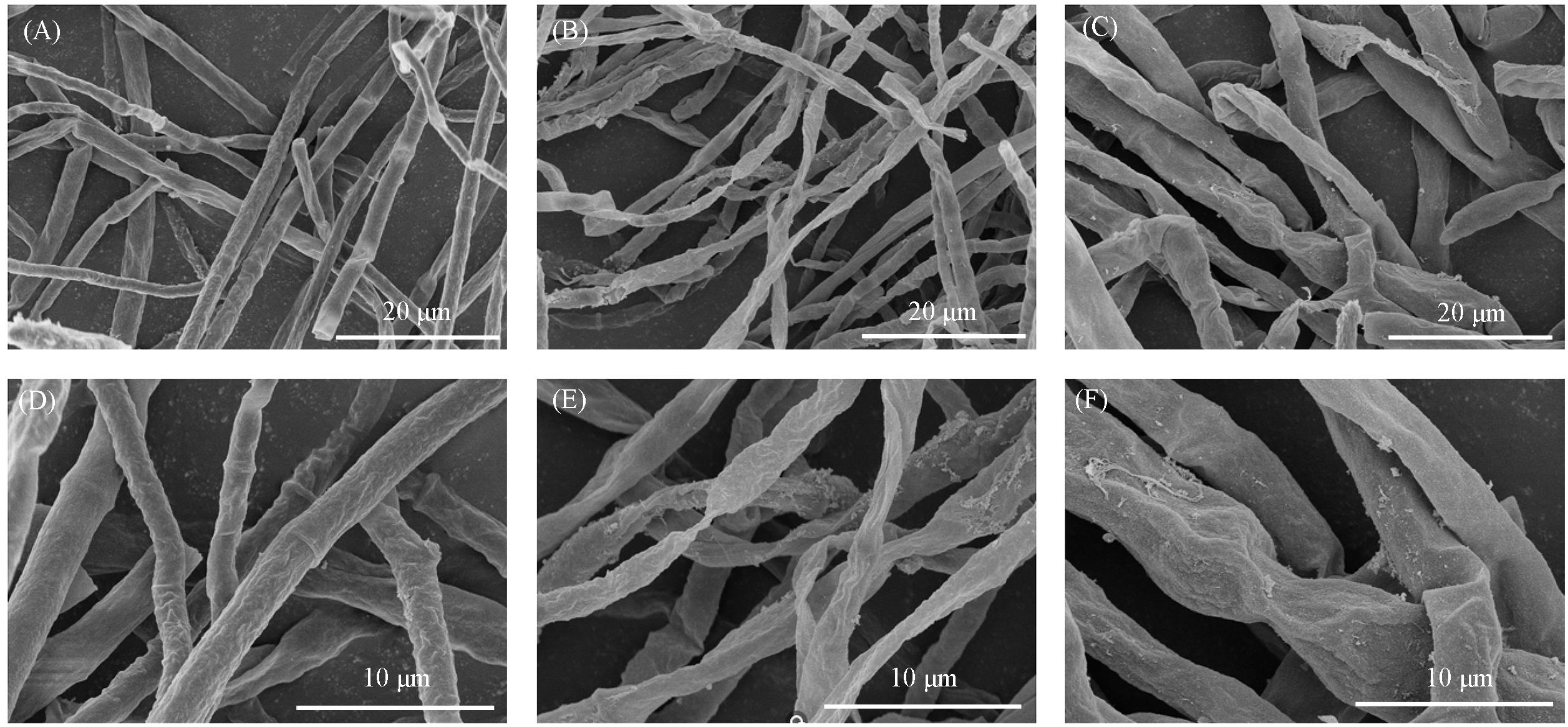
Fig.2 Sanning electron micrographs of A.alternate hyphae in the untreated control(A, D) and 200 μg/mL of compound 8(B, E) and 200 μg/mL of positive control(C, F)
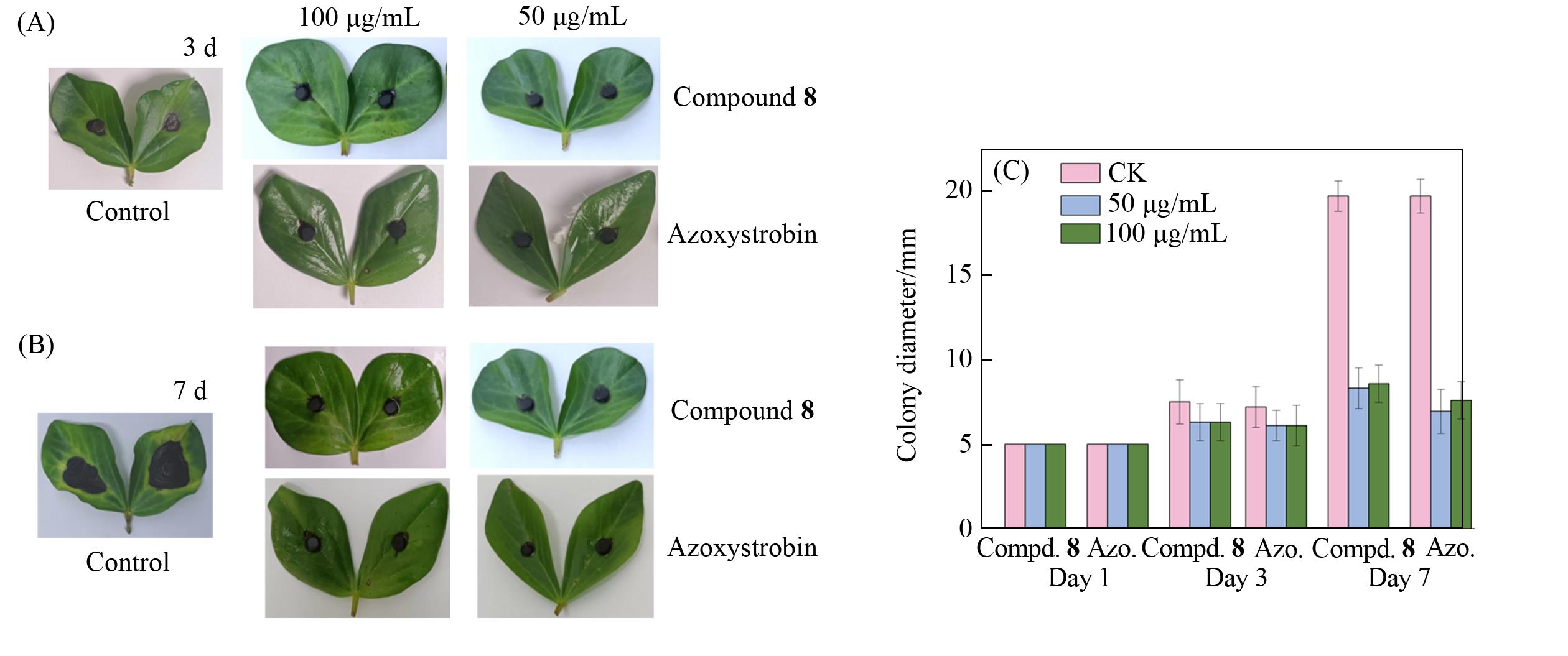
Fig.3 In vivo curative antifungal activities of compound 8 and azoxystrobin against A.alternate(A, B) and colony diameter Statistics of compound 8 and positive control treatments after 3 and 5 days(C)

Fig.5 Efficacy of amino alkyl naphthol analogs against root⁃knot nematodes in the untreated control(A) and 10 μg/mL(D―F) of compound 10(D), 8(E), 4(F) and 10 μg/mL of positive control(B, C)
| [1] | Niu B., Wang W. X., Yuan Z. B., Sederoff R. R., Sederoff H., Chiang V. L., Borriss R., Front. Microbiol., 2020, 11, 585404 |
| [2] | Chen W., Lan Y. X., Jin Y. X., Chen Y., Wu R., Chu C. W., Gao Y. F., Chem. J. Chinese Universities, 2023, 44(10), 20230179 |
| 陈伟, 兰雨欣, 金彦西, 陈阳, 吴润, 储承文, 高嫣凤. 高等学校化学学报, 2023, 44(10), 20230179 | |
| [3] | Salvatore M. M., Andolfi A., Toxins, 2021, 13(10), 689 |
| [4] | Burns A. R., Baker R. J., Kitner M., Knox J., Cooke B., Volpatti J. R., Vaidya A. S., Puumala E., Palmeira B. M., Redman E. M., Snider J., Marwah S., Chung S. W., MacDonald M. H., Tiefenbach J., Hu C., Xiao Q., Finney C. A. M., Krause H. M., MacParland S. A., Stagljar I., Gilleard J. S., Cowen L. E., Meyer S. L. F., Cutler S. R., Dowling J. J., Lautens M., Zasada I., Roy P. J., Nature, 2023, 618(7963), 102—109 |
| [5] | Maqsood A., Aslam M. N., Khaliq H., Shakeel M. T., Wu H. Y., Fahad S., J. Plant Growth Regul., 2024, 43(7), 2454—2469 |
| [6] | Zou Y., Zhu M., Zhu Z. N., Du T. T., Liu X., Jiang Y. J., Chen J. X., J. Agric. Food Chem., 2025, 73(8), 4534—4543 |
| [7] | Peng J., Zhang Y., Yang J. G., Zhou L. L., Zhang S. D., Wu X., Chen J. X., Hu D. Y., Gan X. H., J. Agric. Food Chem., 2024, 72(28), 15561—15571 |
| [8] | Zhang M. H., Feng S., Chen S., Zhou Y. X., Gong C. Y., Xue W., Pest Manag. Sci., 2023, 79(12), 4795—4808 |
| [9] | Larwood D. J., J. Fungi, 2020, 6(4), 261 |
| [10] | Abd El⁃Wahab A. H. F., Pharmaceuticals⁃Base, 2012, 5(7), 745—757 |
| [11] | Petkov H., Simeonov S. P., Appl. Sci.⁃Basel, 2023, 13(11), 6616 |
| [12] | Cimarelli C., Molecules, 2019, 24(13), 2372 |
| [13] | Zhang X., Hu Z., Wang S., Yin F. M., Wei Y. Y., Xie J., Sun R. F., J. Agric. Food Chem., 2023, 71(36), 13209—13219 |
| [14] | Olyaei A., Sadeghpour M., Rsc Advances, 2020, 10(12), 7011—7011 |
| [15] | Shaterian H. R., Yarahmadi H., Arkivoc, 2008, 105—114 |
| [16] | Mulla S. A. R., Salama T. A., Pathan M. Y., Inamdar S. M., Chavan S. S., Tetrahedron Lett., 2013, 54(7), 672—675 |
| [17] | Patil S. B., Singh P. R., Surpur M. P., Samant S. D., Ultrason. Sonochem., 2007, 14(5), 515—518 |
| [18] | Sapkal S. B., Shelke K. F., Madje B. R., Shingate B. B., Shingare M. S., Bull. Korean Chem. Soc., 2009, 30(12), 2887—2889 |
| [19] | Wang X. B., Dong X., Wang R. Y., Zhang J., Wang M. Q., Zhang Z. Q., Yang T. Y., Xu M. H., Chem. J. Chinese Universities, 2024, 45(2), 20230444 |
| 王晓斌, 董雪, 王瑞颖, 张娟, 王濛琪, 张宗群, 杨婷玉, 许梦寒. 高等学校化学学报, 2024, 45(2), 20230444 | |
| [20] | Duanis⁃Assaf D., Alkan N., Shimshoni J. A., Food Control, 2023, 154, 110041 |
| [21] | Hu Z. X., Zhang J., Zhang T., Tian C. Y., An Q., Yi P., Yuan C. M., Zhang Z. K., Zhao L. H., Hao X. J., J. Agric. Food Chem., 2024, 72(14), 8225—8236 |
| [22] | Li Y., Ma T. G., Yang Y., Zhong X., Zhu G. F., Wang J. T., Chen W. Z., Fan J. D., Tang L., Liu W. J., Fan L. L., J. Agric. Food Chem., 2024, 72(48), 26983—26995 |
| [23] | Wang L. S., Qin Y. K., Fan Z. Q., Gao K., Zhan J., Xing R. E., Liu S., Li P. C., J. Agric. Food Chem., 2022, 70(15), 4644—4657 |
| [24] | Cardellicchio C., Capozzi M. A. M., Naso F., Tetrahedron⁃Asymmetr., 2010, 21(5), 507—517 |
| [25] | Shaterian H. R., Azizi K., Fahimi N., Arab. J. Chem., 2017, 10, S42—S55 |
| [26] | Liu Y., Tian J., Zeng W., Wang Y. H., Hu C. M., Luo X. P., Qiu Y. J., Pu H. T., Wu Y. J., Xue W., J. Agric. Food Chem., 2024, 72(43), 23766—23775 |
| [27] | Shanbhag G., Naik M., Wagh D., Autkar S., Hagalavadi M. V., Wachendorff U., Pabba J., Klausener A., Pest Manag. Sci., 2024, https: //doi.org/10.1002/ps.8431 |
| [28] | Yuan Q. L., Fu W., Li X. Y., Xu Z. P., Liu X. L., Li Z., Shao X. S., J. Agric. Food Chem., 2024, 72(29), 16112—16127 |
| [29] | Wang X., Gao D. M., Li J., Wang X. X., Xu J. L., Zhang H., Gao Y. Q., Wu H., Ma Z. Q., J. Agric. Food Chem., 2024, 72(39), 21869—21876 |
| [30] | Chen X. F., Ren T., Mei D. H., Wei X. H., Guo Y. S., Li Y. Z., Nan Z. B., Song Q. Y., J. Agric. Food Chem., 2025, 73(11), 6711—6723 |
| [31] | Fan Z. Q., Qin Y. K., Liu S., Xing R. E., Yu H. H., Chen X. L., Li K. C., Li R. F., Wang X. Q., Li P. C., Carbohydr. Polym., 2019, 224, 115155 |
| [1] | 赵莹, 董继程, 方元, 张立军, 靳琳, 刘波, 程昉. 一锅法对PMMA及聚酯类材料的表面改性及抗生物垢性能评价[J]. 高等学校化学学报, 2025, 46(7): 20240566. |
| [2] | 张兴红, 耿鹏, 向娟娟, 晏佳莹, 毛妙付, 肖述章. 一锅法合成可印刷室温磷光二氟化硼衍生物[J]. 高等学校化学学报, 2024, 45(1): 20230432. |
| [3] | 冯瑞沁, 方云, 樊晔, 夏咏梅. 金纳米花的简易合成及催化硼氢化钠还原对硝基酚性能[J]. 高等学校化学学报, 2023, 44(8): 20230027. |
| [4] | 杨兆华, 成鸿静, 杨弋, 刘辉, 杜飞鹏, 张云飞. 聚乙烯醇载银海绵的制备及界面光热驱动水蒸发性能[J]. 高等学校化学学报, 2022, 43(10): 20220181. |
| [5] | 罗磊, 穆晓清, 吴涛, 聂尧, 徐岩. 一锅法合成去甲基麻黄素[J]. 高等学校化学学报, 2021, 42(8): 2458. |
| [6] | 曹锰, 柳阳, 张尚玺, 王振希, 徐胜. 壳聚糖钴配合物的合成及光催化产氢性能[J]. 高等学校化学学报, 2020, 41(4): 735. |
| [7] | 崔思乾, 卢俊瑞, 谢志强, 卢博为, 刘金彪, 刘梅, 马瑶, 胡新龙, 李贾东. 吡唑并[2,3-c]吡喃酮衍生物的一锅法熔融合成[J]. 高等学校化学学报, 2018, 39(4): 688. |
| [8] | 张金, 史天彩, 罗力文, 刘佳, 刘荣, 刘乐, 梁明, 马养民. 纳米氧化铜催化一锅法合成β-咔啉类化合物[J]. 高等学校化学学报, 2018, 39(11): 2411. |
| [9] | 刘豫龙, 路芳, 路萍. 基于芘并咪唑的电致发光材料的合成与表征[J]. 高等学校化学学报, 2017, 38(4): 583. |
| [10] | 张金, 刘佳, 马养民, 杨秀芳, 程佩, 范超, 卢萍. 纳米TiO2催化一锅法合成喹唑啉酮并酞嗪酮及3-酰胺基异吲哚酮并喹唑啉酮类化合物[J]. 高等学校化学学报, 2016, 37(9): 1629. |
| [11] | 刘楠, 武永刚, 白利斌, 王园, 黄海潮, 赵洪池, 巴信武. 一锅法制备pH响应性聚丙烯酰基乙二胺盐酸盐-卟啉/芴荧光聚合物[J]. 高等学校化学学报, 2014, 35(5): 1111. |
| [12] | 张振宾, 欧俊杰, 林辉, 刘忠山, 董靖, 邹汉法. 有机-硅胶杂化整体柱的制备及应用研究进展[J]. 高等学校化学学报, 2013, 34(9): 2011. |
| [13] | 撒宗朋, 刘彦晶, 李亚鹏, 祝明, 李玉祥, 王静媛. 酶促聚合和原子转移自由基聚合“一锅法”合成嵌段聚合物及其性能表征[J]. 高等学校化学学报, 2013, 34(7): 1770. |
| [14] | 栾贻浩, 武进, 詹茂盛, 张金明, 张军, 何嘉松. “一锅法”均相合成热塑性纤维素丙酸酯接枝聚乳酸共聚物[J]. 高等学校化学学报, 2012, 33(10): 2135. |
| [15] | 高钱, 修洋, 李国栋, 陈接胜. 一步法合成具有高效傅式烷基化催化性能的铁功能化有序介孔催化剂[J]. 高等学校化学学报, 2012, 33(04): 657. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||