

高等学校化学学报 ›› 2024, Vol. 45 ›› Issue (6): 20240054.doi: 10.7503/cjcu20240054
收稿日期:2024-01-29
出版日期:2024-06-10
发布日期:2024-03-12
通讯作者:
鲍雨,崔树勋
E-mail:baoyu@swjtu.edu.cn;cuishuxun@swjtu.edu.cn
基金资助:
SHI Wuyi, BAO Yu( ), CUI Shuxun(
), CUI Shuxun( )
)
Received:2024-01-29
Online:2024-06-10
Published:2024-03-12
Contact:
BAO Yu, CUI Shuxun
E-mail:baoyu@swjtu.edu.cn;cuishuxun@swjtu.edu.cn
Supported by:摘要:
硫属单质(硫、 硒、 碲)是自然界中一类十分重要的物质, 在光电材料、 电池及半导体等领域有着广泛的应用. 硫属单质均具有晶态和无定形态两种结构形态, 其中晶态硫属单质的分子结构已经得到了深入的研究, 但无定形硫属单质的分子结构研究还处于初级阶段. 为了更好地发掘无定形硫属单质的应用潜力, 对其结构与性质进行深入研究十分必要. 本文概述了无定形硫属单质分子结构研究方面的最新进展, 并展望了未来可能的研究方向. 这些工作将有助于更全面地了解无定形硫属单质的性质, 并推动其在不同领域中的应用.
中图分类号:
TrendMD:
时五一, 鲍雨, 崔树勋. 无定形硫属单质分子结构的研究进展. 高等学校化学学报, 2024, 45(6): 20240054.
SHI Wuyi, BAO Yu, CUI Shuxun. Research Progress on the Molecular Structure of Amorphous Chalcogen Elements. Chem. J. Chinese Universities, 2024, 45(6): 20240054.
Common structural characterization technique | Available structural information | Limitation |
|---|---|---|
| XRD | Crystal structure information | Unable to resolve the amorphous structure; Difficulty in sample preparation |
| Neutron diffraction | Neutron scattering length; nuclear density imaging | Unable to resolve the amorphous structure; low resolution |
| Inelastic scattering | Dynamical length scale and other information; excited state information | Complex data analysis; model fitting requirements; difficulty in sample preparation; low resolution |
| EM | Morphology information; composition analysis; local structural analysis | Sample damage; limited sample thickness; difficulty in sample preparation |
| STM | Surface topological information; atomic arrangement structure; local charge density | High sample conductivity requirement; strict environmental requirements; sample damage |
| AFM | Surface topological information; atomic arrangement structure; mechanical property | Slow scanning speed; size effect of probe |
| Infrared spectrum | Chemical composition; molecular structure; impurity detection | Transparent sample; complex spectrum; low reso⁃lution; sensitive to sample surface and thickness |
| EXAFS | Chemical environment analysis; bond length; coordination number and configuration; oxidation states and coordination bond energies | Sensitive to sample surface; low resolution; complex data analysis |
| NMR | Chemical environment analysis; molecular configuration and conformation; molecular dynamics; molecular scale and shape | Low sensitivity; high sample preparation requirements; signal overlap; complex data analysis and simulation |
| XPS | Element composition; chemical state; electronic structure | Unable to obtain deep⁃structure information; low resolution |
| MS | Molecular mass; structural identification; isotopic distribution | Low sensitivity and resolution; high purity of samples |
| Raman spectrum | Molecular vibration information; isotope effect | Weak signal; fluorescent interference; unable to obtain deep⁃structure information |
Table 1 Advantages and disadvantages of common characterization methods for the determination of amorphous substances
Common structural characterization technique | Available structural information | Limitation |
|---|---|---|
| XRD | Crystal structure information | Unable to resolve the amorphous structure; Difficulty in sample preparation |
| Neutron diffraction | Neutron scattering length; nuclear density imaging | Unable to resolve the amorphous structure; low resolution |
| Inelastic scattering | Dynamical length scale and other information; excited state information | Complex data analysis; model fitting requirements; difficulty in sample preparation; low resolution |
| EM | Morphology information; composition analysis; local structural analysis | Sample damage; limited sample thickness; difficulty in sample preparation |
| STM | Surface topological information; atomic arrangement structure; local charge density | High sample conductivity requirement; strict environmental requirements; sample damage |
| AFM | Surface topological information; atomic arrangement structure; mechanical property | Slow scanning speed; size effect of probe |
| Infrared spectrum | Chemical composition; molecular structure; impurity detection | Transparent sample; complex spectrum; low reso⁃lution; sensitive to sample surface and thickness |
| EXAFS | Chemical environment analysis; bond length; coordination number and configuration; oxidation states and coordination bond energies | Sensitive to sample surface; low resolution; complex data analysis |
| NMR | Chemical environment analysis; molecular configuration and conformation; molecular dynamics; molecular scale and shape | Low sensitivity; high sample preparation requirements; signal overlap; complex data analysis and simulation |
| XPS | Element composition; chemical state; electronic structure | Unable to obtain deep⁃structure information; low resolution |
| MS | Molecular mass; structural identification; isotopic distribution | Low sensitivity and resolution; high purity of samples |
| Raman spectrum | Molecular vibration information; isotope effect | Weak signal; fluorescent interference; unable to obtain deep⁃structure information |
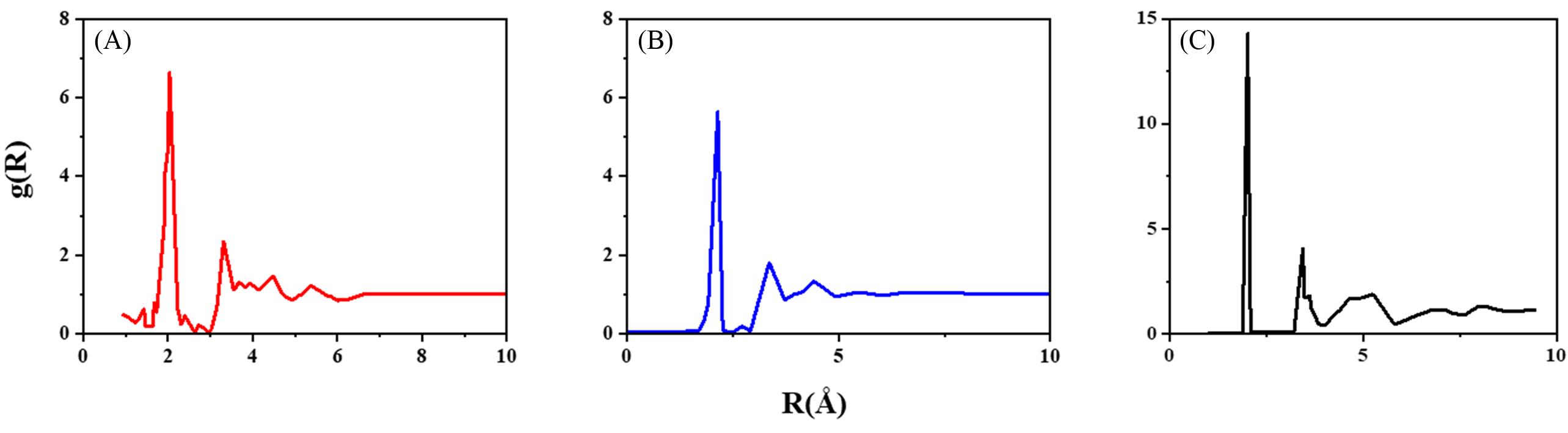
Fig.1 Molecular dynamics simulation diagram of the structure obtained by rapid quenching of amorphous sulfur(A) and liquid sulfur(B)[57] and the Model of S8 propounded by Stillinger et al. at 362 K(C)[58](A, B) Copyright 1990, European Physical Society; (C) Copyright 1986, American Institute of Physics.
| α⁃S8 | a⁃S | ||
|---|---|---|---|
| Raman shift/cm-1 | Raman shift/cm-1 | Raman shift/cm-1 | Raman shift/cm-1 |
| 472.5[ | 475[ | 456.3[ | 460[ |
| 436.7[ | 440[ | 421.7[ | 425[ |
| 246.6[ | 248[ | 274.2[ | 275[ |
| 218.0[ | 220[ | ca. 260, shoulder[ | 260[ |
| 153.6[ | 150—160[ | ||
Table 2 Main Raman shift vibrations and stretching frequencies of sulfur allotropes
| α⁃S8 | a⁃S | ||
|---|---|---|---|
| Raman shift/cm-1 | Raman shift/cm-1 | Raman shift/cm-1 | Raman shift/cm-1 |
| 472.5[ | 475[ | 456.3[ | 460[ |
| 436.7[ | 440[ | 421.7[ | 425[ |
| 246.6[ | 248[ | 274.2[ | 275[ |
| 218.0[ | 220[ | ca. 260, shoulder[ | 260[ |
| 153.6[ | 150—160[ | ||
| Species | Bond length/nm | Bond angle/(°) | Characteristic Raman spectral peak/cm-1 |
|---|---|---|---|
| a⁃S | 0.200~0.210[ | 90~120[ | ca. 460[ |
| a⁃Se | 0.232~0.250[ | >100[ | Se n : ca. 250[ Se8: ca. 260[ |
| a⁃Te | 0.277±0.002[ | 90~120[ | ca. 157[ |
Table 3 Summary of characteristic Raman spectral peaks of amorphous chalcogen elements
| Species | Bond length/nm | Bond angle/(°) | Characteristic Raman spectral peak/cm-1 |
|---|---|---|---|
| a⁃S | 0.200~0.210[ | 90~120[ | ca. 460[ |
| a⁃Se | 0.232~0.250[ | >100[ | Se n : ca. 250[ Se8: ca. 260[ |
| a⁃Te | 0.277±0.002[ | 90~120[ | ca. 157[ |
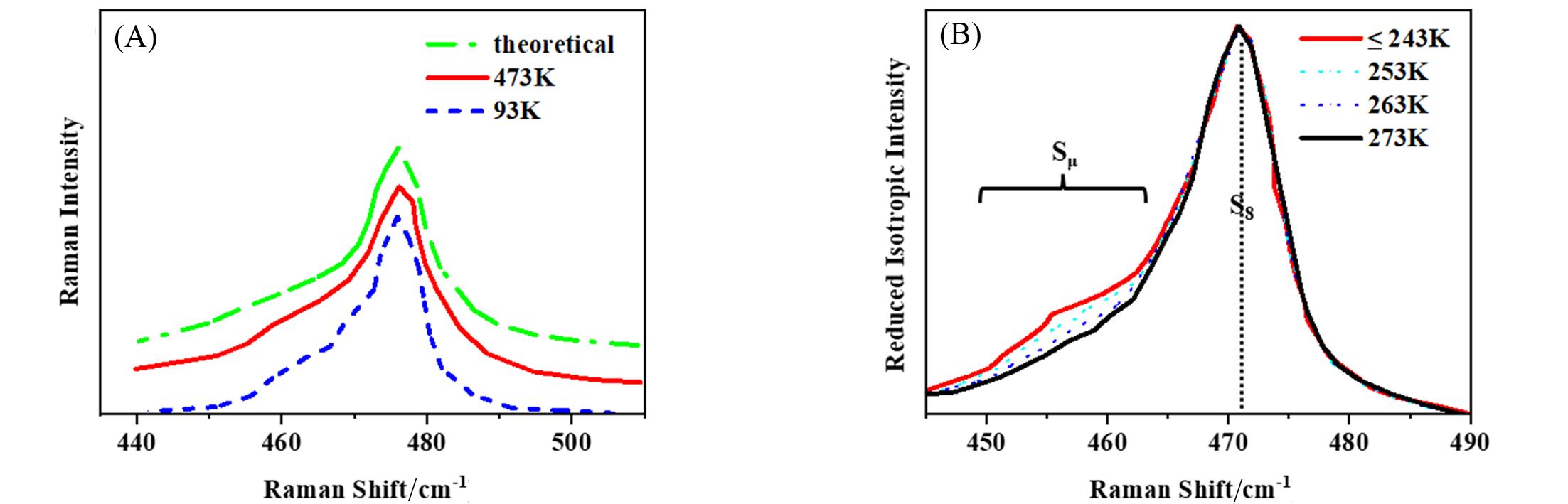
Fig.3 Reduced isotropic intensity of the S—S bond stretching spectral region for the parent liquid at 473 K(solid red curve) and the quenched product at 93 K(dashed blue curve)(A) and Raman spectra of the spectral range representative of the S—S stretching modes obtained at various temperatures(B)[54](A) The upper spectrum(dashed-dotted green curve) is the theoretically calculated spectrum representing the vibrational modes of a mixture of short and long chains and S8 units. (B) Intensities are normalized concerning the maximum of the S8 band. For visual inspection, the spectra have been shifted(along the x-axis) so that the S8 peak maxima coincide.Copyright 2013, American Institute of Physics.
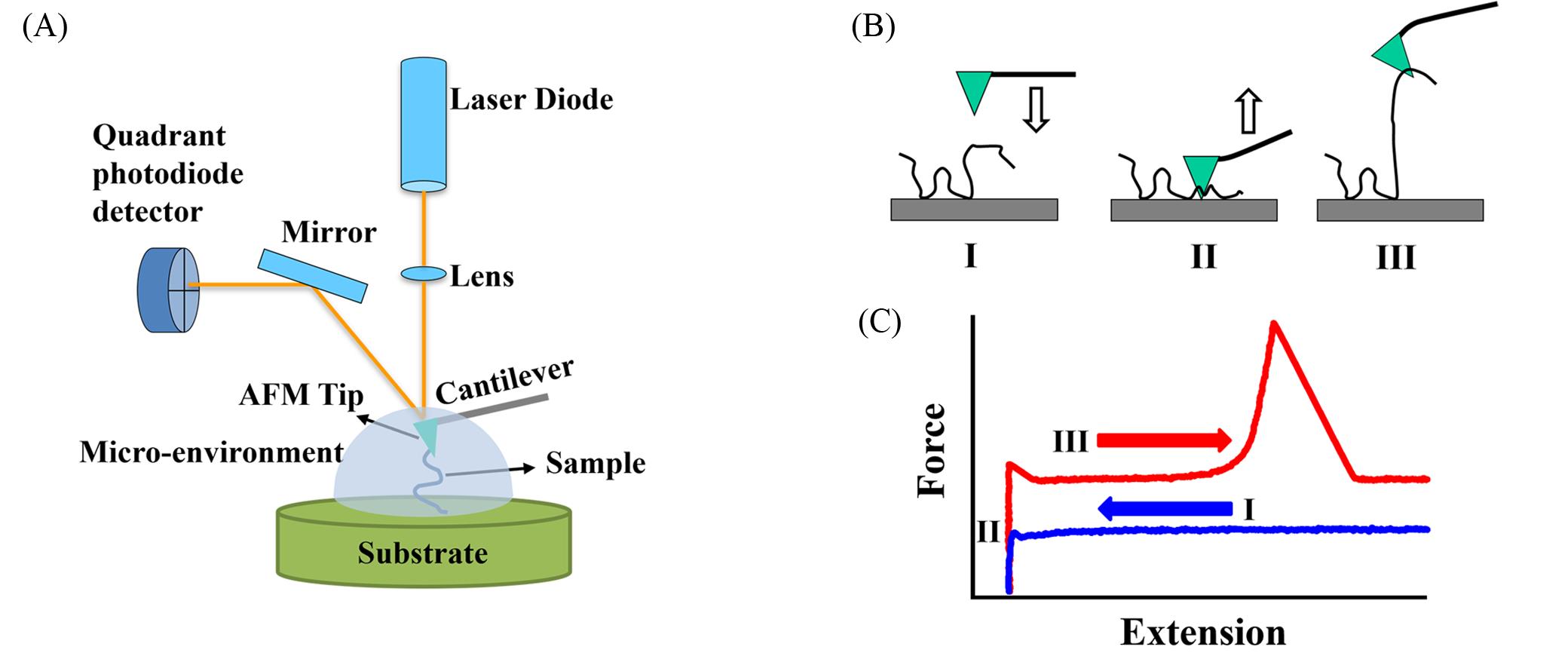
Fig.4 Structural schematic diagram of single⁃molecule atomic force microscopy(A), the working principle of single⁃molecule atomic force microscopy experiments on a sample prepared by physisorption(B) and the approaching(blue) and the retracting(red) F⁃E curves of one experimental cycle(C)[64]Copyright 2020, the Royal Society of Chemistry.
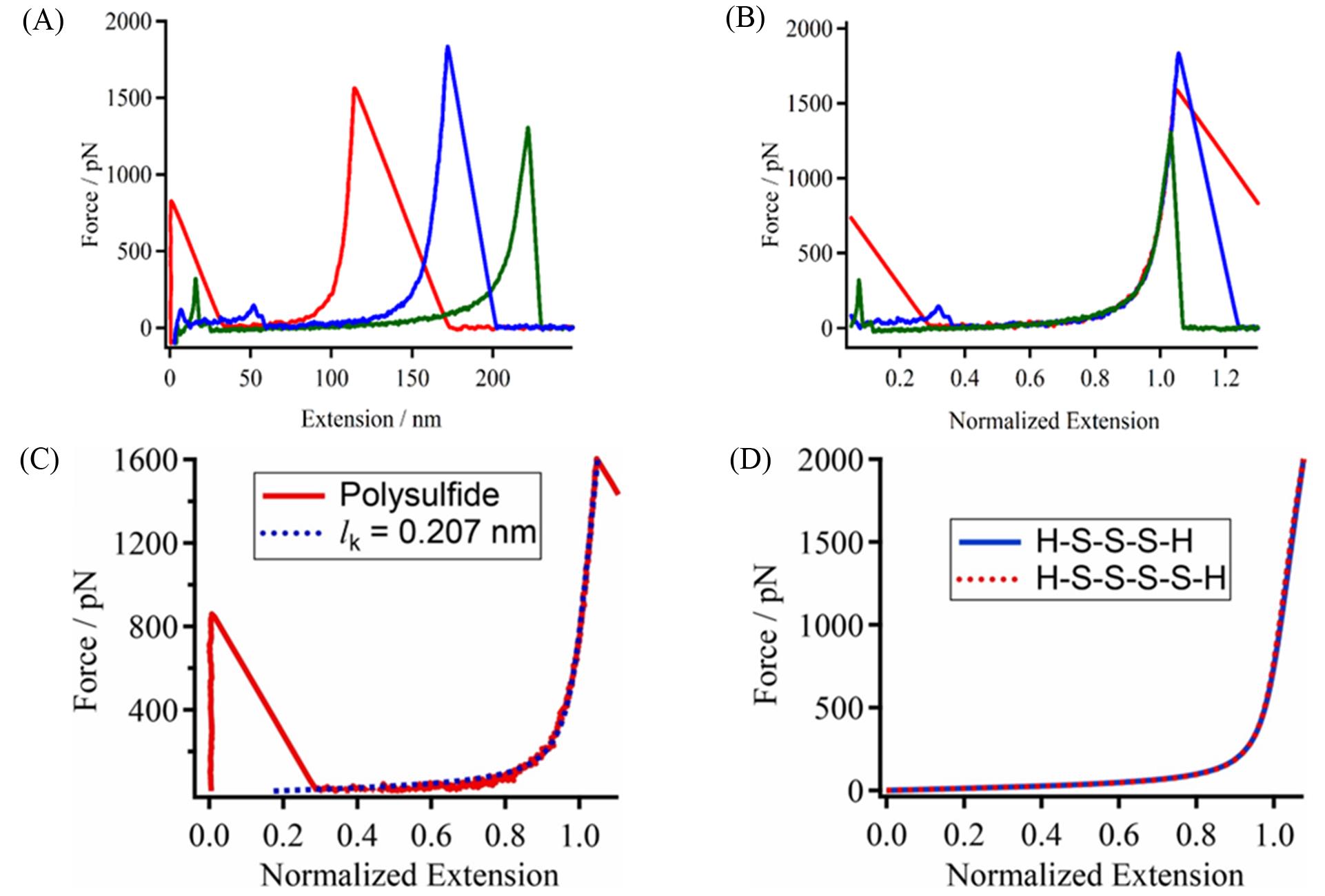
Fig.5 Typical F⁃E curves of polysulfide in nonane obtained by the single⁃molecule atomic force microscopy measurements(A), the normalized F⁃E curves of those shown in (A)(B), normalized experimental curve of polysulfide(red solid line) and the theoretical elastic curve of polysulfide with lk =0.207 nm(blue dotted lines)(C) and comparison of the theoretical curves of polysulfide consisting of three and four sulfur atoms(H⁃S⁃S⁃S⁃H and H⁃S⁃S⁃S⁃S⁃H)(D)[44]Copyright 2022, Elsevier.
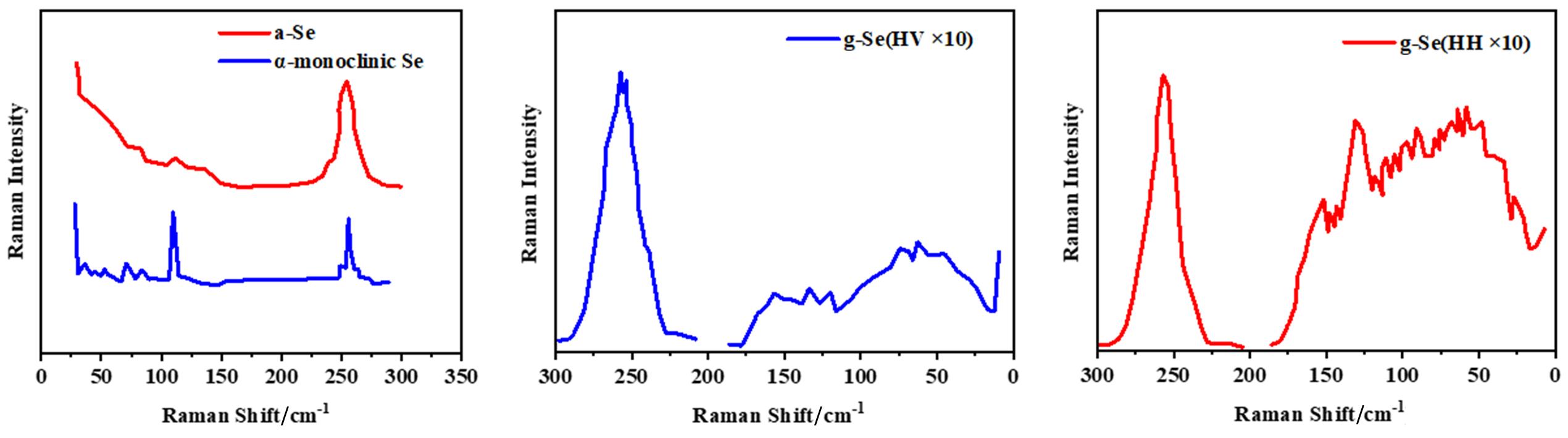
Fig.8 Raman spectra of amorphous and α⁃monoclinic selenium(A)[38], polarized(HV)(B) and depolarized(HH)(C) Raman spectra of bulk glassy Se recorded at 9.8 K using the 799.3 nm laser as the excitation source[76](A) Copyright 1967, Elsevier; (B, C) Copyright 1976, Elsevier.
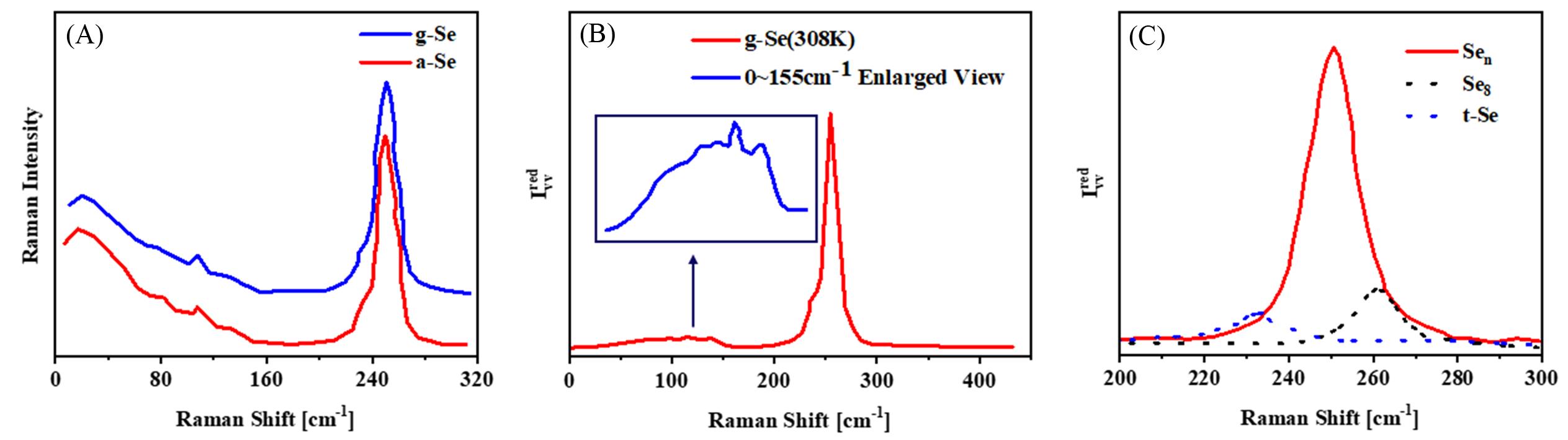
Fig.9 Raman spectra of bulk glass state and evaporated amorphous selenium(A)[79], reduced polarized(IVVred) spectrum of g⁃Se at 308 K(B) and Raman spectra of selenium with different structures(C)[43]Inset of (B): magnified image of the low-frequency part of the spectrum where the Raman peaks associated with the medium-range structural order are seen. (A) Copyright 1981, Elsevier; (B, C) Copyright 2004, American Institute of Physics.
| Species | rintra/nm | Nintra | DWintra/nm |
|---|---|---|---|
| t⁃Te | 0.283±0.002 | 2.00 | 0.0054±0.0002 |
| a⁃Te | 0.277±0.002 | 1.97±0.20 | 0.0049±0.0002 |
Table 4 Structural parameters of t-Te and a-Te
| Species | rintra/nm | Nintra | DWintra/nm |
|---|---|---|---|
| t⁃Te | 0.283±0.002 | 2.00 | 0.0054±0.0002 |
| a⁃Te | 0.277±0.002 | 1.97±0.20 | 0.0049±0.0002 |
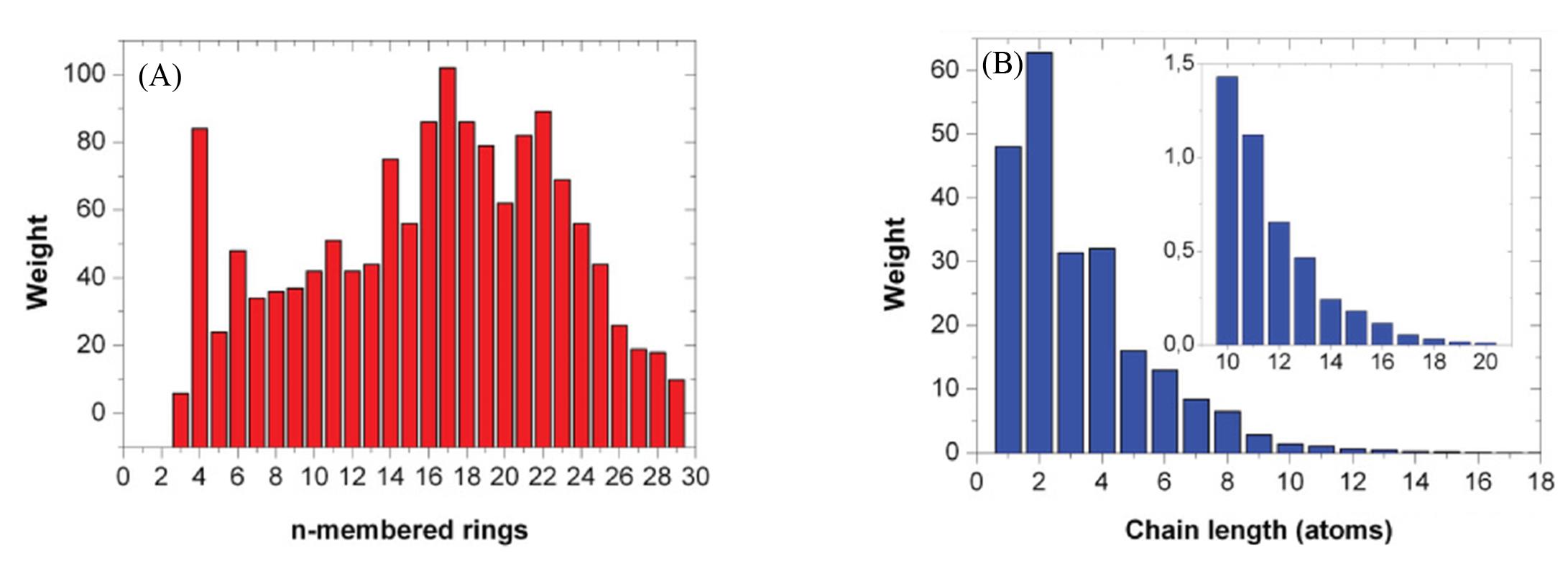
Fig.11 Ring(bond cutoff 0.32 nm, A) and chain(B) distributions of twofold⁃coordinated a⁃Te atoms at 300 K[120]Copyright 2012, American Physical Society.
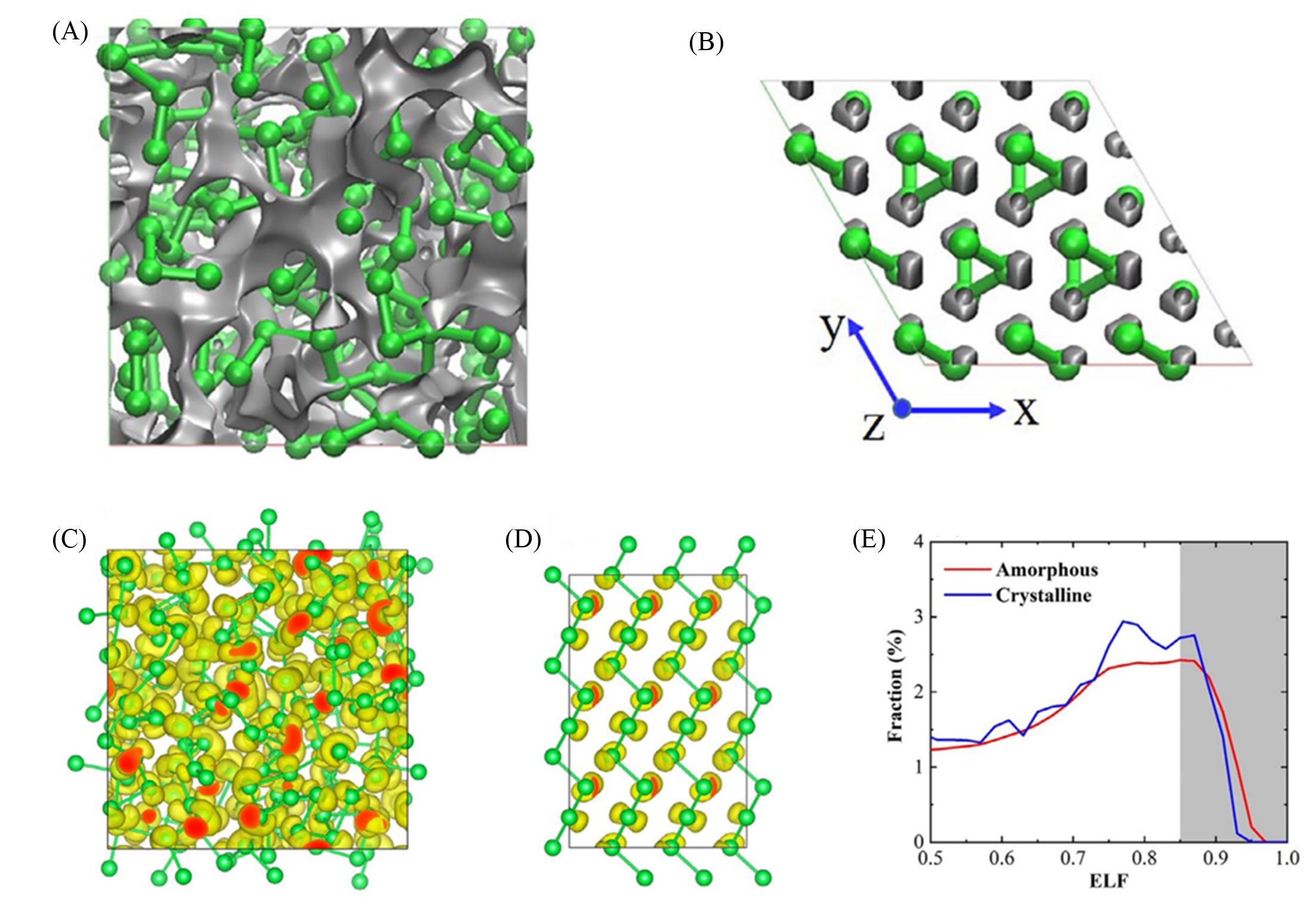
Fig.12 Visualize the voids (grey areas) for a⁃Te(A) and c⁃Te(B), the snapshots of ELF for a⁃Te(C) and c⁃Te(D) as ELF=0.85 and the distribution of ELF(E)[121]Copyright 2021, Elsevier.
| 1 | Yao F. Y., Guo D. W., Gui M. D., Inorganic Chemistry Series, Science Press, Beijing, 1998, 6—276 |
| 姚凤仪, 郭德威, 桂明德. 无机化学丛书, 北京: 科学出版社, 1998, 6—276 | |
| 2 | Krishnamurthi V., Khan H., Ahmed T., Zavabeti A., Tawfik S. A., Jain S. K., Spencer M. J. S., Balendhran S., Crozier K. B., Li Z., Fu L., Mohiuddin M., Low M. X., Shabbir B., Boes A., Mitchell A., McConville C. F., Li Y., KalantarZadeh K., Mahmood N., Walia S., Adv. Mater., 2020, 32(45), 2004247 |
| 3 | Hu C., Lian C., Zheng S., J. Mol. Catal. A: Chem., 2015, 407, 182—188 |
| 4 | Zhou Z., Wang J., Zhou S., Cat. Commun., 2008, 9(5), 568—571 |
| 5 | Syed M. A., Henshaw P. F., J. Chem. Technol. Biotechnol., 2005, 80(2), 119—123 |
| 6 | Yang Z., Jia D., Zhao Q., ACS Appl. Mater. Inter., 2022, 14(28), 32112—32123 |
| 7 | Zhu M., Niu G., Tang J., J. Mater. Chem. C, 2019, 7(8), 2199—2206 |
| 8 | Deka N., Dutta G. K., Curr. Opin. Electrochem., 2023, 38, 101222 |
| 9 | Liu X., Li Y., Xu X., J. Energy Chem., 2021, 61, 104—134 |
| 10 | Kang J. K., Park J. W., Kang J., Bull. Korean Chem. Soc., 2019, 40(6), 517—521 |
| 11 | Li M., Lu J., Amine K., ACS Nano, 2021, 15(5), 8087—8094 |
| 12 | Wu Y. J., Huang W. Z., Pan J. D., Shi K. X., Liu Q. B., Chem. J. Chinese Universities, 2023, 44(1), 20220619 |
| 吴钰洁, 黄文治, 潘俊达, 石凯祥, 刘全兵. 高等学校化学学报, 2023, 44(1), 20220619 | |
| 13 | Geng C. N., Hua W. X., Ling G. W., Tao Y., Zhang C., Yang Q. H., Chem. J. Chinese Universities, 2021, 42(5), 1331—1339 |
| 耿传楠, 化五星, 凌国维, 陶莹, 张辰, 杨全红. 高等学校化学学报, 2021, 42(5), 1331—1339 | |
| 14 | Han F. C., Li F. J., Chen L., He L. Y., Jiang Y. N., Xu S. D., Zhang D., Qi L., Chem. J. Chinese Universities, 2022, 43(8), 20220163 |
| 韩付超, 李福进, 陈良, 贺磊义, 姜玉南, 徐守冬, 张鼎, 其鲁. 高等学校化学学报, 2022, 43(8), 20220163 | |
| 15 | Wen Z., Cao J., Gu Z., Solid State Ion., 2008, 179(27—32), 1697—1701 |
| 16 | Medenbach L., Adelhelm P., Electrochemical Energy Storage, 2019, 375(81), 101—125 |
| 17 | Helms H., Ristau J., Kunz H., Monatsh. Chem., 2000, 131, 165—174 |
| 18 | Ansari Y., Zhang S., Wen B., Adv. Energy Mater., 2019, 9(1), 1802213 |
| 19 | Kasap S., Frey J. B., Belev G., Sensors, 2011, 11, 5112—5157 |
| 20 | Yang Z. N., Yang X. S., J. Guizhou Norm. Univ. Nat. Sci. Ed., 2004, 22(1), 104—112 |
| 杨占南, 杨小生. 贵州师范大学学报, 自然科学版, 2004, 22(1), 104—112 | |
| 21 | Cao W., Xu H. P., Chem. Bull., 2013, 76(4), 291—298 |
| 曹玮, 许华平. 化学通报, 2013, 76(4), 291—298 | |
| 22 | Pan S. J., Xu H. P., Acta Polym. Sin., 2021, 52(8), 857—866 |
| 潘烁炯, 许华平. 高分子学报, 2021, 52(8), 857—866 | |
| 23 | Chang Y., Huang J., Shi S., Xu L., Lin H., Chen T., Adv. Mater., 2023, 35(36), 2212178 |
| 24 | Anderson A., Smith W., Wheeldon J. F., Chem. Phys. Lett., 1996, 263(1/2), 133—137 |
| 25 | Eckert B., Steudel R., Elemental Sulfur and Sulfur⁃Rich Compounds II, 2003, 231, 31—98 |
| 26 | Kalampounias A. G., Andrikopoulos K. S., Yannopoulos S. N., J. Chem. Phys., 2003, 118(18), 8460—8467 |
| 27 | Crapanzano L., Crichton W. A., Monaco G., Nat. Mater., 2005, 4(7), 550—552 |
| 28 | Nims C., Cron B., Wetherington M., Macalady J., Cosmidis J., Sci. Rep., 2019, 9(1), 7971 |
| 29 | Sahu S., Lochab B., ACS Sustain. Chem. Eng., 2022, 10(37), 12355—12364 |
| 30 | Khan N., Baláž M., Burkitbayev M., Appl. Surf. Sci., 2022, 601, 154122 |
| 31 | Martin R. M., Lucovsky G., Helliwell K., Phys. Rev. B, 1976, 13(4), 1383—1395 |
| 32 | Barman S. K., Huda M. N., Asaadi J., Langmuir, 2022, 38(28), 8485—8494 |
| 33 | Liu D., Han L., Wei R., Phys. Rev. Mater., 2022, 6(10), 103403 |
| 34 | Shimakawa K., Kolobov A., Elliott S. R., Adv. Phys., 1995, 44(6), 475—588 |
| 35 | Feltz A. C., Phys. Rev. Lett., 1993, 76, 367—388 |
| 36 | Yannopoulos S. N., J. Mater. Sci. Mater. Electron., 2020, 31(10), 7565—7595 |
| 37 | Smith A., Holmes W. B., Hall E. S., J. Am. Chem. Soc., 1905, 27(7), 797—820 |
| 38 | Lucovsky G., Mooradian A., Taylor W., Solid State Commun., 1967, 5(2), 113—117 |
| 39 | Brodsky M. H., Gambino R. J., Smith J. E., Phys. Status Solid, 1972, 52(2), 609—614 |
| 40 | Eisenberg A., Tobolsky A. V., J. Polym., 1960, 46(147), 19—28 |
| 41 | Steudel R., Eckert B., Elemental Sulfur and Sulfur⁃rich Compounds I, 2003, 2, 1—80 |
| 42 | Steudel R., Eckert B., J. Chem. Phys., 2004, 121(13), 6573—6574 |
| 43 | Yannopoulos S. N., Andrikopoulos K. S., J. Chem. Phys., 2004, 121(10), 4747—4758 |
| 44 | Zhang F., Gong Z., Cai W. H., Polymer, 2022, 240, 124473 |
| 45 | Song T. Y., Xu J. N., Cheng G. Z., Shi S. H., Inorganic Chemistry, Higher Education Press, Beijing, 2010, 121—249 |
| 宋天佑, 徐家宁, 程功臻, 史苏华. 无机化学, 北京: 高等教育出版社, 2010, 121—249 | |
| 46 | Gradie J., Ostro S. J., Thomas P. C., J. Non⁃Cryst. Solids, 1984, 67(1—3), 421—432 |
| 47 | Greer S. C., Annu. Rev. Phys. Chem., 2002, 53(1), 173—200 |
| 48 | Kalampounias A. G., Andrikopoulos K. S., Yannopoulos S. N., J. Chem. Phys., 2003, 119(14), 7543—7553 |
| 49 | Tobolsky A. V., Mac K. W., Beevers R. B., Polymer, 1963, 4, 423—427 |
| 50 | Das S. R., Ghosh K., Indian J. Phys., 1939, 13, 91—105 |
| 51 | Stolz M., Winter R., Howells W. S., J. Phys. Condens. Matter, 1994, 6(20), 3619—3628 |
| 52 | Kalampounias A. G., Kastrissios D. T., Yannopoulos S. N., J. Non⁃Cryst. Solids, 2003, 326, 115—119 |
| 53 | Ruta B., Monaco G., Giordano V. M., J. Phys. Chem. B, 2011, 115(48), 14052—14063 |
| 54 | Andrikopoulos K. S., Kalampounias A. G., Falagara O., Yannopoulos S. N., J. Chem. Phys., 2013, 139(12), 124501 |
| 55 | Rankin G. A., J. Phys. Chem., 1907, 11(1), 1—8 |
| 56 | Luo H., Ruoff A. L., Phys. Rev. B, 1993, 48(1), 569—572 |
| 57 | Winter R., Pilgrim W. C., Egelstaff P. A., EPL, 1990, 11(3), 225—228 |
| 58 | Stillinger F. H., Weber T. A., LaViolette R. A., J. Chem. Phys., 1986, 85, 6460—6469 |
| 59 | Eckert B., Schumacher R., Jodl H. J., Int. J. High Press. Res., 2000, 17(2), 113—146 |
| 60 | Blight K. R., Candy R. M., Ralph D. E., Hydrometallurgy, 2009, 99(1/2), 100—104 |
| 61 | Zhou H. T., Gao X., Zheng P., Qin M., Cao Y., Wang W., Acta Phys. Sin., 2016, 65, 188703 |
| 周浩天, 高翔, 郑鹏, 秦猛, 曹毅, 王炜. 物理学报, 2016, 65, 188703 | |
| 62 | Li X., Xue Y. R., Song Y., Zhang W. K., Chem. J. Chinese Universities, 2018, 39(12), 2774—2780 |
| 李逊, 薛玉瑞, 宋宇, 张文科. 高等学校化学学报, 2018, 39(12), 2774—2780 | |
| 63 | Lü X. J., Song Y., Zhang W. K., Chem. J. Chinese Universities, 2018, 39(1), 166—171 |
| 吕秀娟, 宋宇, 张文科. 高等学校化学学报, 2018, 39(1), 166—171 | |
| 64 | Bao Y., Luo Z. L., Cui S. X., Chem. Soc. Rev., 2020, 49(9), 2799—2827 |
| 65 | Junker J. P., Rief M., Angew. Chem. Int. Ed., 2010, 49(19), 3306—3309 |
| 66 | Fu L., Wang H., Li H., CCS Chem., 2019, 1(1), 138—147 |
| 67 | Geisler M., Netz R., Hugel T., Angew. Chem. Int. Ed., 2010, 49(28), 4730—4733 |
| 68 | Zhang Y., Xu J., Bao Y., Cui S. X., Chem. J. Chinese Universities, 2022, 43(4), 20210863 |
| 张勇, 许俊, 鲍雨, 崔树勋. 高等学校化学学报, 2022, 43(4), 20210863 | |
| 69 | Wan P. C., Lu S., Bao Y., Cui S. X., Chem. J. Chinese Universities, 2023, 44(8), 20230126 |
| 万鹏程, 陆松, 鲍雨, 崔树勋. 高等学校化学学报, 2023, 44(8), 20230126 | |
| 70 | Zhang S., Li Z., Bao Y., Lu S., Gong Z., Qian H. J., Cui S. X., ACS Nano, 2023, 17(11), 10958—10964 |
| 71 | Zhang S., Qian H. J., Liu Z., Ju H., Lu Z. Y., Zhang H., Cui S. X., Angew. Chem. Int. Ed., 2019, 58(6), 1659—1663 |
| 72 | Boyd R., Nat. Chem., 2011, 3(7), 570 |
| 73 | Santi C., Bangoli L., Molecules, 2017, 22(12), 1—4 |
| 74 | Dittrich G., Angew. Chem. Int. Ed., 1975, 87(18), 681—689 |
| 75 | Schottmiller J., Tabak M., Lucovsky G., J. Non⁃Cryst. Solids, 1970, 4, 80—96 |
| 76 | Gorman M., Solin S. A., Solid State Commun., 1976, 18(11/12), 1401—1404 |
| 77 | Lucovsky G., the Physics of Selenium and Tellurium, Springer, New York, 1979, 178—192 |
| 78 | Carini G., Cutroni M., Galli G., Solid State Commun., 1980, 33(11), 1139—1141 |
| 79 | Carroll P. J., Lannin J. S., Solid State Commun., 1981, 40(1), 81—84 |
| 80 | Yannopoulos S. N., Andrikopoulos K. S., Phys. Rev. B, 2004, 69(14), 144206 |
| 81 | Dash S., Chen P., Boolchand P., J. Chem. Phys., 2017, 146(22), 224506 |
| 82 | Gomp F., J. Phys. Chem. Sol., 1981, 42(6), 539—544 |
| 83 | Phillips W. A., Buchenau U., Nücker N., Phys. Rev. Lett., 1989, 63(21), 2381—2384 |
| 84 | Kamitakahara W. A., Cappelletti R. L., Boolchand P., Halfpap B., Gompf F., Neumann D. A., Mutka H., Phys. Rev. B, 1991, 44(1), 94—100 |
| 85 | Scopigno T., Di Leonardo R., Ruocco G., Baron A. Q. R., Tsutsui S., Bossard F., Yannopoulos S. N., Phys. Rev. Lett., 2004, 92(2), 025503 |
| 86 | Yang C. Y., Sayers D. E., Paesler M. A., J. Non⁃Cryst. Solids, 1989, 114, 67—69 |
| 87 | Depanfilis S., Filipponi A., EPL, 1997, 37(6), 397 |
| 88 | Kaplow R., Rowe T. A., Averbach B. L., Phys. Rev., 1968, 168(3), 1068—1079 |
| 89 | Andonov P., J. Non⁃Cryst. Solids, 1982, 47(3), 297—339 |
| 90 | Stephens R. B., J. Appl. Phys., 1978, 49(12), 5855—5864 |
| 91 | Stephens R. B., Phys. Rev. B, 1984, 30(9), 5195—5202 |
| 92 | Echeverrı́a I., Kolek P. L., Plazek D. J., J. Non⁃Cryst. Solids, 2003, 324(3), 242—255 |
| 93 | Shevchik N. J., Phys. Rev. Lett., 1974, 33(26), 1572—1576 |
| 94 | Warren W., Dupree R., Phys. Rev. B, 1980, 22(5), 2257 |
| 95 | Jóvári P., Pusztai L., Phys. Rev. B, 2001, 64(1), 14205 |
| 96 | Jóvári P., Delaplane R. G., Pusztai L., Phys. Rev. B, 2003, 67(17), 172201 |
| 97 | Caprion D., Schober H. R., Phys. Rev. B, 2000, 62(6), 3709—3716 |
| 98 | Hohl D., Jones R. O., Phys. Rev. B, 1991, 43(5), 3856—3870 |
| 99 | Oligschleger C., Jones R. O., Reimann S. M., Phys. Rev. B, 1996, 53(10), 6165—6173 |
| 100 | Kirchhoff F., Kresse G., Gillan M. J., Phys. Rev. B, 1998, 57(17), 10482 |
| 101 | Zhang X., Drabold D. A., Phys. Rev. Lett., 1999, 83(24), 5042—5045 |
| 102 | Nakamura K., Ikawa A., Phys. Rev. B, 2003, 67(10), 104203 |
| 103 | Richter H., J. Non⁃Cryst. Solids, 1972, 8, 388—394 |
| 104 | Griffiths J. E., Solid State Commun., 1982, 43(4), 253—255 |
| 105 | Misawa M., Suzuki K., J. Phys. Soc. Jpn., 1978, 44(5), 1612—1618 |
| 106 | Kobashi Y. K. Y., Kodera S. K. S., Jpn. J. Appl. Phys., 1998, 37(5R), 2590—2592 |
| 107 | Guarneros A. C., Calzadilla O., Barón M. J. A., Mater. Res. Express, 2019, 6(6), 066412 |
| 108 | Marple M., Badger J., Hung I., Angew. Chem. Int. Ed., 2017, 56(33), 9777—9781 |
| 109 | Zare B., Nami M., Shahverdi A. R., Biol. Trace. Elem. Res., 2017, 180, 171—181 |
| 110 | Sarrach D. J., Deneufville J. P., Haworth W. L., J. Non⁃Cryst. Solids, 1976, 22(2), 245—267 |
| 111 | Housecroft C. E., Catherine H., Alan G., Vib. Spectrosc., 2012, 71, 3—7 |
| 112 | Vasileiadis T., Dracopoulos V., Kollia M., Sci. Rep., 2013, 3(1), 1209 |
| 113 | Vasileiadis T., Yannopoulos S. N., J. Appl. Phys., 2014, 116(10), 103510 |
| 114 | Koma A., Mizuno O., Tanaka S., Phys. Status Solid, 1971, 46(1), 225—233 |
| 115 | Ruiz⁃Martín M. D., Jiménez R. M., Bermejo F. J., Phys. Rev. B, 2006, 73(9), 094201 |
| 116 | Pine A. S., Dresselhaus G., Phys. Rev. B, 1971, 4(2), 356—371 |
| 117 | Ichikawa T., Phys. Status Solid, 1973, 56(2), 707—715 |
| 118 | Stuke J., J. Non⁃Cryst. Solids, 1970, 4, 1—26 |
| 119 | Ikemoto H., Miyanaga T., J. Synchrotron Radiat., 2014, 21(2), 409—412 |
| 120 | Akola J., Jones R. O., Phys. Rev. B, 2012, 85(13), 134103 |
| 121 | Qiao C., Xu M., Wang S., Scr. Mater., 2021, 202, 114011 |
| 122 | Lu W., Li Z., Feng M., Zheng L., Liu S., Yan B., Hu J., Xue D., J. Am. Chem. Soc., 2024, 146(9), 6345—6351 |
| [1] | 张慧莲, 杨新杰, 李军, 李泉, 张福娟, 张艳丽, 王红斌, 杨文荣, 庞鹏飞. 基于N掺杂Ti3C2 MXene量子点的荧光探针用于Hg2+和S2-的传感检测[J]. 高等学校化学学报, 2024, 45(5): 20230504. |
| [2] | 任传清, 季晓晖, 张强, 曹小燕, 季建伟, 刘波. 钯催化合成噻吩并[2,3-b]噻喃-4-酮类化合物[J]. 高等学校化学学报, 2024, 45(5): 20240033. |
| [3] | 闫晓雨, 陈钰莹, 赵媚, 袁贻云, 卞佳坤, 马献涛. 水相中烷基硫代酯的绿色合成[J]. 高等学校化学学报, 2024, 45(4): 20240006. |
| [4] | 衡盼盼, 张咪, 侯华, 王宝山. 气体介质绝缘强度的化学键组合理论[J]. 高等学校化学学报, 2024, 45(1): 20230418. |
| [5] | 张煜, 陈介焕, 周家东, 刘琳琳, 解增旗. 苝酰亚胺受体的聚集形态对有机太阳电池中激子过程与电子传输的影响[J]. 高等学校化学学报, 2023, 44(9): 20230092. |
| [6] | 桑丽霞, 马梦楠. (Ag, Au)/MoS2的电解水性能及等离激元光热作用[J]. 高等学校化学学报, 2023, 44(6): 20220768. |
| [7] | 朱影影, 闫晓雨, 于静, 满文鑫, 刘凤, 周颖, 马献涛. 无催化剂下二硫醚的高效合成[J]. 高等学校化学学报, 2023, 44(6): 20230045. |
| [8] | 任思远, 郭玮, 付永柱. 有机硫化物在可充电电池中的研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220729. |
| [9] | 王日, 边超, 谢勇, 韩明杰, 李洋, 夏善红. 基于二硫化钼和钴基金属有机骨架的痕量汞离子电化学传感器[J]. 高等学校化学学报, 2023, 44(4): 20220719. |
| [10] | 韩旭, 白雪, 张仲, 杨琰莉, 崔红, 刘术侠. PW11M@Cu3(BTC)2杂化物的合成及燃料油深度脱硫活性[J]. 高等学校化学学报, 2023, 44(4): 20220702. |
| [11] | 薛转, 穆钟林, 何润合, 李永兵, 张兴祥. 不同氨基含量碳纳米管对锂硫电池正极比容量的影响[J]. 高等学校化学学报, 2023, 44(4): 20220709. |
| [12] | 曹舒杰, 李泓君, 管文丽, 任梦田, 周传政. 硫代磷酸酯寡聚核苷酸的立体控制合成研究进展[J]. 高等学校化学学报, 2023, 44(3): 20220304. |
| [13] | 胡平澳, 张琪, 张会茹. 锂硫电池中硒缺陷WSe2催化性能的理论研究[J]. 高等学校化学学报, 2023, 44(2): 20220595. |
| [14] | 郭昊天, 鲁新环, 孙凡棋, 陶艺元, 段金贵, 张望, 周丹, 夏清华. 纳米球型Mo-MOF材料的调控合成及催化硫醚选择性氧化[J]. 高等学校化学学报, 2023, 44(12): 20230408. |
| [15] | 姚田浩, 马语和, 柳博龙, 马玉强, 张聪, 李嘉辰, 马海霞. 偶氮四唑钾盐的绿色电合成反应耦合WS2纳米片催化电解水制氢[J]. 高等学校化学学报, 2023, 44(12): 20230347. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||