

高等学校化学学报 ›› 2026, Vol. 47 ›› Issue (1): 20250262.doi: 10.7503/cjcu20250262
收稿日期:2025-09-15
出版日期:2026-01-10
发布日期:2025-11-21
通讯作者:
赵成吉
E-mail:zhaochengji@jlu.edu.cn
基金资助:
LIU Binghui1,2, ZHAO Chengji1( )
)
Received:2025-09-15
Online:2026-01-10
Published:2025-11-21
Contact:
ZHAO Chengji
E-mail:zhaochengji@jlu.edu.cn
Supported by:摘要:
质子交换膜燃料电池(PEMFC)具有能量转化效率高、 启动速度快及操作维护方便等突出优势. 在 120~250 ℃范围内运行的高温质子交换膜燃料电池(HT-PEMFC)无需依靠水进行质子传导, 可以有效简化水管理系统, 同时提升电极反应动力学并强化铂基电催化剂的抗中毒能力. 目前, 磷酸(PA)掺杂的聚苯并咪唑(PBI)膜是HT-PEMFC的首选隔膜材料, 但其面临着抗氧化稳定性不佳以及PA流失等关键挑战. 本文首先阐明了PA掺杂型高温质子交换膜(HT-PEM)的传输机制, 并基于近十年的研究进展对此类材料进行了系统分类; 然后, 重点剖析了HT-PEM面临的关键技术挑战及其应对策略, 并展望了未来的发展趋势.
中图分类号:
TrendMD:
刘炳辉, 赵成吉. 磷酸掺杂型高温质子交换膜的研究进展与改进策略. 高等学校化学学报, 2026, 47(1): 20250262.
LIU Binghui, ZHAO Chengji. Research Progress and Improvement Strategies of Phosphoric Acid-doped High-temperature Proton Exchange Membranes. Chem. J. Chinese Universities, 2026, 47(1): 20250262.
| Polymer type | Representative structure | Advantage | Disadvantage |
|---|---|---|---|
| Polybenzimidazoles |  | Excellent thermal stability and mechanical properties | Complex preparation process; poor solubility; severe phosphoric acid loss |
| Polyaryl ethers |  | Good mechanical strength; simple preparation process; high modifiability | Poor chemical stability |
| Polyphenyls |
| High chemical stability and excellent thermal stability | Metal⁃catalyzed process; poor solubility |
Phenylated polyphenylenes | 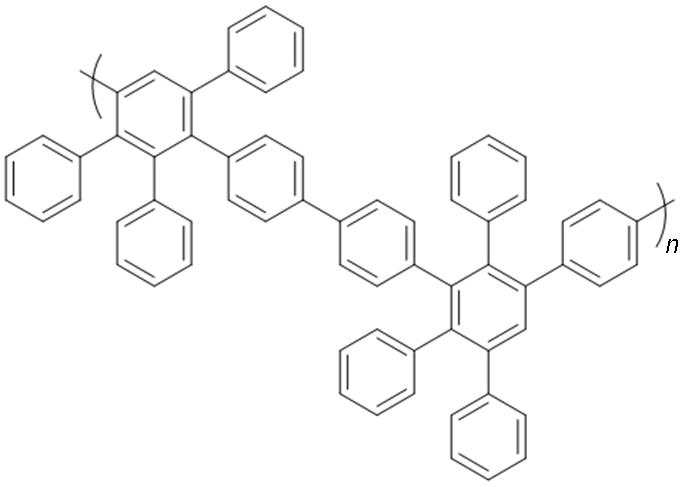 | Excellent chemical stability and mechanical properties; excellent solubility | Limited molecular design. |
| Poly(arylene⁃alkane)s | 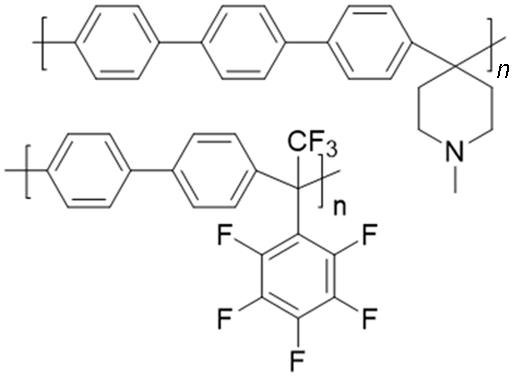 | No noble metal catalysts required; excellent thermal and chemical stability | The use of strong acid catalysts may cause environmental pollution |
| Polymers of intrinsic microporosity | 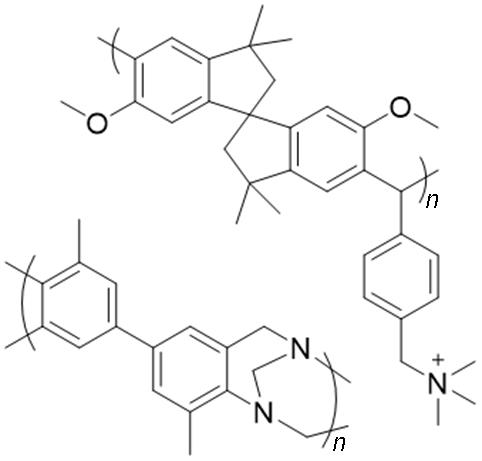 | Strong phosphoric acid retention capacity | Poor mechanical strength; Limited molecular design |
Table 1 Summary of the relevant properties of polymers with different backbone structures
| Polymer type | Representative structure | Advantage | Disadvantage |
|---|---|---|---|
| Polybenzimidazoles |  | Excellent thermal stability and mechanical properties | Complex preparation process; poor solubility; severe phosphoric acid loss |
| Polyaryl ethers |  | Good mechanical strength; simple preparation process; high modifiability | Poor chemical stability |
| Polyphenyls |
| High chemical stability and excellent thermal stability | Metal⁃catalyzed process; poor solubility |
Phenylated polyphenylenes |  | Excellent chemical stability and mechanical properties; excellent solubility | Limited molecular design. |
| Poly(arylene⁃alkane)s |  | No noble metal catalysts required; excellent thermal and chemical stability | The use of strong acid catalysts may cause environmental pollution |
| Polymers of intrinsic microporosity |  | Strong phosphoric acid retention capacity | Poor mechanical strength; Limited molecular design |
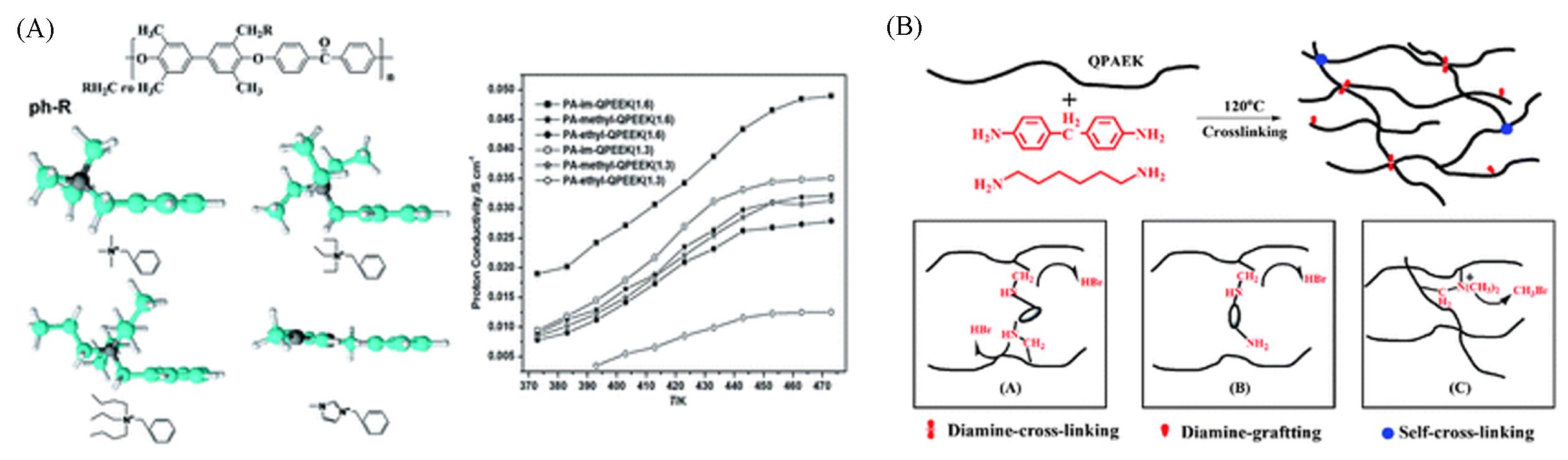
Fig.3 Structure of polyaryletherketone(QPAEK) containing different quaternary ammonium groups and proton conductivity of phosphoric acid⁃doped membranes(A)[32] and schematic representation of QPAEK cross⁃linked with diamines(B)[17](A) Copyright 2014, the Royal Society of Chemistry; (B) Copyright 2012, the Royal Society of Chemistry.
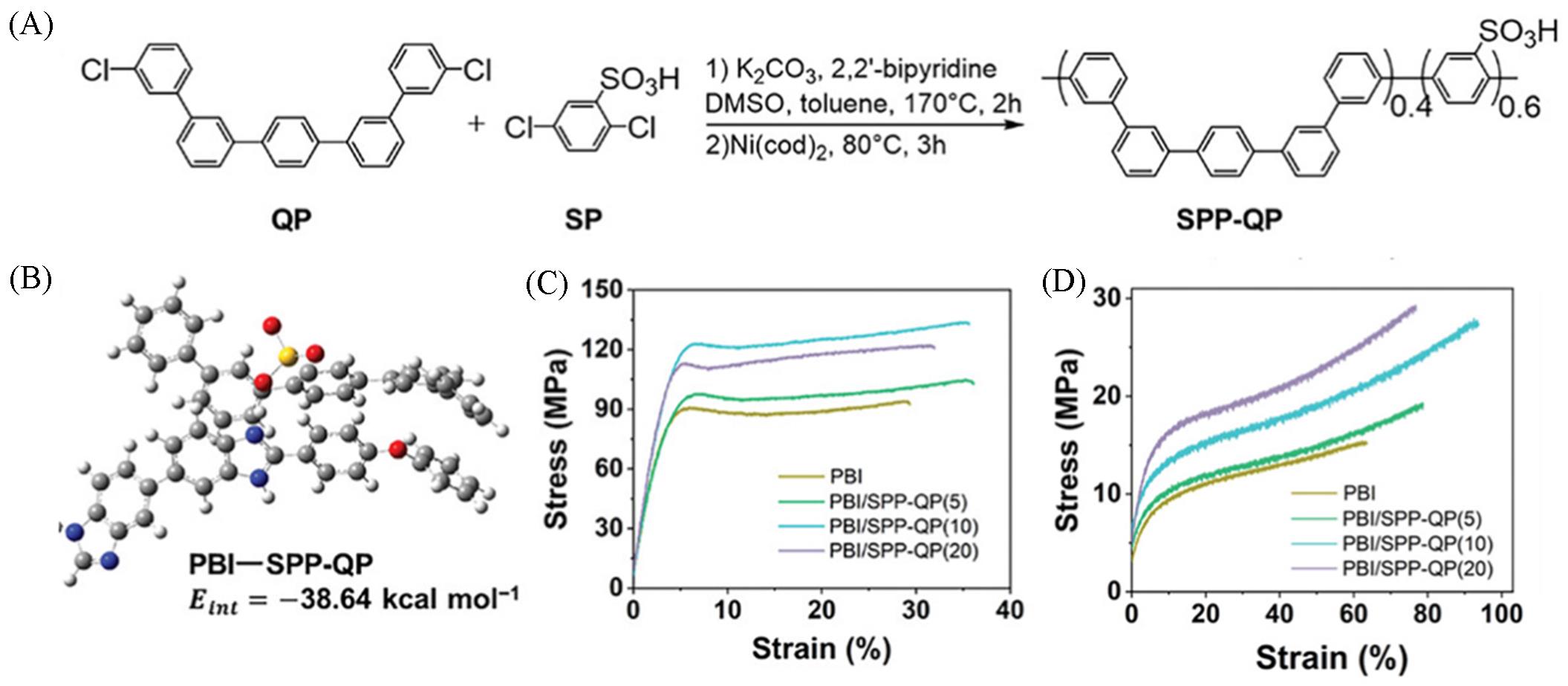
Fig.4 Synthetic process of the polyphenylene polymer SPP⁃QP(A), intermolecular interaction energy between PBI and SPP⁃QP(B), and stress⁃strain curves of the PBI/SPP⁃QP composite membranes before (C) and after (D) PA doping[39]Copyright 2024, John Wiley & Sons, Inc.
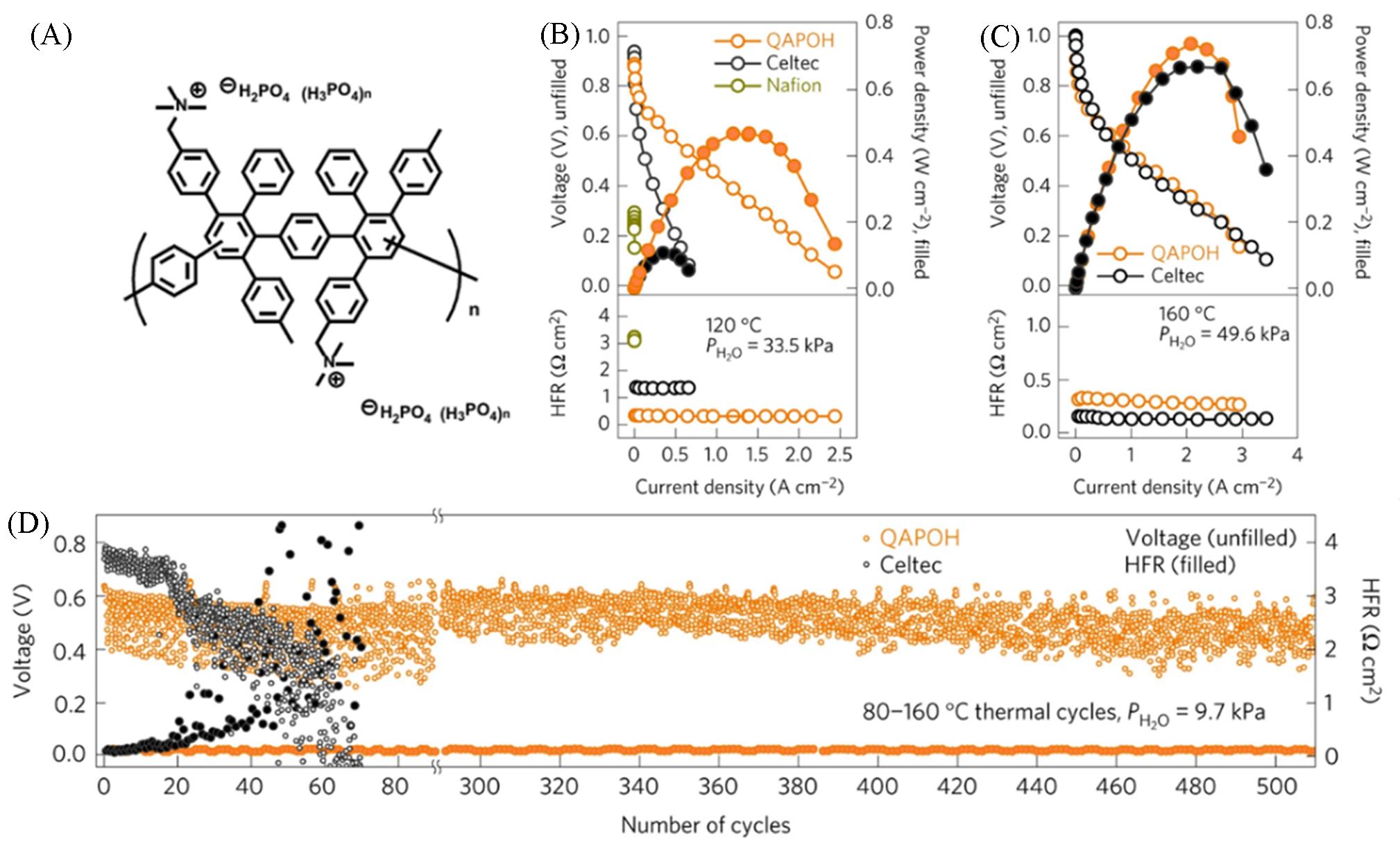
Fig.5 Structure of phenylated polyphenylene and its performance in the single cell[42](A) Chemical structure of phosphoric acid-doped phenyl polyphenylene QAPOH; (B, C) i⁃Voltage curve, power density, and HFR of QAPOH, Celtec®, and Nafion at 120 ℃(B) and 160 ℃(C); (D) variations of high frequency impedance and cell voltage for QAPOH electrodes and Celtec® electrodes during accelerated stress testing.Copyright 2016, Springer Nature.
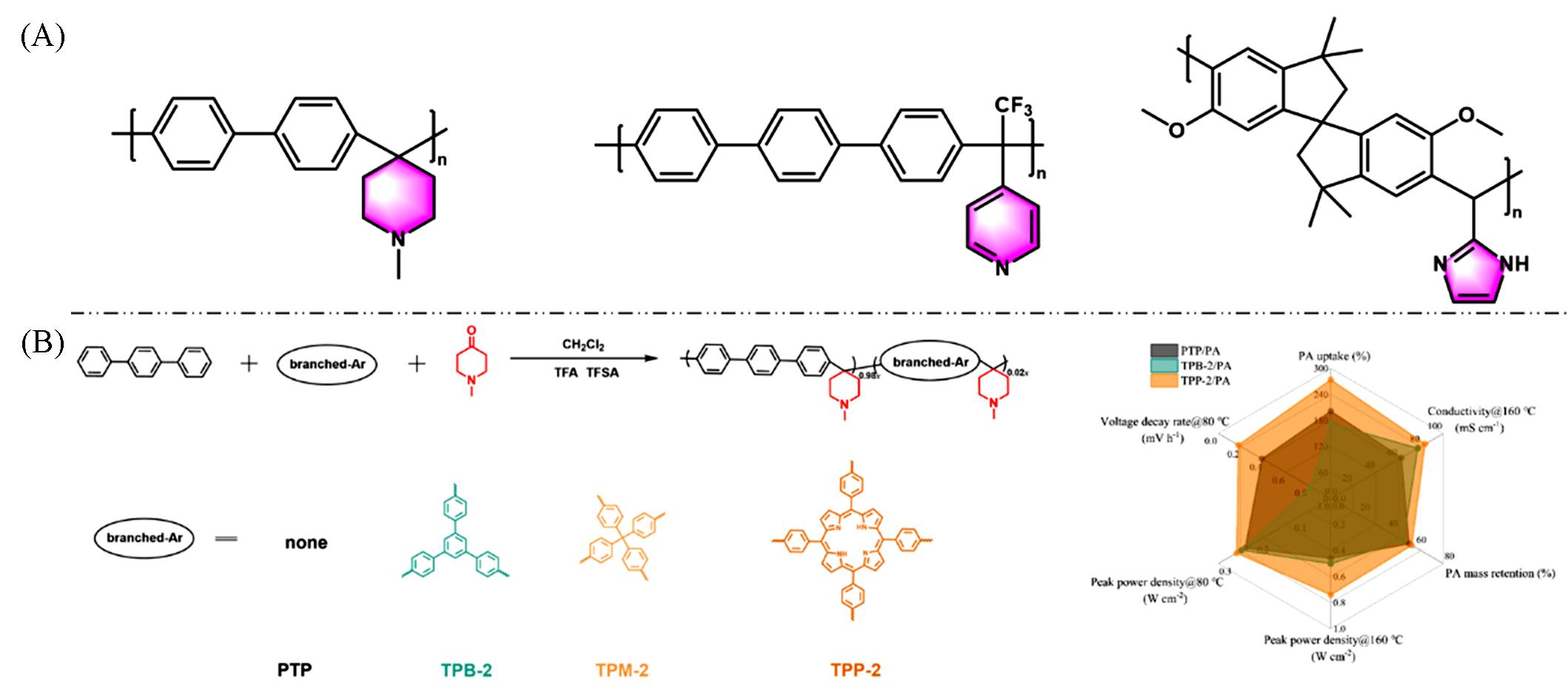
Fig.6 Chemical structures of representative polyarylene⁃alkane materials(A) and preparation and key performance comparison of phenyl⁃branched polyarylpiperidine polymers(B)[47](B) Copyright 2025, Elsevier.
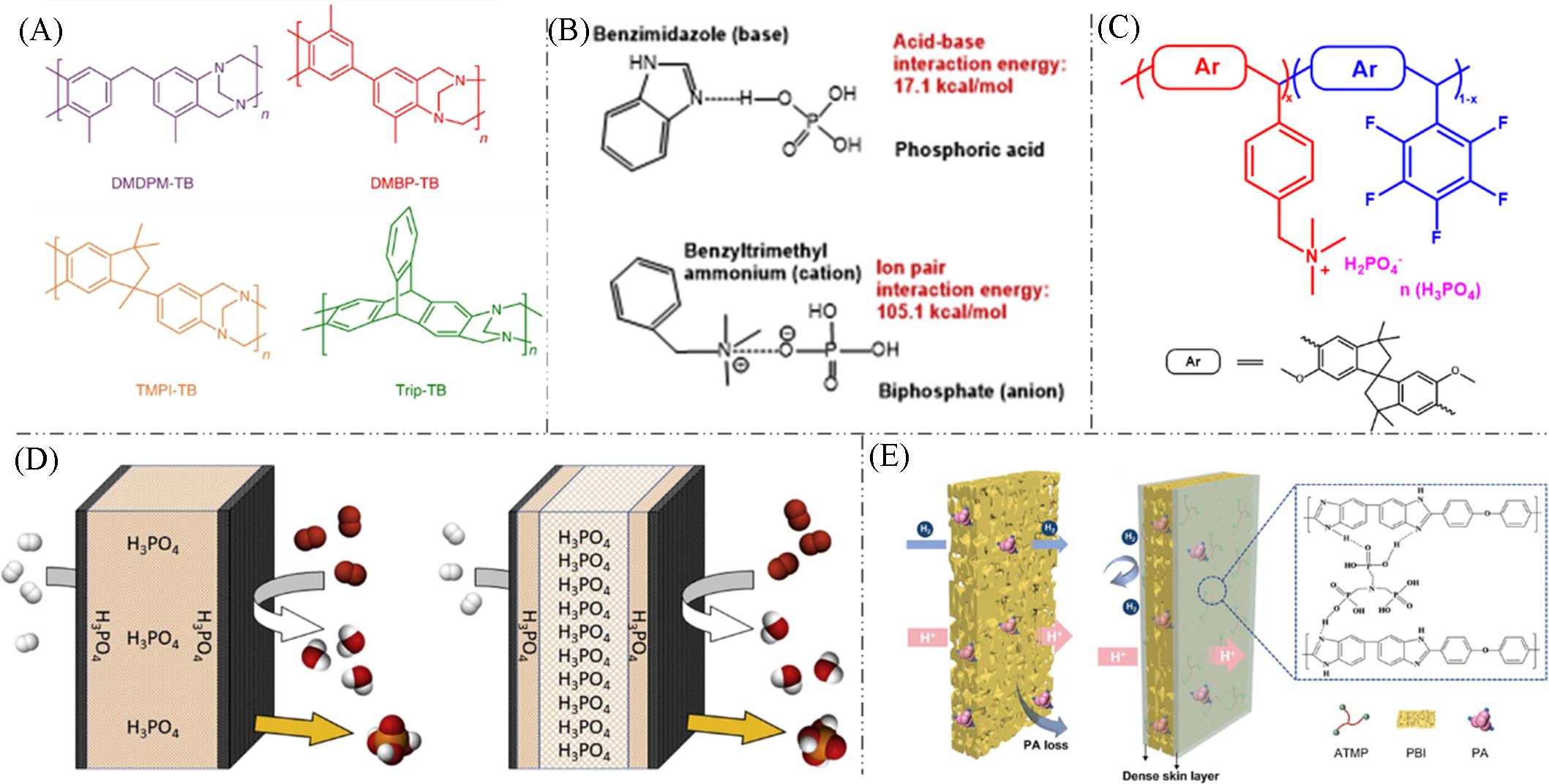
Fig.7 The ways to enhance phosphoric acid retention capacity(A) Designing Tröger base polymers with microporous structures[55]; (B) introducing strong ion-pair interactions between QA+ groups and H2PO4- ions[42]; (C) developing multi-block copolymers QPSBI-b-xTMA with ultramicroporous characteristics and QA+ groups[57]; (D) obtaining multilayer PBI membranes via surface curing processes[58]; (E) designing sandwich structures with dense surfaces and porous interiors[59].(A) Copyright 2022, Springer Nature; (B) Copyright 2016, Springer Nature; (C) Copyright 2024, American Chemical Society; (D) Copyright 2019, Elsevier; (E) Copyright 2022, John Wiley & Sons, Inc.
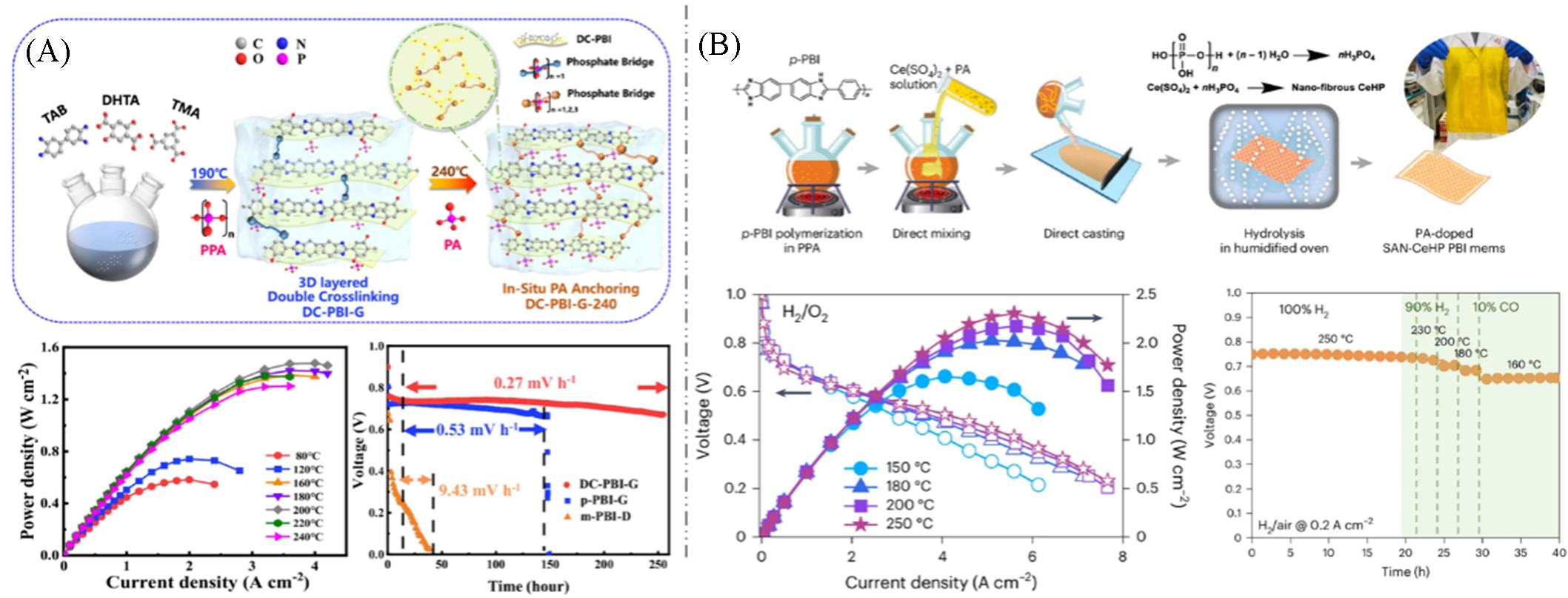
Fig.8 Preparation process of PA⁃poped Gel⁃state membrane DC⁃PBI⁃G and cell performance(A)[61] and polarization curves and long⁃term durability testing of SAN⁃CeHP⁃PBI membranes within the temperature range of 150—250 ℃(B)[62](A) Copyright 2024, Springer Nature; (B) Copyright 2024, Springer Nature.
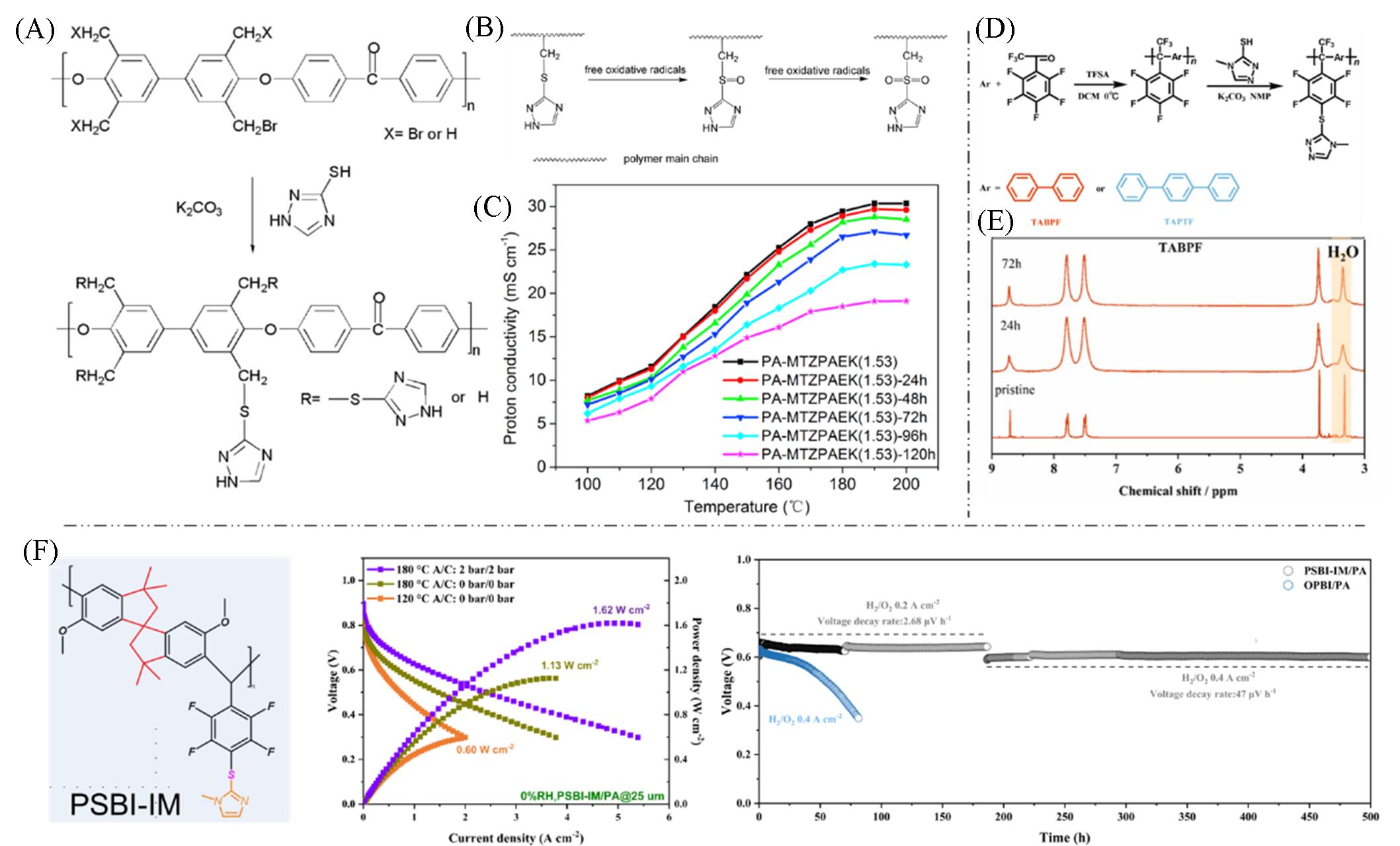
Fig.10 Applications of thioether bonds in polymers(A) Preparation process of PAEK polymer containing thiol-triazole groups; (B) antioxidant mechanism of the sulfide bond; (C) proton conductivity of the membrane before and after Fenton reagent treatment[68]; (D) preparation process of polyarylene-alkane polymers containing thiol triazoles; (E) 1H NMR structure of the membrane before and after Fenton reagent treatment[69]; (F) structural formula, single-cell performance, and stability of the intrinsically microporous polymer PSBI-IM containing imidazole groups[70].(A—C) Copyright 2017, Elsevier; (D, E) Copyright 2023, Elsevier; (F) Copyright 2025, John Wiley & Sons, Inc.
| [1] | Thomas J., Edwards P., Dobson P., Owen G., J. Energy Chem., 2020, 51, 405—415 |
| [2] | Zhu Y. H., Liu Y., Zhang F., Fan Z. H., Kang Z. Y., Wan X. H., Wang G. X., Li J., Tian C., Lei H., Wang W. N., Tian X. L., Chem. Res. Chinese Universities, 2025, 41(3), 484—494 |
| [3] | Hou M., Yi B. L., J. Electrochem., 2012, 18(1), 1—13 |
| 侯明, 衣宝廉. 电化学, 2012, 18(1), 1—13 | |
| [4] | Meng H., Song J., Guan P., Wang H., Zhao W., Zou Y., Ding H., Wu X., He P., Liu F., Zhang Y., J. Power Sources, 2024, 602, 234205 |
| [5] | Cheng H. L., Han K. H., Li A., Tao L. J., Yi F. Y., Sun J. J., Chem. J. Chinese Universities, 2024, 45(8), 20240217 |
| 程海龙, 韩康辉, 李奥, 陶璐静, 易飞扬, 孙娇娇. 高等学校化学学报, 2024, 45(8), 20240217 | |
| [6] | Wang S., Jiang S. P., Natl. Sci. Rev., 2017, 4(2), 163—166 |
| [7] | Dong W. Y., Pan J. X., Guo W., Chem. J. Chinese Universities, 2024, 45(2), 20230397 |
| 董文雅, 潘建欣, 郭伟. 高等学校化学学报, 2024, 45(2), 20230397 | |
| [8] | Gong L., Tao L., Wang L., Fu X., Wang S., Chinese J. Catal., 2025, 68, 155—176 |
| [9] | Aili D., Henkensmeier D., Martin S., Singh B., Hu Y., Jensen J. O., Cleemann L. N., Li Q., Electrochem. Energy Rev., 2020, 3(4), 793—845 |
| [10] | Wang Z. Q., Yang L. L., Sun H., Chem. Ind. Eng. Pro., 2020, 39(6), 20 |
| 王子乾, 杨林林, 孙海. 化工进展, 2020, 39(6), 20 | |
| [11] | Han S., Lv Y., Yang M. Y., Li Y. P., Tan C., Liu F., Yang H., Chu J. N., Liu M., Zhu C. Y., Gao R., Song Y. J., Chem. Res. Chinese Universities, 2025, 41(5), 1217—1224 |
| [12] | Adamski M., Peressin N., Holdcroft S., Mater. Adv., 2021, 2(15), 4966—5005 |
| [13] | Mauritz K. A., Moore R. B., Chem. Rev., 2004, 104(10), 4535—4585 |
| [14] | Amiinu I. S., Li W., Wang G., Tu Z., Tang H., Pan M., Zhang H., Electrochim. Acta, 2015, 160, 185—194 |
| [15] | Yang J., Xu H., Li J., Gong K., Yue F., Han X., Wu K., Shao P., Fu Q., Zhu Y., Xu W., Huang X., Xie J., Wang F., Yang W., Zhang T., Xu Z., Feng X., Wang B., Science, 2024, 385(6713), 1115—1120 |
| [16] | Ma W., Zhao C., Lin H., Zhang G., Ni J., Wang J., Wang S., Na H., J. Power Sources, 2011, 196(22), 9331—9338 |
| [17] | Ma W., Zhao C., Yang J., Ni J., Wang S., Zhang N., Lin H., Wang J., Zhang G., Li Q., Na H., Energy Environ. Sci., 2012, 5(6), 7617—7625 |
| [18] | Staiti P., Minutoli M., J. Power Sources, 2001, 94(1), 9—13 |
| [19] | Wu X., Scott K., Fuel Cells, 2012, 12(4), 583—588 |
| [20] | Li Q., Jensen J. O., Savinell R. F., Bjerrum N. J., Prog. Polym. Sci., 2009, 34(5), 449—477 |
| [21] | Wainright J. S., Wang J. T., Weng D., Savinell R. F., Litt M., J. Electrochem. Soc., 1995, 142(7), L121 |
| [22] | Melchior J. P., Majer G., Kreuer K. D., Phys. Chem. Chem. Phys., 2017, 19(1), 601—612 |
| [23] | Vogel H., Marvel C. S., Prog. Polym. Sci., 1961, 50(154), 511—539 |
| [24] | Seselj N., Aili D., Celenk S., Cleemann L. N., Hjuler H. A., Jensen J. O., Azizi K., Li Q., Chem. Soc. Rev, 2023, 52(12), 4046—4070 |
| [25] | Asensio J. A., Gómez⁃Romero P., Fuel Cells, 2005, 5(3), 336—343 |
| [26] | Wu A., Liu J., Wei G., Liu D., Wang L., J. Power Sources, 2022, 545, 231925 |
| [27] | Wang S., Zhao C., Ma W., Zhang G., Liu Z., Ni J., Li M., Zhang N., Na H., J. Membr. Sci., 2012, 411/412, 54—63 |
| [28] | Luo Y., Yu D., Gao T., Bai W., Zhang S., Guan X., Wu W., Wang S., Int. J. Hydrogen Energy, 2024, 77, 784—794 |
| [29] | Cao K. Y., Peng J. W., Li H. B., Shi C. Y., Wang P., Liu B. J., Chem. J. Chinese Universities, 2021, 42(6), 2049—2055 |
| 曹凯悦, 彭金武, 李宏斌, 石埕荧, 王鹏, 刘佰军. 高等学校化学学报, 2021, 42(6), 2049—2055 | |
| [30] | Dai J., Zhang Y., Gong C., Wan Y., Zhuang Y., Chem. Eng. J., 2023, 466, 143151 |
| [31] | Xu Z., Wang Q., Guo L., Li Y., Wang J., Yu S., Liao J., Xu Y., Shen J., Adv. Funct. Mater., 2023, 34, 2310762 |
| [32] | Zhang N., Wang B., Zhao C., Wang S., Zhang Y., Bu F., Cui Y., Li X., Na H., J. Mater. Chem. A, 2014, 2(34), 13996—14003 |
| [33] | Li Q., Liu L., Liang S., Li Q., Jin B., Bai R., Polym. Chem., 2014, 5(7), 2425—2432 |
| [34] | Wu W., Zou G., Fang X., Cong C., Zhou Q., Ind. Eng. Chem. Res., 2017, 56(37), 10227—10234 |
| [35] | Zhao Y. Y., Tsuchida E., Choe Y. K., Wang J., Ikeshoji T., Ohira A., J. Membr. Sci., 2015, 487, 229—239 |
| [36] | Pang Y., Duan Y., Li Q., Liu B., Hu X., Liu Q., Zhao C., J. Membr. Sci., 2023, 686, 121999 |
| [37] | Yamamoto T., Hayashi Y., Yamamoto A., Bull. Chem. Soc. Jpn, 2006, 51(7), 2091—2097 |
| [38] | Li T. T., Li H. B., Liu B. H., Zhao C. J., Li H. L., Prog. Chem., 2023, 35(11), 1559—1578 |
| 李婷婷, 李海宾, 刘炳辉, 赵成吉, 李昊龙. 化学进展, 2023, 35(11), 1559—1578 | |
| [39] | Bai Y., Xiao M., Wang C., Wang S., Meng Y., Miyatake K., Adv. Energy Mater., 2024, 14(33), 2400751 |
| [40] | Holmes T., Skalski T. J. G., Adamski M., Holdcroft S., Chem. Mater., 2019, 31(4), 1441—1449 |
| [41] | Peressin N., Adamski M., Schibli E. M., Ye E., Frisken B. J., Holdcroft S., Macromolecules, 2020, 53(8), 3119—3138 |
| [42] | Lee K., Spendelow J., Choe Y., Fujimoto C., Kim Y., Nat. Energy, 2016, 1(9), 16120 |
| [43] | Wang J., Zhao Y., Setzler B. P., Rojas⁃Carbonell S., Ben Yehuda. C., Amel A., Page M., Wang L., Hu K., Shi L., Gottesfeld S., Xu B., Yan Y., Nat. Energy, 2019, 4(5), 392—398 |
| [44] | Jin Y., Wang T., Che X., Dong J., Li Q., Yang J., J. Power Sources, 2022, 526, 231131 |
| [45] | Bai H., Peng H., Xiang Y., Zhang J., Wang H., Lu S., Zhuang L., J. Power Sources, 2019, 443, 227219 |
| [46] | Liu B., Duan Y., Li T., Pang Y., Liu Q., Li Q., Hu X., Zhao C., J. Membr. Sci., 2024, 692, 122273 |
| [47] | Liu B., Mu T., Liu Q., Pang Y., Lou J., Cao J., Zhao C., J. Membr. Sci., 2025, 733, 124327 |
| [48] | Feng X., Zhu J., Jin J., Wang Y., Zhang Y., van der Bruggen B., Prog. Mater Sci., 2024, 144, 101285 |
| [49] | Carta M., Malpass⁃Evans R., Croad M., Rogan Y., Jansen J C., Bernardo P., Bazzarelli F., Mckeown N. B., Science, 2013, 339(6117), 303—307 |
| [50] | Chen X., Wu L., Yang H., Qin Y., Ma X., Li N., Angew. Chem. Int. Ed., 2021, 60(33), 17875—17880 |
| [51] | Olvera L. I., Zolotukhin M. G., Hernández⁃Cruz O., Fomine S., Cárdenas J., Gaviño⁃Ramírez R. L., Ruiz⁃Trevino F. A., ACS Macro Lett., 2015, 4(5), 492—494 |
| [52] | Yang S., Li H., Zou W., Ling R., Ma X., Chen S., Yang Z., Xu T., JACS Au, 2024, 4(8), 3277—3283 |
| [53] | Guo Z., Perez⁃Page M., Chen J., Ji Z., Holmes S. M., J. Energy Chem., 2021, 63, 393—429 |
| [54] | Li J., Yang C., Zhang X., Xia Z., Wang S., Yu S., Sun G., J. Mater. Chem. A, 2023, 11(34), 18409—18418 |
| [55] | Tang H., Geng K., Wu L., Liu J., Chen Z., You W., Yan F., Guiver M. D., Li N., Nat. Energy, 2022, 7(2), 153—162 |
| [56] | Li J., Yang C., Lin H., Huang J., Wang S., Sun G., J. Energy Chem., 2024, 92, 572—578 |
| [57] | Liu B., Liu Q., Pang Y., Mu T., Zhao C., Macromolecules, 2024, 57(21), 10338—10348 |
| [58] | Kannan A., Aili D., Cleemann L. N., Li Q., Jensen J. O., Int. J. Hydrogen Energy, 2020, 45(1), 1008—1017 |
| [59] | Li W., Liu W., Zhang J., Wang H., Lu S., Xiang Y., Adv. Funct. Mater., 2023, 33(6), 2210036 |
| [60] | Zeng L., Dong D., Lu J., He K., Liu X., Wang J., Wei Z., Gresil M., Ratcliffe J., Li Z., Wang H., Adv. Funct. Mater., 2025, 35(31), 2424662 |
| [61] | Zhang L., Liu M., Zhu D., Tang M., Zhu T., Gao C., Huang F., Xue L., Nat. Commun., 2024, 15(1), 3409 |
| [62] | Lee S., Seong J., Jo Y., Hwang S., Gwak G., Park Y., Kim Y., Lim K., Park H., Jang J., Kim H., Nam S., Lee S. Y., Nat. Energy, 2024, 9(7), 849—861 |
| [63] | Liao J., Li Q., Rudbeck H., Jensen J., Chromik A., Bjerrum N., Kerres J., Xing W., Fuel Cells, 2011, 11(6), 745—755 |
| [64] | Ju Q., Chao G., Wang Y., Lv Z., Geng K., Li N., J. Membr. Sci., 2023, 686, 121970 |
| [65] | Liu B., Duan Y., Pang Y., Li Q., Zhao C., Chem. Eng. J., 2023, 477, 146955 |
| [66] | Wang J., Dai Y., Wan R., Wei W., Xu S., Zhai F., He R., Chem. Eng. J., 2021, 413, 127541 |
| [67] | Duan Y., Pang Y., Liu B., Wu L., Hu X., Li Q., Zhao C., ACS Sustainable Chem. Eng., 2023, 11(13), 5270—5283 |
| [68] | Bu F., Zhang Y., Hong L., Zhao W., Li D., Li J., Na H., Zhao C., J. Membr. Sci., 2018, 545, 167—175 |
| [69] | Hu X., Ao Y., Gao Y., Liu B., Zhao C., J. Membr. Sci., 2023, 687, 122102 |
| [70] | Liu B., Liu Q., Pang Y., Duan Y., Zhao C., Adv. Funct. Mater., 2025, 35(1), 2408291 |
| [71] | Yue Z., Cai Y. B., Xu S., Int. J. Hydrogen Energy, 2016, 41(24), 10421—10429 |
| [72] | Zhang W., Wang W., Xie D., Li J., Li H., Dai J., Tang Y., Yang T., Jin W., Zhou P., Gong C., J. Power Sources, 2024, 623, 235410 |
| [1] | 程海龙, 韩康辉, 李奥, 陶璐静, 易飞扬, 孙娇娇. 磺化聚苯乙烯/侧链交联型磺化聚芳醚酮砜复合质子交换膜的制备与性能[J]. 高等学校化学学报, 2024, 45(8): 20240217. |
| [2] | 贾林瀚, 杨代军, 明平文, 闵峻英, 冷宇. 质子交换膜燃料电池服役环境对TA1腐蚀行为的影响[J]. 高等学校化学学报, 2024, 45(2): 20230436. |
| [3] | 董文雅, 潘建欣, 郭伟. 质子交换膜燃料电池电堆阳极饥饿反极的研究[J]. 高等学校化学学报, 2024, 45(2): 20230397. |
| [4] | 李世宣, 蒙化, 尹学虎, 易锦飞, 马丽红, 张艳丽, 王红斌, 杨文荣, 庞鹏飞. 基于石墨烯/金纳米粒子/碳化钛复合材料构建双室酶生物燃料电池自供能葡萄糖生物传感器[J]. 高等学校化学学报, 2024, 45(12): 20240301. |
| [5] | 赵倩, 李赏, 程矿伟, 文智勇, 张晓宇, 易少杰, 潘牧. 氮掺杂PtCo/C合金催化剂的研究[J]. 高等学校化学学报, 2023, 44(6): 20230016. |
| [6] | 陈亚锋, 曾刘莉, 郭伟. 质子交换膜燃料电池阴极侧不同部位水淹对性能的影响[J]. 高等学校化学学报, 2023, 44(6): 20230003. |
| [7] | 王军, 杜石谦, 陶李. 高温聚合物电解质膜燃料电池催化剂的研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220722. |
| [8] | 仇心声, 吴芹, 史大昕, 张耀远, 陈康成, 黎汉生. 离子型交联磺化聚酰亚胺质子交换膜的制备及高温燃料电池性能[J]. 高等学校化学学报, 2022, 43(8): 20220140. |
| [9] | 陈长利, 米万良, 李煜璟. 单原子催化材料在电化学氢循环应用中的研究进展[J]. 高等学校化学学报, 2022, 43(5): 20220065. |
| [10] | 罗昪, 周芬, 潘牧. 层级多孔碳载铂催化剂的制备及可达性[J]. 高等学校化学学报, 2022, 43(4): 20210853. |
| [11] | 贾红军, 张佳涛, 马卓利, 王恒, 杨欣瑜, 杨加志. 丙烯酸水溶液聚合法制备PTFE/PAA/Nafion膜及其性能[J]. 高等学校化学学报, 2022, 43(11): 20220350. |
| [12] | 刘杰, 李金晟, 柏景森, 金钊, 葛君杰, 刘长鹏, 邢巍. 降低直接甲醇燃料电池浓差极化的含磺化碳管阻水夹层的构建[J]. 高等学校化学学报, 2022, 43(11): 20220420. |
| [13] | 付志男, 谈云龙, 肖谷雨, 颜德岳. 含全氟联苯结构的磺化聚二氮杂萘酮醚氧膦质子交换膜的制备与性能[J]. 高等学校化学学报, 2021, 42(8): 2635. |
| [14] | 曹凯悦, 彭金武, 李宏斌, 石埕荧, 王鹏, 刘佰军. 基于聚苯并咪唑/超支化聚合物的交联共混体系的高温质子交换膜[J]. 高等学校化学学报, 2021, 42(6): 2049. |
| [15] | 蒲阳阳, 宁聪, 陆瑶, 刘莉莉, 李娜, 胡朝霞, 陈守文. 新型共混交联磺化聚醚醚酮/部分氟化磺化聚芳醚砜质子交换膜的制备与表征[J]. 高等学校化学学报, 2021, 42(6): 2002. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||