

高等学校化学学报 ›› 2025, Vol. 46 ›› Issue (10): 20250123.doi: 10.7503/cjcu20250123
张灵芝1( ), 鞠秋荣2, 管哲3, 朱启华2, 张婷婷1, 杨诗勤1, 徐云根2
), 鞠秋荣2, 管哲3, 朱启华2, 张婷婷1, 杨诗勤1, 徐云根2
收稿日期:2025-04-25
出版日期:2025-10-10
发布日期:2025-06-25
通讯作者:
张灵芝
E-mail:zhanglingzhicpu@163.com
基金资助:
ZHANG Lingzhi1( ), JU Qiurong2, GUAN Zhe3, ZHU Qihua2, ZHANG Tingting1, YANG Shiqin1, XU Yungen2
), JU Qiurong2, GUAN Zhe3, ZHU Qihua2, ZHANG Tingting1, YANG Shiqin1, XU Yungen2
Received:2025-04-25
Online:2025-10-10
Published:2025-06-25
Contact:
ZHANG Lingzhi
E-mail:zhanglingzhicpu@163.com
Supported by:摘要:
以ERK抑制剂EK-I-22和MK-8353为先导化合物, 利用药效团融合策略, 设计合成了14个异吲哚啉酮类目标化合物. 初步的药理活性测试结果显示, 化合物19a(IC50=16 nmol/L), 19b(IC50=15 nmol/L), 19e(IC50= 20 nmol/L), 27a(IC50=19 nmol/L)和27b(IC50=56 nmol/L)对ERK2激酶具有较好的抑制活性, 其中化合物27b对 4种人肿瘤细胞(Colo-205, A375, A2058和HT-29)均具有一定的抑制活性.
中图分类号:
TrendMD:
张灵芝, 鞠秋荣, 管哲, 朱启华, 张婷婷, 杨诗勤, 徐云根. 异吲哚啉酮类ERK抑制剂的设计、合成及初步生物活性研究. 高等学校化学学报, 2025, 46(10): 20250123.
ZHANG Lingzhi, JU Qiurong, GUAN Zhe, ZHU Qihua, ZHANG Tingting, YANG Shiqin, XU Yungen. Design, Synthesis and Biological Evaluation of ERK Inhibitors with Isoindolin-1-one Structure. Chem. J. Chinese Universities, 2025, 46(10): 20250123.
| Compd. | Appearance | Yield(%) | m. p./℃ | Compd. | Appearance | Yield(%) | m. p./℃ |
|---|---|---|---|---|---|---|---|
| 3 | Yellow solid | 39.4 | 128—130 | 17b | White solid | 54.3 | 222—224 |
| 6 | Yellow solid | 86.2 | 150—152 | 17c | White solid | 80.2 | 192—194 |
| 7* | Yellow solid | — | — | 17d | White solid | 50.5 | 181—183 |
| 8 | White solid | 37.4 | 130—132 | 17e | White solid | 89.2 | 177—179 |
| 11a | White solid | 69.4 | 140—142 | 17f | White solid | 25.5 | 243—245 |
| 11b | White solid | 95.6 | 129—130 | 17g | Yellow solid | 59.3 | 134—136 |
| 14a | Yellow solid | 46.3 | 182—184 | 17h | Yellow solid | 50.8 | 126—128 |
| 14b | Yellow solid | 78.0 | 113—115 | 21 | White solid | 64.7 | 222—224 |
| 15a | Yellow solid | 81.7 | >250 | 22 | White solid | 78.7 | 176—178 |
| 15b | White solid | 40.5 | 209—211 | 24a | Yellow solid | 62.8 | 190—192 |
| 17a | White solid | 63.4 | 246—248 | 24b | Yellow solid | 94.2 | 240—242 |
Table 1 Appearances and yields of compounds11a, 11b, 14a, 14b, 15a, 15b, 17a—17h, 21, 24a, 24b
| Compd. | Appearance | Yield(%) | m. p./℃ | Compd. | Appearance | Yield(%) | m. p./℃ |
|---|---|---|---|---|---|---|---|
| 3 | Yellow solid | 39.4 | 128—130 | 17b | White solid | 54.3 | 222—224 |
| 6 | Yellow solid | 86.2 | 150—152 | 17c | White solid | 80.2 | 192—194 |
| 7* | Yellow solid | — | — | 17d | White solid | 50.5 | 181—183 |
| 8 | White solid | 37.4 | 130—132 | 17e | White solid | 89.2 | 177—179 |
| 11a | White solid | 69.4 | 140—142 | 17f | White solid | 25.5 | 243—245 |
| 11b | White solid | 95.6 | 129—130 | 17g | Yellow solid | 59.3 | 134—136 |
| 14a | Yellow solid | 46.3 | 182—184 | 17h | Yellow solid | 50.8 | 126—128 |
| 14b | Yellow solid | 78.0 | 113—115 | 21 | White solid | 64.7 | 222—224 |
| 15a | Yellow solid | 81.7 | >250 | 22 | White solid | 78.7 | 176—178 |
| 15b | White solid | 40.5 | 209—211 | 24a | Yellow solid | 62.8 | 190—192 |
| 17a | White solid | 63.4 | 246—248 | 24b | Yellow solid | 94.2 | 240—242 |
| Compd. | 1H NMR |
|---|---|
| 3 | (300 MHz, CDCl3), δ: 8.00(s, 1H), 7.92(d, J=8.8 Hz, 2H), 6.67(d, J=8.7 Hz, 2H), 4.07(s, 2H), 3.93(s, 3H), 3.60(q, J=5.9 Hz, 2H), 3.39(t, J=5.8 Hz, 2H) |
| 6 | (300 MHz, CDCl3), δ: 8.08—7.95(m, 3H), 7.25(d, J=8.0 Hz, 2H), 4.78(s, 1H), 3.92(s, 3H), 3.44—3.35(m, 2H), 2.82(t, J=7.0 Hz, 2H), 1.43(s, 9H) |
| 8 | (300 MHz, CDCl3), δ: 8.10(s, 1H), 8.08(d, J=8.2 Hz, 2H), 7.34—7.31(d, J=2.0 Hz, 2H), 6.68(s, 1H), 4.07(s, 2H), 4.01(s, 3H), 3.64(q, J=6.7 Hz, 2H), 2.93(t, J=7.0 Hz, 2H). |
| 11a | (300 MHz, CDCl3), δ: 8.65(d, J=5.3 Hz, 1H), 8.61—8.60(m, 1H), 8.16(dd, J=9.8, 1.8 Hz, 1H), 7.68(d, J=5.3 Hz, 1H), 7.41(d, J=8.1 Hz, 1H), 3.95(s, 3H), 2.68(s, 3H) |
| 11b | (400 MHz, CDCl3), δ: 8.65(s, 1H), 8.61-8.60(d, J=2.0 Hz, 1H), 7.96(dd, J=10.0, 2.0 Hz, 1H), 7.41(d, J=8.0 Hz, 1H), 3.93(s, 3H), 2.69(s, 3H) |
| 14a | (300 MHz, CDCl3), δ: 8.71(d, J=5.3 Hz, 1H), 8.51—8,50(m, 1H), 8.46(dd, J=9.7, 1.7 Hz, 1H), 7.76(d, J=5.3 Hz, 1H), 7.64(d, J=8.0 Hz, 1H), 4.64(s, 2H), 4.46(s, 2H), 3.80(s, 3H) |
| 14b | (300 MHz, CDCl3), δ: 8.69(s, 1H), 8.44(s, 1H), 8.11(d, J=8.1 Hz, 1H), 7.63(d, J=8.1 Hz, 1H), 4.63(s, 2H), 4.46(s, 2H), 3.78(s, 3H) |
| 15a | (300 MHz, DMSO⁃d6), δ: 8.84(d, J=5.3 Hz, 1H), 8.48—8.43(m, 2H), 8.30(d, J=5.3 Hz, 1H), 7.82(d, J=8.0 Hz, 1H), 4.62(s, 2H), 4.32(s, 2H). |
| 15b | (300 MHz, DMSO⁃d6), δ: 12.98(s, 1H), 9.03(s, 1H), 8.14(s, 1H), 8.07(d, J=7.6 Hz, 1H), 7.83(d, J=8.3 Hz, 1H), 4.64(s, 2H), 4.33(s, 2H) |
| 17a | (300 MHz, DMSO⁃d6), δ: 8.85(d, J=5.2 Hz, 1H), 8.50—8.44(m, 3H), 8.31(d, J=5.3 Hz, 1H), 7.97(d, J=8.1 Hz, 2H), 7.82(d, J=8.3 Hz, 1H), 7.57—7.53(m, 2H), 6.30—6.28(m, 1H), 4.61—4.54(m, 3H), 4.30—4.15(m, 2H), 3.93—3.91(m, 4H), 3.76—3.70(m, 2H), 2.72—2.65(m, 2H) |
| 17b | (300 MHz, CDCl3), δ: 8.69(d, J=5.1 Hz, 1H), 8.41—8.38(m, 2H), 7.97(s, 1H), 7.78(d, J=8.5 Hz, 2H), 7.71(d, J=5.4 Hz, 1H), 7.56(d, J=8.2 Hz, 1H), 6.57(d, J=8.9 Hz, 2H), 4.54(s, 2H), 4.27(s, 2H), 3.94(s, 3H), 3.59—3.53(m, 2H), 3.40—3.35(m, 2H) |
| 17c | (300 MHz, CDCl3), δ: 8.71(d, J=5.3 Hz, 1H), 8.52(s, 1H), 8.46(dd, J=8.0, 1.7 Hz, 1H), 7.76(d, J=5.3 Hz, 1H), 7.65(d, J=8.0 Hz, 1H), 4.70(s, 2H), 4.51(s, 2H), 3.70—3.64(m, 6H), 2.63—2.55(m, 6H) |
| 17d | (300 MHz, CDCl3), δ: 8.71(d, J=5.3 Hz, 1H), 8.54—8.51(m, 1H), 8.45(dd, J=9.7, 1.7 Hz, 1H), 7.76(d, J=5.3 Hz, 1H), 7.64(d, J=8.4 Hz, 1H), 4.68(s, 2H), 4.50(s, 2H), 3.75—3.63(m, 10H), 2.70—2.58(m, 6H) |
| 17e | (300 MHz, CDCl3), δ: 8.72(d, J=5.3 Hz, 1H), 8.51—8.47(m, 2H), 7.78(d, J=5.2 Hz, 1H), 7.67(d, J=7.8 Hz, 1H), 4.70(s, 2H), 4.29(s, 2H), 3.77—3.74(m, 2H), 3.59(t, J=4.6 Hz, 4H), 3.52—3.48(m, 2H) |
| 17f | (400 MHz, CDCl3), δ: 8.70—8.67(m, 1H), 8.45—8.44(m, 1H), 8.08—8.06(m, 4H), 7.63—7.62 m, 1H), 7.46—7.44(m, 2H), 6.18—6.13(m, 1H), 4.70(s, 2H), 4.58—4.53(m, 2H), 4.28(s, 2H), 3.98(s, 3H), 3.86—3.82(m, 2H), 2.68—2.61(m, 2H) |
| 17g | (300 MHz, DMSO⁃d6), δ: 9.05(s, 1H), 8.14(s, 1H), 8.08—8.04(m, 1H), 7.83(d, J=8.0 Hz, 1H), 7.32—7.28(m, 2H), 6.70(d, J=9.6 Hz, 2H), 6.02—6.00(m, 1H), 4.62—4.53(m, 4H), 4.24—4.10(m, 2H), 3.71—3.69(m, 2H), 2.90(s, 6H), 2.60—2.56(m, 2H) |
| 17h | (300 MHz, DMSO⁃d6), δ: 9.05(s, 1H), 8.14(s, 1H), 8.07(dd, J=8.0, 1.6 Hz, 1H), 7.83(d, J=8.4 Hz, 1H), 6.91—6.84(m, 2H), 6.74—6.68(m, 2H), 4.61(s, 2H), 4.55(s, 2H), 3.66—3.49(m,8H), 2.80(s, 6H) |
| 21 | (300 MHz, DMSO⁃d6⁃D2O), δ: 8.86(d, J=5.3 Hz, 1H, ArH), 8.50—8.43(m, 2H, ArH), 8.22(d, J=6.2 Hz, 1H, ArH), 7.87(d, J=7.7 Hz, 1H, ArH), 4.68(s, 2H, CONC |
| Compd. | 1H NMR |
| 22 | (300 MHz, CDCl3), δ: 8.72(d, J=5.3 Hz, 1H), 8.52(s, 1H), 8.46(dd, J=8.0, 1.6 Hz, 1H), 7.75(d, J=5.2 Hz, 1H), 7.66(d, J=8.2 Hz, 1H), 6.64(s, 1H), 4.68(s, 2H), 4.33(s, 2H), 3.52—3.44(m, 4H). |
| 24a | (300 MHz, DMSO⁃d6), δ: 8.85(d, J=5.3 Hz, 1H), 8.48(s, 1H), 8.45(dd, J=8.0, 1.6 Hz, 1H), 8.30(d, J=5.3 Hz, 1H), 8.28—8.26(m, 1H), 8.06(s, 1H), 7.80(d, J=8.1 Hz, 1H), 4.55(s, 2H), 4.51(s, 2H), 4.43(t, J=5.8 Hz, 2H), 4.16(s, 2H), 3.53—3.46(m, 6H) |
| 24b | (300 MHz, DMSO⁃d6), δ: 8.85(d, J=5.7 Hz, 1H), 8.48(s, 1H), 8.45(dd, J=7.9, 1.6 Hz, 1H), 8.30—8.26(m, 2H), 7.84(s, 1H), 7.79(d, J=8.0 Hz, 1H), 5.02(s, 1H), 4.55(s, 2H), 4.39(t, J=6.1 Hz, 2H), 4.17(s, 2H), 3.53—3.46(m, 2H), 1.45(s, 6H) |
Table 2 1 H NMR data of compounds 11a, 11b, 14a, 14b, 15a, 15b, 17a—17h, 21, 22, 24a, 24b
| Compd. | 1H NMR |
|---|---|
| 3 | (300 MHz, CDCl3), δ: 8.00(s, 1H), 7.92(d, J=8.8 Hz, 2H), 6.67(d, J=8.7 Hz, 2H), 4.07(s, 2H), 3.93(s, 3H), 3.60(q, J=5.9 Hz, 2H), 3.39(t, J=5.8 Hz, 2H) |
| 6 | (300 MHz, CDCl3), δ: 8.08—7.95(m, 3H), 7.25(d, J=8.0 Hz, 2H), 4.78(s, 1H), 3.92(s, 3H), 3.44—3.35(m, 2H), 2.82(t, J=7.0 Hz, 2H), 1.43(s, 9H) |
| 8 | (300 MHz, CDCl3), δ: 8.10(s, 1H), 8.08(d, J=8.2 Hz, 2H), 7.34—7.31(d, J=2.0 Hz, 2H), 6.68(s, 1H), 4.07(s, 2H), 4.01(s, 3H), 3.64(q, J=6.7 Hz, 2H), 2.93(t, J=7.0 Hz, 2H). |
| 11a | (300 MHz, CDCl3), δ: 8.65(d, J=5.3 Hz, 1H), 8.61—8.60(m, 1H), 8.16(dd, J=9.8, 1.8 Hz, 1H), 7.68(d, J=5.3 Hz, 1H), 7.41(d, J=8.1 Hz, 1H), 3.95(s, 3H), 2.68(s, 3H) |
| 11b | (400 MHz, CDCl3), δ: 8.65(s, 1H), 8.61-8.60(d, J=2.0 Hz, 1H), 7.96(dd, J=10.0, 2.0 Hz, 1H), 7.41(d, J=8.0 Hz, 1H), 3.93(s, 3H), 2.69(s, 3H) |
| 14a | (300 MHz, CDCl3), δ: 8.71(d, J=5.3 Hz, 1H), 8.51—8,50(m, 1H), 8.46(dd, J=9.7, 1.7 Hz, 1H), 7.76(d, J=5.3 Hz, 1H), 7.64(d, J=8.0 Hz, 1H), 4.64(s, 2H), 4.46(s, 2H), 3.80(s, 3H) |
| 14b | (300 MHz, CDCl3), δ: 8.69(s, 1H), 8.44(s, 1H), 8.11(d, J=8.1 Hz, 1H), 7.63(d, J=8.1 Hz, 1H), 4.63(s, 2H), 4.46(s, 2H), 3.78(s, 3H) |
| 15a | (300 MHz, DMSO⁃d6), δ: 8.84(d, J=5.3 Hz, 1H), 8.48—8.43(m, 2H), 8.30(d, J=5.3 Hz, 1H), 7.82(d, J=8.0 Hz, 1H), 4.62(s, 2H), 4.32(s, 2H). |
| 15b | (300 MHz, DMSO⁃d6), δ: 12.98(s, 1H), 9.03(s, 1H), 8.14(s, 1H), 8.07(d, J=7.6 Hz, 1H), 7.83(d, J=8.3 Hz, 1H), 4.64(s, 2H), 4.33(s, 2H) |
| 17a | (300 MHz, DMSO⁃d6), δ: 8.85(d, J=5.2 Hz, 1H), 8.50—8.44(m, 3H), 8.31(d, J=5.3 Hz, 1H), 7.97(d, J=8.1 Hz, 2H), 7.82(d, J=8.3 Hz, 1H), 7.57—7.53(m, 2H), 6.30—6.28(m, 1H), 4.61—4.54(m, 3H), 4.30—4.15(m, 2H), 3.93—3.91(m, 4H), 3.76—3.70(m, 2H), 2.72—2.65(m, 2H) |
| 17b | (300 MHz, CDCl3), δ: 8.69(d, J=5.1 Hz, 1H), 8.41—8.38(m, 2H), 7.97(s, 1H), 7.78(d, J=8.5 Hz, 2H), 7.71(d, J=5.4 Hz, 1H), 7.56(d, J=8.2 Hz, 1H), 6.57(d, J=8.9 Hz, 2H), 4.54(s, 2H), 4.27(s, 2H), 3.94(s, 3H), 3.59—3.53(m, 2H), 3.40—3.35(m, 2H) |
| 17c | (300 MHz, CDCl3), δ: 8.71(d, J=5.3 Hz, 1H), 8.52(s, 1H), 8.46(dd, J=8.0, 1.7 Hz, 1H), 7.76(d, J=5.3 Hz, 1H), 7.65(d, J=8.0 Hz, 1H), 4.70(s, 2H), 4.51(s, 2H), 3.70—3.64(m, 6H), 2.63—2.55(m, 6H) |
| 17d | (300 MHz, CDCl3), δ: 8.71(d, J=5.3 Hz, 1H), 8.54—8.51(m, 1H), 8.45(dd, J=9.7, 1.7 Hz, 1H), 7.76(d, J=5.3 Hz, 1H), 7.64(d, J=8.4 Hz, 1H), 4.68(s, 2H), 4.50(s, 2H), 3.75—3.63(m, 10H), 2.70—2.58(m, 6H) |
| 17e | (300 MHz, CDCl3), δ: 8.72(d, J=5.3 Hz, 1H), 8.51—8.47(m, 2H), 7.78(d, J=5.2 Hz, 1H), 7.67(d, J=7.8 Hz, 1H), 4.70(s, 2H), 4.29(s, 2H), 3.77—3.74(m, 2H), 3.59(t, J=4.6 Hz, 4H), 3.52—3.48(m, 2H) |
| 17f | (400 MHz, CDCl3), δ: 8.70—8.67(m, 1H), 8.45—8.44(m, 1H), 8.08—8.06(m, 4H), 7.63—7.62 m, 1H), 7.46—7.44(m, 2H), 6.18—6.13(m, 1H), 4.70(s, 2H), 4.58—4.53(m, 2H), 4.28(s, 2H), 3.98(s, 3H), 3.86—3.82(m, 2H), 2.68—2.61(m, 2H) |
| 17g | (300 MHz, DMSO⁃d6), δ: 9.05(s, 1H), 8.14(s, 1H), 8.08—8.04(m, 1H), 7.83(d, J=8.0 Hz, 1H), 7.32—7.28(m, 2H), 6.70(d, J=9.6 Hz, 2H), 6.02—6.00(m, 1H), 4.62—4.53(m, 4H), 4.24—4.10(m, 2H), 3.71—3.69(m, 2H), 2.90(s, 6H), 2.60—2.56(m, 2H) |
| 17h | (300 MHz, DMSO⁃d6), δ: 9.05(s, 1H), 8.14(s, 1H), 8.07(dd, J=8.0, 1.6 Hz, 1H), 7.83(d, J=8.4 Hz, 1H), 6.91—6.84(m, 2H), 6.74—6.68(m, 2H), 4.61(s, 2H), 4.55(s, 2H), 3.66—3.49(m,8H), 2.80(s, 6H) |
| 21 | (300 MHz, DMSO⁃d6⁃D2O), δ: 8.86(d, J=5.3 Hz, 1H, ArH), 8.50—8.43(m, 2H, ArH), 8.22(d, J=6.2 Hz, 1H, ArH), 7.87(d, J=7.7 Hz, 1H, ArH), 4.68(s, 2H, CONC |
| Compd. | 1H NMR |
| 22 | (300 MHz, CDCl3), δ: 8.72(d, J=5.3 Hz, 1H), 8.52(s, 1H), 8.46(dd, J=8.0, 1.6 Hz, 1H), 7.75(d, J=5.2 Hz, 1H), 7.66(d, J=8.2 Hz, 1H), 6.64(s, 1H), 4.68(s, 2H), 4.33(s, 2H), 3.52—3.44(m, 4H). |
| 24a | (300 MHz, DMSO⁃d6), δ: 8.85(d, J=5.3 Hz, 1H), 8.48(s, 1H), 8.45(dd, J=8.0, 1.6 Hz, 1H), 8.30(d, J=5.3 Hz, 1H), 8.28—8.26(m, 1H), 8.06(s, 1H), 7.80(d, J=8.1 Hz, 1H), 4.55(s, 2H), 4.51(s, 2H), 4.43(t, J=5.8 Hz, 2H), 4.16(s, 2H), 3.53—3.46(m, 6H) |
| 24b | (300 MHz, DMSO⁃d6), δ: 8.85(d, J=5.7 Hz, 1H), 8.48(s, 1H), 8.45(dd, J=7.9, 1.6 Hz, 1H), 8.30—8.26(m, 2H), 7.84(s, 1H), 7.79(d, J=8.0 Hz, 1H), 5.02(s, 1H), 4.55(s, 2H), 4.39(t, J=6.1 Hz, 2H), 4.17(s, 2H), 3.53—3.46(m, 2H), 1.45(s, 6H) |
| Compd. | Appearance | m. p./℃ | Yield(%) | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 19a | White solid | >250 | 17.8 | 591.2832(591.2833) |
| 19b | White solid | 254—256 | 58.6 | 625.2437(625.2431) |
| 19c | Yellow solid | >250 | 46.7 | 587.2626(587.2622) |
| 19d | Yellow solid | 279—281 | 68.4 | 621.2236(621.2247) |
| 19e | White solid | 219—221 | 61.7 | 568.2784(568.2784) |
| 19f | Yellow solid | 170—172 | 48.6 | 481.2563(481.2555) |
| 19g | White solid | 139—141 | 44.5 | 525.2825(525.2828) |
| 19h | White solid | 188—190 | 59.1 | 456.2247(456.2234) |
| 19i | White solid | 215—217 | 65.3 | 587.2532(587.2530) |
| 19j | White solid | 222—224 | 77.2 | 612.2460(612.2441) |
| 25a | Yellow solid | 100—102 | 85.1 | 537.2574(537.2571) |
| 25b | White solid | 118—120 | 90.2 | 521.2625(521.2617) |
| 27a | White solid | 139—141 | 52.9 | 669.3078(669.3085) |
| 27b | White solid | 140—142 | 41.2 | 654.2969(654.2977) |
Table 3 Appearances, melting points, yields and HRMS data of compounds 19a—19j, 25a, 25b, 27a and 27b
| Compd. | Appearance | m. p./℃ | Yield(%) | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 19a | White solid | >250 | 17.8 | 591.2832(591.2833) |
| 19b | White solid | 254—256 | 58.6 | 625.2437(625.2431) |
| 19c | Yellow solid | >250 | 46.7 | 587.2626(587.2622) |
| 19d | Yellow solid | 279—281 | 68.4 | 621.2236(621.2247) |
| 19e | White solid | 219—221 | 61.7 | 568.2784(568.2784) |
| 19f | Yellow solid | 170—172 | 48.6 | 481.2563(481.2555) |
| 19g | White solid | 139—141 | 44.5 | 525.2825(525.2828) |
| 19h | White solid | 188—190 | 59.1 | 456.2247(456.2234) |
| 19i | White solid | 215—217 | 65.3 | 587.2532(587.2530) |
| 19j | White solid | 222—224 | 77.2 | 612.2460(612.2441) |
| 25a | Yellow solid | 100—102 | 85.1 | 537.2574(537.2571) |
| 25b | White solid | 118—120 | 90.2 | 521.2625(521.2617) |
| 27a | White solid | 139—141 | 52.9 | 669.3078(669.3085) |
| 27b | White solid | 140—142 | 41.2 | 654.2969(654.2977) |
| Compd. | 1H NMR | 13C NMR |
|---|---|---|
| 19a | (300 MHz, CDCl3), δ: 8.50(s, 1H), 8.38(d, J=5.3 Hz, 1H), 8.30(dd, J=8.1, 1.7 Hz, 1H), 8.11—8.08(m, 3H), 7.59(d, J=8.0 Hz, 1H), 7.49—7.44(m, 2H), 7.08(d, J=5.3 Hz, 1H), 6.20—6.15(m, 1H), 5.26(d, J=8.4 Hz, 1H), 4.69(s, 2H), 4.60(s, 1H), 4.55(s, 1H), 4.31—4.30(m, 2H), 4.23—4.19(m, 1H), 4.07—4.0(m, 5H), 3.90—3.82(m, 2H), 3.66—3.58(m, 2H), 2.69—2.64(m, 2H), 2.15—2.11(m, 2H), 1.63— 1.58(m, 2H) | (75 MHz, DMSO⁃d6), δ: 167.99, 166.75, 166.58, 162.27, 161.27, 159.65, 146.15, 144.91, 140.60, 134.82, 134.38, 133.14, 130.31, 126.21, 125.47, 124.34, 121.72, 121.43, 121.14,106.14, 72.75, 66.59, 60.72, 51.03, 47.21, 44.05, 36.44, 32.98, 27.33 |
| 19b | (300 MHz, DMSO⁃d6), δ: 8.51(s, 1H), 8.44(s, 1H), 8.04—7.96(m, 4H), 7.75(d, J=8.4 Hz, 1H), 7.57—7.54(m, 2H), 6.29(s, 1H), 4.59—4.54(m, 4H), 4.29—4.15(m, 2H), 3.92—3.85(m, 6H), 3.76—3.73(m, 2H), 3.41—3.37(m, 2H), 2.66—2.54(m, 2H), 1.87—1.83(m, 2H), 1.55—1.51(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.74, 167.74, 160.38, 145.52, 144.50, 141.34, 135.50, 134.44, 132.86, 131.57, 128.40, 126.53, 125.14, 124.67, 122.99, 120.76, 115.97, 115.73, 104.31, 99.06, 66.06, 51.68, 47.26, 35.99, 31.92, 26.88, 19.64, 19.38, 19.12 |
| 19c | (400 MHz, DMSO⁃d6), δ: 9.56(s, 1H), 8.52—8.40(m, 4H), 8.48(s, 1H), 7.98(d, J=8.3 Hz, 2H), 7.92(s, 1H), 7.79(d, J=8.0 Hz, 1H), 7.57(t, J=7.2 Hz, 3H), 7.42(d, J=5.2 Hz, 1H), 6.30(s, 1H), 4.61—4.55(m, 4H), 4.30(s, 1H), 4.16(s, 1H), 3.92(s, 3H), 3.83(s, 3H), 3.76—3.70(m, 2H), 2.67—2.54(m, 2H) | (75 MHz, aectic⁃d4), δ: 169.65, 167.47, 160.51, 145.56, 143.16, 141.20, 140.14, 139.16, 136.38, 135.48, 134.41, 132.39, 131.43, 128.45, 126.49, 125.09, 123.97, 123.11, 121.59, 114.73, 107.02, 96.29, 90.58, 51.74, 38.06, 35.93, 35.45, 19.64, 19.38, 19.12 |
| 19d | (400 MHz, DMSO⁃d6), δ: 9.83(s, 1H), 8.58(s, 1H), 8.51(s, 1H), 8.11(s, 1H), 8.06(d, J=8.0 Hz, 1H), 7.98(d, J=8.5 Hz, 2H), 7.83—7.79(m, 2H), 7.56(dd, J=8.2, 6.2 Hz, 2H), 7.52(s, 1H), 6.30(s, 1H), 4.61—4.55(m, 4H), 4.30(s, 1H), 4.16(s, 1H), 3.92(s, 3H), 3.79—3.71(m, 5H), 2.66—2.54(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.76, 167.58, 159.65, 157.54, 149.08, 145.21, 144.61, 141.60, 134.40, 132.93, 131.68, 126.63, 125.18, 124.70, 123.17, 122.56, 120.93, 119.97, 116.95, 101.59, 98.04, 90.16, 86.42, 64.75, 51.73, 37.95, 36.24, 19.65, 19.38, 19.12 |
| 19e | (300 MHz, CDCl3), δ: 8.44(s, 1H), 8.38(d, J=5.3 Hz, 1H), 8.26(dd, J=7.5, 1.7 Hz, 1H), 7.99(s, 1H), 7.86(d, J=8.5 Hz, 2H), 7.52(d, J=8.2 Hz, 1H), 7.04(d, J=5.0 Hz, 1H), 6.62(d, J=8.5 Hz, 2H), 4.48(s, 1H), 4.28(s, 2H), 4.21—4.15(m, 1H), 4.06—4.02(m, 2H), 3.94(s, 3H), 3.65—3.53(m, 4H), 3.36(t, J=6.2 Hz, 2H), 2.15—2.09(m, 2H), 1.66—1.60(m, 2H) | (75 MHz, DMSO⁃d6), δ: 168.70, 168.35, 163.20, 162.11, 162.04, 149.70, 145.42, 144.93, 137.35, 132.83, 130.49, 127.37, 124.37, 121.45, 118.97, 114.94, 112.21, 106.16, 66.58, 51.22, 47.10, 45.43, 42.29, 36.14, 32.83, 27.27 |
| 19f | (300 MHz, CDCl3), δ: 8.42(s, 1H), 8.33(d, J=5.2 Hz, 1H), 8.24(d, J=9.8 Hz, 1H), 7.53(d, J=8.9 Hz, 1H), 7.01(d, J=5.5 Hz, 1H), 5.15(d, J=6.8 Hz, 1H), 4.60(s, 2H), 4.44(s, 2H), 4.19—4.09(m, 1H), 4.01—3.97(m, 2H), 3.64—3.52(m, 8H), 2.57—2.50(m, 6H), 2.08—2.04(m, 2H), 1.84(brs, 1H), 1.63—1.52(m, 2H) | (75 MHz, DMSO⁃d6), δ: 167.92, 166.32, 163.17, 162.27, 159.61, 144.87, 137.47, 133.10, 130.30, 124.31, 121.43, 106.14, 66.59, 60.54, 58.96, 53.63, 53.23, 50.99, 47.21, 44.59, 43.87, 41.93, 32.98(Affected by steric hindrance, the four carbon atoms in piperazine show different displacements) |
| 19g | (300 MHz, CDCl3), δ: 8.48(s, 1H, ArH), 8.39(d, J=5.2 Hz, 1H), 8.30(dd, J=8.0, 1.7 Hz, 1H), 7.58(d, J=8.2 Hz, 1H), 7.07(d, J=6.0 Hz, 1H), 5.18(d, J=7.8 Hz, 1H), 4.65(s, 2H), 4.49(s, 2H), 4.24—4.17(m, 1H), 4.06—4.02(m, 2H), 3.75—3.58(m, 12H), 2.72—2.64(m, 6H), 2.16—2.09(m, 2H), 1.65—1.61(m, 3H) | (75 MHz, DMSO⁃d6), δ:167.92, 166.33, 162.27, 159.72, 144.87, 137.48, 133.11, 130.31, 124.33, 121.44, 116.58, 106.15, 72.69, 68.67, 66.59, 60.71, 57.57, 53.59, 53.18, 50.99, 47.21, 44.57, 43.88, 32.98. |
| 19h | (300 MHz, CDCl3), δ: 8.50(s, 1H), 8.39(d, J=5.5 Hz, 1H), 8.32(dd, J=8.0, 1.7 Hz, 1H), 7.61(d, J=7.7 Hz, 1H), 7.18—7.15(m, 1H), 7.08(d, J=5.3 Hz, 1H), 5.46—5.42(m, 1H), 4.67(s, 2H), 4.29(s, 2H), 4.22—4.18(m, 1H), 4.07—4.03(m, 2H), 3.75(t, J=4.5 Hz, 2H), 3.67—3.58(m, 6H), 3.51—3.48(m, 2H), 2.16—2.10(m, 2H), 1.69—1.63(m, 3H) | (75 MHz, DMSO⁃d6), δ: 168.34, 167.88, 163.17, 162.27, 159.64, 144.88, 137.44, 133.14, 130.29, 124.28, 121.44, 106.12, 72.58, 69.35, 66.60, 60.65, 51.08, 47.22, 45.25, 36.44, 32.97 |
| 19i | (300 MHz, DMSO⁃d6), δ: 8.45(s, 1H), 8.03(s, 1H), 7.99(d, J=6.5 Hz, 1H), 7.76(d, J=9.0 Hz, 1H), 7.63(s, 1H), 7.33—7.28(m, 2H), 6.71(d, J=9.3 Hz, 2H), 6.02—6.00(m, 1H), 4.58—4.45(m, 4H), 4.23—4.10(m, 2H), 3.90—3.85(m, 3H), 3.74—3.67(m, 4H), 2.90(s, 6H), 2.59—2.55(m, 2H), 1.88—1.82(m, 2H), 1.58—1.49(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.76, 163.66, 157.73, 146.51, 145.71, 144.43, 132.93, 131.53, 126.45, 124.63, 122.99, 120.45, 120.09, 115.69, 110.54, 109.86, 108.66, 98.84, 66.06, 51.64, 47.23, 45.42, 31.94, 25.79, 19.64, 19.38, 19.11 |
| 19j | (300 MHz, DMSO⁃d6), δ: 8.45(s, 1H), 8.04(s, 1H), 7.99(d, J=8.4 Hz, 1H), 7.75(d, J=7.9 Hz, 1H), 7.63(s, 1H), 6.90—6.86(m, 2H), 6.73—6.68(s, 2H), 4.56(d, J=11.9 Hz, 4H), 3.95—3.84(m, 3H), 3.63(d, J=15.9 Hz, 4H), 3.41—3.37(m, 2H), 3.05—2.96(m, 4H), 2.78(s, 6H), 1.86—1.81(m, 2H), 1.59—1.46(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.74, 167.59, 163.30, 144.42, 143.85, 136.64, 135.56, 135.16, 132.94, 131.48, 126.61, 124.62, 123.01, 120.62, 117.38, 110.20, 66.06, 47.24, 45.56, 31.93, 25.89, 19.65, 19.38, 19.12 |
| 25a | (300 MHz, DMSO⁃d6), δ: 8.40(s, 1H), 8.37(d, J=5.2 Hz, 1H | (75 MHz, DMSO⁃d6), δ: 168.24, 167.44, 161.73, 159.12, 151.81, 148.31, 144.41, 143.97, 132.59, 129.84, 124.17, 123.78, 120.96, 105.63, 77.24, 71.49, 66.09, 63.51, 60.12, 50.51, 48.54, 46.72, 44.89, 32.47 |
| 25b | (300 MHz, DMSO⁃d6), δ: 8.40—8.24(m, 4H), 7.83(s, 1H), 7.70(d, J=8.1 Hz, 1H), 7.26(s, 1H), 7.26(s, 1H), 7.23(d, J=5.2 Hz, 1H), 5.01(s, 1H), 4.51(s, 2H), 4.39(t, J=5.9 Hz, 2H), 4.15(s, 2H), 4.05—3.98(m, 1H), 3.90—3.87(m, 2H), 3.54—3.37(m, 4H), 1.90—1.85(m, 2H), 1.59—1.51(m, 2H), 1.45(s, 6H) | (75 MHz, DMSO⁃d6), δ: 167.56, 167.14, 160.75, 158.35, 155.10, 154.71, 143.63, 136.03, 131.47, 129.23, 123.04, 120.10, 104.90, 99.87, 66.26, 65.29, 49.86, 47.58, 45.82, 44.07, 31.52, 29.54, 21.84 |
| 27a | (300 MHz, CDCl3), δ: 9.03(s, 1H), 8.13(t, J=1.3 Hz, 1H), 7.98—7.94(m, 3H), 7.89(dd, J=9.3, 2.5 Hz, 1H), 7.75(d, J=8.7 Hz, 2H), 7.44(d, J=1.3 Hz, 2H), 6.69(d, J=9.3 Hz, 1H), 6.51(d, J=8.7 Hz, 1H), 5.34(hept, J=6.8 Hz, 1H), 4.81(s, 1H), 3.89(s, 3H), 3.74—3.67(m, 2H), 3.49—3.32(m, 4H), 3.13—2.99(m, 2H), 2.843—2.53(m, 3H), 2.06—1.99(m, 4H), 1.42—1.40(m, 6H) | (75 MHz, CDCl3), δ: 172.20, 170.86, 163.17, 161.73, 149.06, 143.99, 142.20, 139.37, 138.19, 131.61, 127.51, 125.25, 121.85, 120.73, 120.59, 119.75, 113.31, 112.30, 111.49, 110.99, 63.37, 57.65, 56.97, 53.15, 46.86, 43.70, 37.28, 36.16, 22.04, 13.93 |
| 27b | (300 MHz, CDCl3), δ: 9.04(s, 1H), 8.31(s, 1H), 8.06—7.94(m, 6H), 7.55—7.45(m, 3H), 7.34(s, 1H), 6.77(d, J=9.3 Hz, 1H), 5.41(hept, J=7.4 Hz, 1H), 3.97(s, 3H), 3.69—3.62(m, 2H), 3.50—3.47(m, 1H), 3.38—3.32(m, 1H), 2.99 — 2.71(m, 6H), 2.68—2.64(m, 1H), 2.11(s, 3H), 2.05—2.03(m, 1H), 1.51—1.49(m, 6H) | (75 MHz, CDCl3), δ: 171.13, 170.33, 162.54, 161.78, 144.33, 141.88, 140.13, 139.21, 138.28, 131.90, 131.49,129.18, 129.09, 126.46, 121.28, 120.71, 120.46, 113.64, 110.89, 110.81, 63.35, 58.36, 57.68, 53.56, 46.90, 40.02, 36.65, 36.28, 35.33, 22.07, 13.90 |
Table 4 1H NMR and 13C NMR data of compounds 19a—19j, 25a, 25b, 27a and 27b
| Compd. | 1H NMR | 13C NMR |
|---|---|---|
| 19a | (300 MHz, CDCl3), δ: 8.50(s, 1H), 8.38(d, J=5.3 Hz, 1H), 8.30(dd, J=8.1, 1.7 Hz, 1H), 8.11—8.08(m, 3H), 7.59(d, J=8.0 Hz, 1H), 7.49—7.44(m, 2H), 7.08(d, J=5.3 Hz, 1H), 6.20—6.15(m, 1H), 5.26(d, J=8.4 Hz, 1H), 4.69(s, 2H), 4.60(s, 1H), 4.55(s, 1H), 4.31—4.30(m, 2H), 4.23—4.19(m, 1H), 4.07—4.0(m, 5H), 3.90—3.82(m, 2H), 3.66—3.58(m, 2H), 2.69—2.64(m, 2H), 2.15—2.11(m, 2H), 1.63— 1.58(m, 2H) | (75 MHz, DMSO⁃d6), δ: 167.99, 166.75, 166.58, 162.27, 161.27, 159.65, 146.15, 144.91, 140.60, 134.82, 134.38, 133.14, 130.31, 126.21, 125.47, 124.34, 121.72, 121.43, 121.14,106.14, 72.75, 66.59, 60.72, 51.03, 47.21, 44.05, 36.44, 32.98, 27.33 |
| 19b | (300 MHz, DMSO⁃d6), δ: 8.51(s, 1H), 8.44(s, 1H), 8.04—7.96(m, 4H), 7.75(d, J=8.4 Hz, 1H), 7.57—7.54(m, 2H), 6.29(s, 1H), 4.59—4.54(m, 4H), 4.29—4.15(m, 2H), 3.92—3.85(m, 6H), 3.76—3.73(m, 2H), 3.41—3.37(m, 2H), 2.66—2.54(m, 2H), 1.87—1.83(m, 2H), 1.55—1.51(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.74, 167.74, 160.38, 145.52, 144.50, 141.34, 135.50, 134.44, 132.86, 131.57, 128.40, 126.53, 125.14, 124.67, 122.99, 120.76, 115.97, 115.73, 104.31, 99.06, 66.06, 51.68, 47.26, 35.99, 31.92, 26.88, 19.64, 19.38, 19.12 |
| 19c | (400 MHz, DMSO⁃d6), δ: 9.56(s, 1H), 8.52—8.40(m, 4H), 8.48(s, 1H), 7.98(d, J=8.3 Hz, 2H), 7.92(s, 1H), 7.79(d, J=8.0 Hz, 1H), 7.57(t, J=7.2 Hz, 3H), 7.42(d, J=5.2 Hz, 1H), 6.30(s, 1H), 4.61—4.55(m, 4H), 4.30(s, 1H), 4.16(s, 1H), 3.92(s, 3H), 3.83(s, 3H), 3.76—3.70(m, 2H), 2.67—2.54(m, 2H) | (75 MHz, aectic⁃d4), δ: 169.65, 167.47, 160.51, 145.56, 143.16, 141.20, 140.14, 139.16, 136.38, 135.48, 134.41, 132.39, 131.43, 128.45, 126.49, 125.09, 123.97, 123.11, 121.59, 114.73, 107.02, 96.29, 90.58, 51.74, 38.06, 35.93, 35.45, 19.64, 19.38, 19.12 |
| 19d | (400 MHz, DMSO⁃d6), δ: 9.83(s, 1H), 8.58(s, 1H), 8.51(s, 1H), 8.11(s, 1H), 8.06(d, J=8.0 Hz, 1H), 7.98(d, J=8.5 Hz, 2H), 7.83—7.79(m, 2H), 7.56(dd, J=8.2, 6.2 Hz, 2H), 7.52(s, 1H), 6.30(s, 1H), 4.61—4.55(m, 4H), 4.30(s, 1H), 4.16(s, 1H), 3.92(s, 3H), 3.79—3.71(m, 5H), 2.66—2.54(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.76, 167.58, 159.65, 157.54, 149.08, 145.21, 144.61, 141.60, 134.40, 132.93, 131.68, 126.63, 125.18, 124.70, 123.17, 122.56, 120.93, 119.97, 116.95, 101.59, 98.04, 90.16, 86.42, 64.75, 51.73, 37.95, 36.24, 19.65, 19.38, 19.12 |
| 19e | (300 MHz, CDCl3), δ: 8.44(s, 1H), 8.38(d, J=5.3 Hz, 1H), 8.26(dd, J=7.5, 1.7 Hz, 1H), 7.99(s, 1H), 7.86(d, J=8.5 Hz, 2H), 7.52(d, J=8.2 Hz, 1H), 7.04(d, J=5.0 Hz, 1H), 6.62(d, J=8.5 Hz, 2H), 4.48(s, 1H), 4.28(s, 2H), 4.21—4.15(m, 1H), 4.06—4.02(m, 2H), 3.94(s, 3H), 3.65—3.53(m, 4H), 3.36(t, J=6.2 Hz, 2H), 2.15—2.09(m, 2H), 1.66—1.60(m, 2H) | (75 MHz, DMSO⁃d6), δ: 168.70, 168.35, 163.20, 162.11, 162.04, 149.70, 145.42, 144.93, 137.35, 132.83, 130.49, 127.37, 124.37, 121.45, 118.97, 114.94, 112.21, 106.16, 66.58, 51.22, 47.10, 45.43, 42.29, 36.14, 32.83, 27.27 |
| 19f | (300 MHz, CDCl3), δ: 8.42(s, 1H), 8.33(d, J=5.2 Hz, 1H), 8.24(d, J=9.8 Hz, 1H), 7.53(d, J=8.9 Hz, 1H), 7.01(d, J=5.5 Hz, 1H), 5.15(d, J=6.8 Hz, 1H), 4.60(s, 2H), 4.44(s, 2H), 4.19—4.09(m, 1H), 4.01—3.97(m, 2H), 3.64—3.52(m, 8H), 2.57—2.50(m, 6H), 2.08—2.04(m, 2H), 1.84(brs, 1H), 1.63—1.52(m, 2H) | (75 MHz, DMSO⁃d6), δ: 167.92, 166.32, 163.17, 162.27, 159.61, 144.87, 137.47, 133.10, 130.30, 124.31, 121.43, 106.14, 66.59, 60.54, 58.96, 53.63, 53.23, 50.99, 47.21, 44.59, 43.87, 41.93, 32.98(Affected by steric hindrance, the four carbon atoms in piperazine show different displacements) |
| 19g | (300 MHz, CDCl3), δ: 8.48(s, 1H, ArH), 8.39(d, J=5.2 Hz, 1H), 8.30(dd, J=8.0, 1.7 Hz, 1H), 7.58(d, J=8.2 Hz, 1H), 7.07(d, J=6.0 Hz, 1H), 5.18(d, J=7.8 Hz, 1H), 4.65(s, 2H), 4.49(s, 2H), 4.24—4.17(m, 1H), 4.06—4.02(m, 2H), 3.75—3.58(m, 12H), 2.72—2.64(m, 6H), 2.16—2.09(m, 2H), 1.65—1.61(m, 3H) | (75 MHz, DMSO⁃d6), δ:167.92, 166.33, 162.27, 159.72, 144.87, 137.48, 133.11, 130.31, 124.33, 121.44, 116.58, 106.15, 72.69, 68.67, 66.59, 60.71, 57.57, 53.59, 53.18, 50.99, 47.21, 44.57, 43.88, 32.98. |
| 19h | (300 MHz, CDCl3), δ: 8.50(s, 1H), 8.39(d, J=5.5 Hz, 1H), 8.32(dd, J=8.0, 1.7 Hz, 1H), 7.61(d, J=7.7 Hz, 1H), 7.18—7.15(m, 1H), 7.08(d, J=5.3 Hz, 1H), 5.46—5.42(m, 1H), 4.67(s, 2H), 4.29(s, 2H), 4.22—4.18(m, 1H), 4.07—4.03(m, 2H), 3.75(t, J=4.5 Hz, 2H), 3.67—3.58(m, 6H), 3.51—3.48(m, 2H), 2.16—2.10(m, 2H), 1.69—1.63(m, 3H) | (75 MHz, DMSO⁃d6), δ: 168.34, 167.88, 163.17, 162.27, 159.64, 144.88, 137.44, 133.14, 130.29, 124.28, 121.44, 106.12, 72.58, 69.35, 66.60, 60.65, 51.08, 47.22, 45.25, 36.44, 32.97 |
| 19i | (300 MHz, DMSO⁃d6), δ: 8.45(s, 1H), 8.03(s, 1H), 7.99(d, J=6.5 Hz, 1H), 7.76(d, J=9.0 Hz, 1H), 7.63(s, 1H), 7.33—7.28(m, 2H), 6.71(d, J=9.3 Hz, 2H), 6.02—6.00(m, 1H), 4.58—4.45(m, 4H), 4.23—4.10(m, 2H), 3.90—3.85(m, 3H), 3.74—3.67(m, 4H), 2.90(s, 6H), 2.59—2.55(m, 2H), 1.88—1.82(m, 2H), 1.58—1.49(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.76, 163.66, 157.73, 146.51, 145.71, 144.43, 132.93, 131.53, 126.45, 124.63, 122.99, 120.45, 120.09, 115.69, 110.54, 109.86, 108.66, 98.84, 66.06, 51.64, 47.23, 45.42, 31.94, 25.79, 19.64, 19.38, 19.11 |
| 19j | (300 MHz, DMSO⁃d6), δ: 8.45(s, 1H), 8.04(s, 1H), 7.99(d, J=8.4 Hz, 1H), 7.75(d, J=7.9 Hz, 1H), 7.63(s, 1H), 6.90—6.86(m, 2H), 6.73—6.68(s, 2H), 4.56(d, J=11.9 Hz, 4H), 3.95—3.84(m, 3H), 3.63(d, J=15.9 Hz, 4H), 3.41—3.37(m, 2H), 3.05—2.96(m, 4H), 2.78(s, 6H), 1.86—1.81(m, 2H), 1.59—1.46(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.74, 167.59, 163.30, 144.42, 143.85, 136.64, 135.56, 135.16, 132.94, 131.48, 126.61, 124.62, 123.01, 120.62, 117.38, 110.20, 66.06, 47.24, 45.56, 31.93, 25.89, 19.65, 19.38, 19.12 |
| 25a | (300 MHz, DMSO⁃d6), δ: 8.40(s, 1H), 8.37(d, J=5.2 Hz, 1H | (75 MHz, DMSO⁃d6), δ: 168.24, 167.44, 161.73, 159.12, 151.81, 148.31, 144.41, 143.97, 132.59, 129.84, 124.17, 123.78, 120.96, 105.63, 77.24, 71.49, 66.09, 63.51, 60.12, 50.51, 48.54, 46.72, 44.89, 32.47 |
| 25b | (300 MHz, DMSO⁃d6), δ: 8.40—8.24(m, 4H), 7.83(s, 1H), 7.70(d, J=8.1 Hz, 1H), 7.26(s, 1H), 7.26(s, 1H), 7.23(d, J=5.2 Hz, 1H), 5.01(s, 1H), 4.51(s, 2H), 4.39(t, J=5.9 Hz, 2H), 4.15(s, 2H), 4.05—3.98(m, 1H), 3.90—3.87(m, 2H), 3.54—3.37(m, 4H), 1.90—1.85(m, 2H), 1.59—1.51(m, 2H), 1.45(s, 6H) | (75 MHz, DMSO⁃d6), δ: 167.56, 167.14, 160.75, 158.35, 155.10, 154.71, 143.63, 136.03, 131.47, 129.23, 123.04, 120.10, 104.90, 99.87, 66.26, 65.29, 49.86, 47.58, 45.82, 44.07, 31.52, 29.54, 21.84 |
| 27a | (300 MHz, CDCl3), δ: 9.03(s, 1H), 8.13(t, J=1.3 Hz, 1H), 7.98—7.94(m, 3H), 7.89(dd, J=9.3, 2.5 Hz, 1H), 7.75(d, J=8.7 Hz, 2H), 7.44(d, J=1.3 Hz, 2H), 6.69(d, J=9.3 Hz, 1H), 6.51(d, J=8.7 Hz, 1H), 5.34(hept, J=6.8 Hz, 1H), 4.81(s, 1H), 3.89(s, 3H), 3.74—3.67(m, 2H), 3.49—3.32(m, 4H), 3.13—2.99(m, 2H), 2.843—2.53(m, 3H), 2.06—1.99(m, 4H), 1.42—1.40(m, 6H) | (75 MHz, CDCl3), δ: 172.20, 170.86, 163.17, 161.73, 149.06, 143.99, 142.20, 139.37, 138.19, 131.61, 127.51, 125.25, 121.85, 120.73, 120.59, 119.75, 113.31, 112.30, 111.49, 110.99, 63.37, 57.65, 56.97, 53.15, 46.86, 43.70, 37.28, 36.16, 22.04, 13.93 |
| 27b | (300 MHz, CDCl3), δ: 9.04(s, 1H), 8.31(s, 1H), 8.06—7.94(m, 6H), 7.55—7.45(m, 3H), 7.34(s, 1H), 6.77(d, J=9.3 Hz, 1H), 5.41(hept, J=7.4 Hz, 1H), 3.97(s, 3H), 3.69—3.62(m, 2H), 3.50—3.47(m, 1H), 3.38—3.32(m, 1H), 2.99 — 2.71(m, 6H), 2.68—2.64(m, 1H), 2.11(s, 3H), 2.05—2.03(m, 1H), 1.51—1.49(m, 6H) | (75 MHz, CDCl3), δ: 171.13, 170.33, 162.54, 161.78, 144.33, 141.88, 140.13, 139.21, 138.28, 131.90, 131.49,129.18, 129.09, 126.46, 121.28, 120.71, 120.46, 113.64, 110.89, 110.81, 63.35, 58.36, 57.68, 53.56, 46.90, 40.02, 36.65, 36.28, 35.33, 22.07, 13.90 |
| Compd. | R1a | R2 | R3a | ERK2 IC50/(nmol∙L-1) |
|---|---|---|---|---|
| 19a | 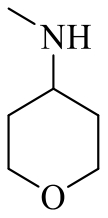 | H | 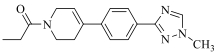 | 16 |
| 19b | Cl | 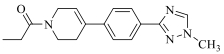 | 15 | |
| 19c | 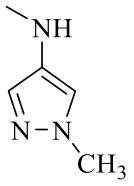 | H | 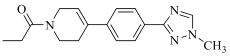 | 97 |
| 19d | Cl | 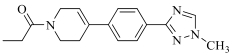 | 121 | |
| 19e | 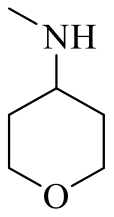 | H | 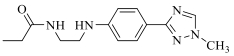 | 20 |
| 19f | 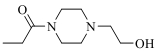 | 9085 | ||
| 19g | 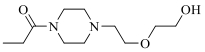 | >10000 | ||
| 19h | 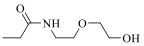 | 3475 | ||
| 19i | 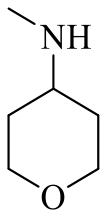 | Cl | 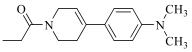 | 96 |
| 19j | 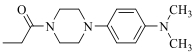 | 150 | ||
| 25a | 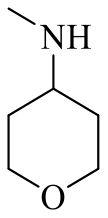 | H | 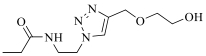 | 2152 |
| 25b |  | 2254 | ||
| MK⁃8353 b | — | — | — | 1.7 |
Table 5 Inhibitory activities of compounds 19a—19j, 25a and 25b on EKR2
| Compd. | R1a | R2 | R3a | ERK2 IC50/(nmol∙L-1) |
|---|---|---|---|---|
| 19a |  | H |  | 16 |
| 19b | Cl |  | 15 | |
| 19c |  | H |  | 97 |
| 19d | Cl |  | 121 | |
| 19e |  | H |  | 20 |
| 19f |  | 9085 | ||
| 19g |  | >10000 | ||
| 19h |  | 3475 | ||
| 19i |  | Cl |  | 96 |
| 19j |  | 150 | ||
| 25a |  | H |  | 2152 |
| 25b |  | 2254 | ||
| MK⁃8353 b | — | — | — | 1.7 |
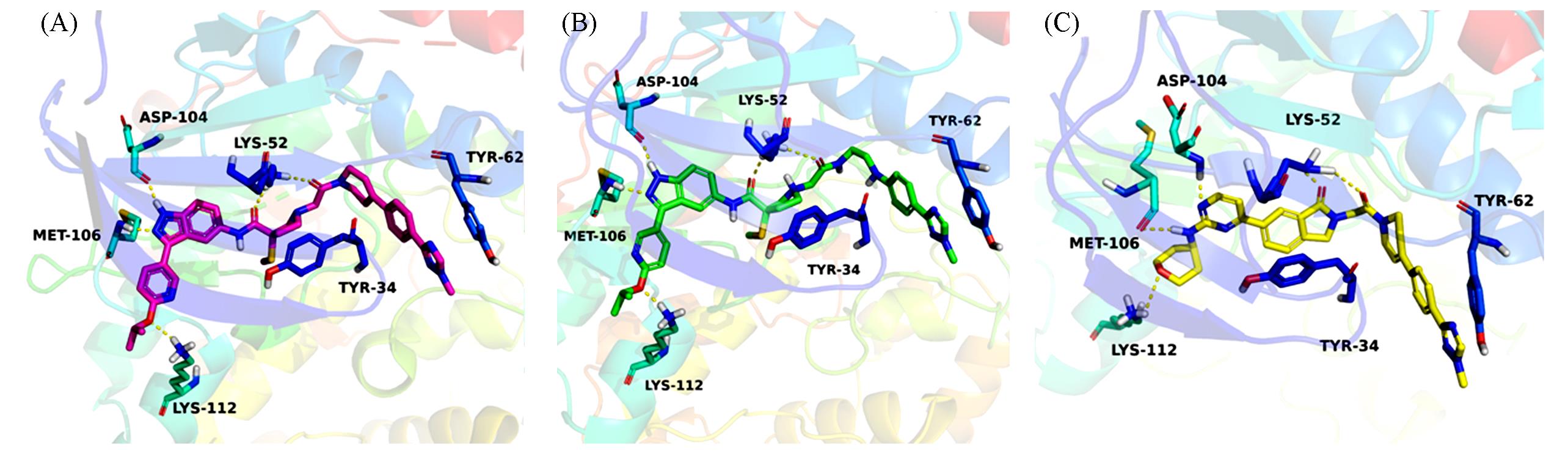
Fig.4 X⁃ray crystal structure of MK⁃8353(A, in red) and proposed binding modes for compounds 27a(B, in green, IC50=19 nmol/L) and 19a(C, in yellow, IC50=16 nmol/L) in the active site of ERK2(PDB: 6DCG)
| Compd. | IC50 a /(μmol∙L-1) | |||
|---|---|---|---|---|
| A375 | A2058 | COLO⁃205 | HT⁃29 | |
| 19a | 1.13±0.05 | 7.82±0.06 | 9.30±0.09 | >50 |
| 19b | 2.89±0.04 | 2.69±0.04 | 2.29±0.05 | 5.62±0.06 |
| 19e | 3.29±0.01 | 7.22±0.03 | 2.82±0.04 | >50 |
| 30a | >50 | 9.72±0.05 | 21.96±0.03 | 26.14±0.01 |
| 30b | 3.04±0.03 | 1.73±0.04 | 1.08±0.05 | 3.16±0.04 |
| MK-8353 b | 0.04±0.04 | 0.04±0.03 | 0.01±0.05 | 0.57±0.06 |
Table 6 Anti-proliferation activities of five selected compounds
| Compd. | IC50 a /(μmol∙L-1) | |||
|---|---|---|---|---|
| A375 | A2058 | COLO⁃205 | HT⁃29 | |
| 19a | 1.13±0.05 | 7.82±0.06 | 9.30±0.09 | >50 |
| 19b | 2.89±0.04 | 2.69±0.04 | 2.29±0.05 | 5.62±0.06 |
| 19e | 3.29±0.01 | 7.22±0.03 | 2.82±0.04 | >50 |
| 30a | >50 | 9.72±0.05 | 21.96±0.03 | 26.14±0.01 |
| 30b | 3.04±0.03 | 1.73±0.04 | 1.08±0.05 | 3.16±0.04 |
| MK-8353 b | 0.04±0.04 | 0.04±0.03 | 0.01±0.05 | 0.57±0.06 |
| [1] | Zhang W., Liu H. T., Cell Res., 2002, 12(1), 9—18 |
| [2] | Raman M., Chen W., Cobb M. H., Oncogene, 2007, 26(22), 3100—3112 |
| [3] | Lavoie H., Gagnon J., Therrien M., Nat. Rev. Mol. Cell Biol., 2020, 21(10), 607—632 |
| [4] | Guo Y. J., Pan W. W., Liu S. B., Shen Z. F., Xu Y., Hu L. L., Exp. Ther. Med., 2020, 19(3), 1997—2007 |
| [5] | Degirmenci U., Wang M., Hu J. C., Cells, 2020, 9(1), 198 |
| [6] | Maik⁃Rachline G., Hacohen⁃Lev⁃Ran A., Seger R., Int. J. Mol. Sci., 2019, 20(5),1194 |
| [7] | Liang T. T., Wang W. J., Hao S. Y., He G. C., Xu Y. G., J. China Pharm. Univ., 2020, 51(3), 260—269 |
| 梁停停, 王文杰, 郝思远, 何光超, 徐云根. 中国药科大学学报, 2020, 51(3), 260—269 | |
| [8] | Song Y. L., Bi Z. F., Liu Y., Qin F. R., Wei Y. Q., Wei X. W., Genes Dis., 2023, 10(1), 76—88 |
| [9] | Smorodinsky⁃Atias K., Soudah N., Engelberg D., Cells, 2020, 9(1), 129 |
| [10] | Pan X. L., Pei J. P., Wang A. X., Shuai W., Feng L., Bu F. Q., Zhu Y. M., Zhang L., Wang G., Ouyang L., Acta Pharm. Sin. B, 2022, 12(5), 2171—2192 |
| [11] | Timofeev O., Giron P., Lawo S., Pichler M., Noeparast M., NPJ Precis. Oncol., 2024, 8(1), 70 |
| [12] | Xiao H., Wang A. X., Shuai W., Qian Y. P., Wu C. Y., Wang X., Yang P. P., Sun Q., Wang G., Ouyang L., Sun Q., Signal Transduct. Target. Ther., 2025, 10(1), 70 |
| [13] | McCubrey J. A., Steelman L. S., Chappell W. H., Abrams S. L., Franklin R. A., Montalto G., Cervello M., Libra M., Candido S., Malaponte G., Mazzarino M. C., Fagone P., Nicoletti F., Bäsecke J., Mijatovic S., Maksimovic⁃Ivanic D., Milella M., Tafuri A., Chiarini F., Evangelisti C., Cocco L., Martelli A. M., Oncotarget, 2012, 3(10), 1068—1111 |
| [20] | Heightman T. D., Berdini V., Braithwaite H., Buck I. M., Cassidy M., Castro J., Courtin A., Day J. E. H., East C., Fazal L., Graham B., Griffiths⁃Jones C. M., Lyons J. F., Martins V., Muench S., Munck J. M., Norton D., O'Reilly M., Palmer N., Pathuri P., Reader M., Rees D. C., Rich S. J., Richardson C., Saini H., Thompson N. T., Wallis N. G., Walton H., Wilsher N. E., Woolford A. J. A., Cooke M., Cousin D., Onions S., Shannon J., Watts J., Murray C. W., J. Med. Chem., 2018, 61(11), 4978—4992 |
| [21] | Kampmann S. S., Skelton B. W., Yeoh G. C., Abraham L. J., Lengkeek N. A., Stubbs K. A., Heath C. H., Stewart S.G., Tetrahedron, 2015, 71(42), 8140—8149 |
| [22] | Zhang L., Chen Y. Q., Li W. H., Chem. Res. Chinese Universities, 2025, 41(1), 146—154 |
| [14] | Ji D. Z., Zhang L. Z., Zhu Q. H., Bai Y., Wu Y. Y., Xu Y. G., Eur. J. Med. Chem., 2019, 164, 334—341 |
| [15] | Cevik U. A., Saglik B. N., Osmaniye D., Levent S., Cavusoglu B. K., Karaduman A. B., Atlid O., Eklioglu O. A., Kaplancikli Z. A., J. Enzyme Inhib. Med. Chem., 2020, 35(1), 1657—1673 |
| [16] | Giampietro N. C., Demeter D. A., Diagne A. B., Esguerra K. V. N., Heemstra R. J., Schuldt R. A., Barton T. J., Horty L. G., Sparks T. C., Watson G. B., Molecules Having Certain Pesticidal Utilities, and Intermediates, Compositions, and Processes Rrelated Thereto, WO 2021011722 A1, 2021⁃01⁃21 |
| [17] | Boyington A. J., Seath C. P., Zearfoss A. M., Xu Z. H., Jui N. T., J. Am. Chem. Soc., 2019, 141(9), 4147—4153 |
| [18] | Jin B., Tao Y., Yang H. L., Chem. Res. Chinese Universities, 2018, 34(6), 912—917 |
| [19] | Tan S., Li F., Park S., Kim S., Org. Chem. Front., 2019, 6(23), 3854—3858 |
| [1] | 陈阳密, 佘慧娴, 汪晴, 杨家强. 蛇床子素肟醚衍生物的合成与抗菌活性[J]. 高等学校化学学报, 2025, 46(9): 20250147. |
| [2] | 李栋, 濮雪, 邓力, 吴琪琳, 巨安奇. ZIF-67衍生空心花状Ni0.3Co2.7S/MoS2复合催化剂的制备及电解水制氢应用[J]. 高等学校化学学报, 2025, 46(9): 20250144. |
| [3] | 佘慧娴, 陈阳密, 杨家强. 含硫肽结构的噻肟酰胺衍生物的设计、合成与抗菌活性[J]. 高等学校化学学报, 2025, 46(5): 20240548. |
| [4] | 吴霜, 林思雨, 李楠, 林艺涵, 丁实, 陈烨, 刘举, 沈继伟. 含缩氨基脲结构的4-苯氧基喹啉类c-Met激酶抑制剂的合成与抗肿瘤活性[J]. 高等学校化学学报, 2025, 46(4): 20240439. |
| [5] | 刘冬梅, 徐元强, 夏超, 郑晶晶, 苏贤斌. 氟离子敏感疏水标签辅助连续流动绿色合成胸腺五肽[J]. 高等学校化学学报, 2025, 46(4): 20240544. |
| [6] | 刘奕萱, 胡慧敏, 范晓强, 于学华, 孔莲, 肖霞, 解则安, 赵震. Pt/Mn-silicalite-1催化剂的制备及丙烷脱氢性能[J]. 高等学校化学学报, 2025, 46(3): 20240460. |
| [7] | 王适豪, 石万瑞, 刘轶, 张皓. cGAS-STING通路在肿瘤免疫治疗中的作用机制与研究进展[J]. 高等学校化学学报, 2025, 46(1): 20240241. |
| [8] | 王璐. 离子液体辅助水热合成1T-MoS2及其锌离子储存性能[J]. 高等学校化学学报, 2024, 45(9): 20240145. |
| [9] | 许玲, 尹盼盼, 鲁显福, 李宜明. 连接-脱硫策略在蛋白质化学合成中的发展与应用[J]. 高等学校化学学报, 2024, 45(8): 20240196. |
| [10] | 刘豪, 刘冬梅, 孙浩田, 夏超, 苏贤斌. 利用脂溶性硅基载体连续流动液相合成维洛斯肽[J]. 高等学校化学学报, 2024, 45(7): 20240024. |
| [11] | 孔雪, 张海平, 夏文生, 张庆红, 万惠霖. 石墨烯负载单原子Mo上合成气催化转化机理: 碳组分和Mo-C作用的影响[J]. 高等学校化学学报, 2024, 45(7): 20240038. |
| [12] | 龙磊, 韦伟, 罗运军, 李霄羽. 高效叠氮化方法研究进展[J]. 高等学校化学学报, 2024, 45(5): 20230511. |
| [13] | 董远, 马养民, 马思悦, 孙任伟. 基于薯蓣皂苷元催化合成N-甲基吲哚孕烯醇酮化合物及其抗肿瘤活性[J]. 高等学校化学学报, 2024, 45(4): 20230449. |
| [14] | 赵晓光, 王云龙, 尹海波, 曲亚坤, 苏海伟, 房韡. 不同氮源用于电催化合成氨的研究进展[J]. 高等学校化学学报, 2024, 45(3): 20230527. |
| [15] | 王刚, 梁爽, 单忠刚, 英君伍, 吕亮, 李斌, 杨辉斌. 吡嗪酰胺类似物的设计、 合成及杀菌活性[J]. 高等学校化学学报, 2024, 45(10): 20240369. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||