

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (7): 2111.doi: 10.7503/cjcu20210093
收稿日期:2021-02-08
出版日期:2021-07-10
发布日期:2021-05-31
通讯作者:
李磊
E-mail:lei.li@sdu.edu.cn;fsy@sdu.edu.cn
作者简介:冯圣玉, 男, 博士, 教授, 主要从事有机硅材料研究. E-mail: 基金资助:
WANG Linlin, LI Lei( ), FENG Shengyu(
), FENG Shengyu( )
)
Received:2021-02-08
Online:2021-07-10
Published:2021-05-31
Contact:
LI Lei
E-mail:lei.li@sdu.edu.cn;fsy@sdu.edu.cn
摘要:
有机硅材料具有优异的热稳定性、 生物相容性、 电绝缘性能和透气性等, 在国民经济各个领域已得到广泛应用. 近年来, 具有自修复和可塑性的有机硅超分子材料引起了人们的广泛关注, 各种各样的功能材料不断被开发出来. 本文综合评述了有机硅超分子材料的合成、 性能和应用等方面的近期研究进展, 重点阐述了氢键型、 金属配位键型、 Lewis酸碱对型、 离子键型及π-π堆积型有机硅超分子材料, 并对其发展前景进行了展望.
中图分类号:
TrendMD:
王琳琳, 李磊, 冯圣玉. 有机硅超分子材料研究进展. 高等学校化学学报, 2021, 42(7): 2111.
WANG Linlin, LI Lei, FENG Shengyu. Research Progress of Silicone Supramolecular Materials. Chem. J. Chinese Universities, 2021, 42(7): 2111.
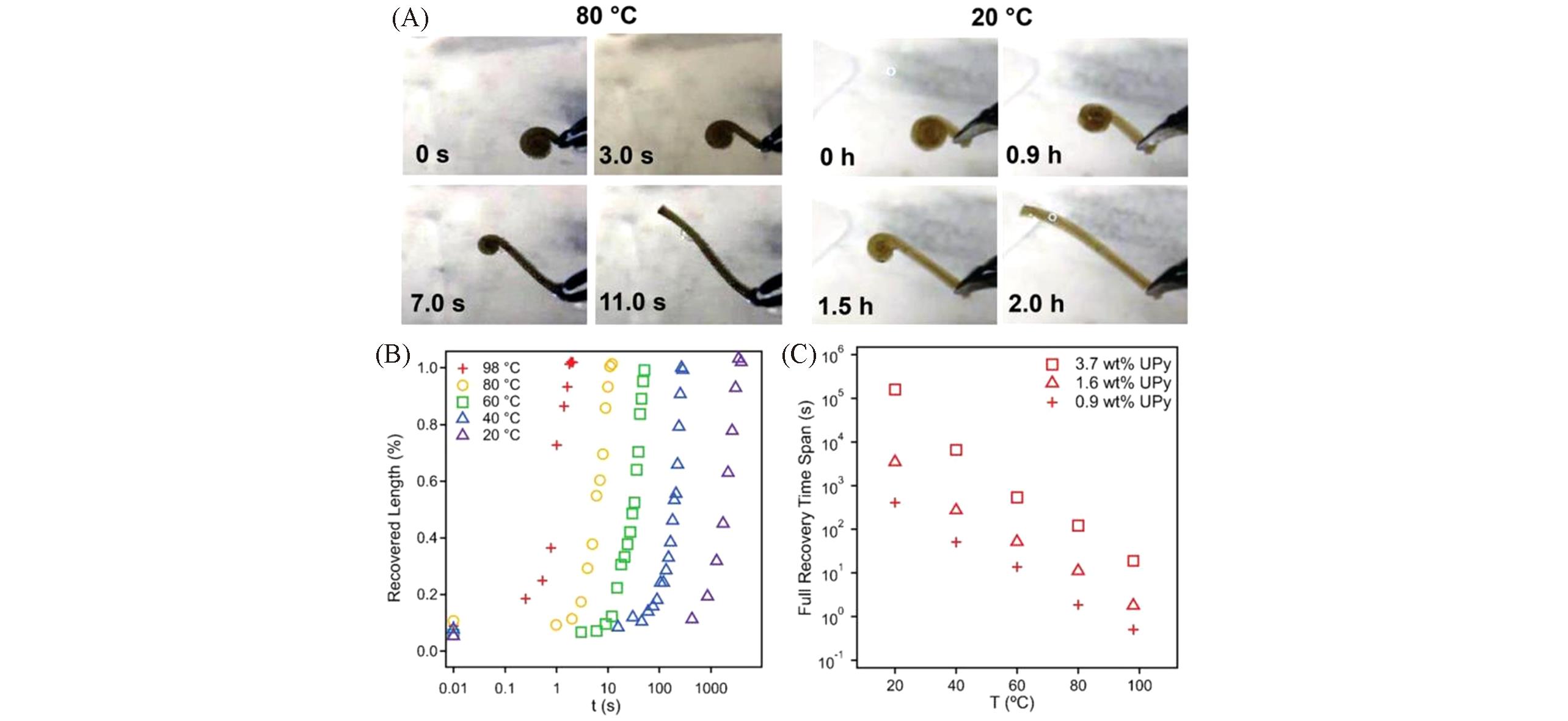
Fig.2 Temperature‐dependent shape‐memory capability of UPY?containing PDMS networks[16](A) Temperature dependent shape‐memory demonstration of a temporary, coiled piece(XDN‐25k‐1.6) recovering to its permanent, stripe geometry(30 mm×2 mm×1 mm). For a specimen immersed under water bath maintained at 80 ℃, the recovery took 11 s to complete; for a specimen immersed under water bath at 20 ℃, the recovery took 2 h to complete. (B) Shape recovery of a coiled XDN‐25k‐1.6 piece at 98, 80, 60, 40, and 20 °C, the recovered length is characterized as the straight segment that has spread out from the curved coiled state. (C) Full shape recovery time span of three coiled XDN‐25k pieces each with 0.9%, 1.6% and 3.7%(mass fraction) UPy loading, under different temperatures. The recovery time span of this shape‐memory silicone material can be tuned from seconds to days.Copyright 2019, Wiley-VCH.
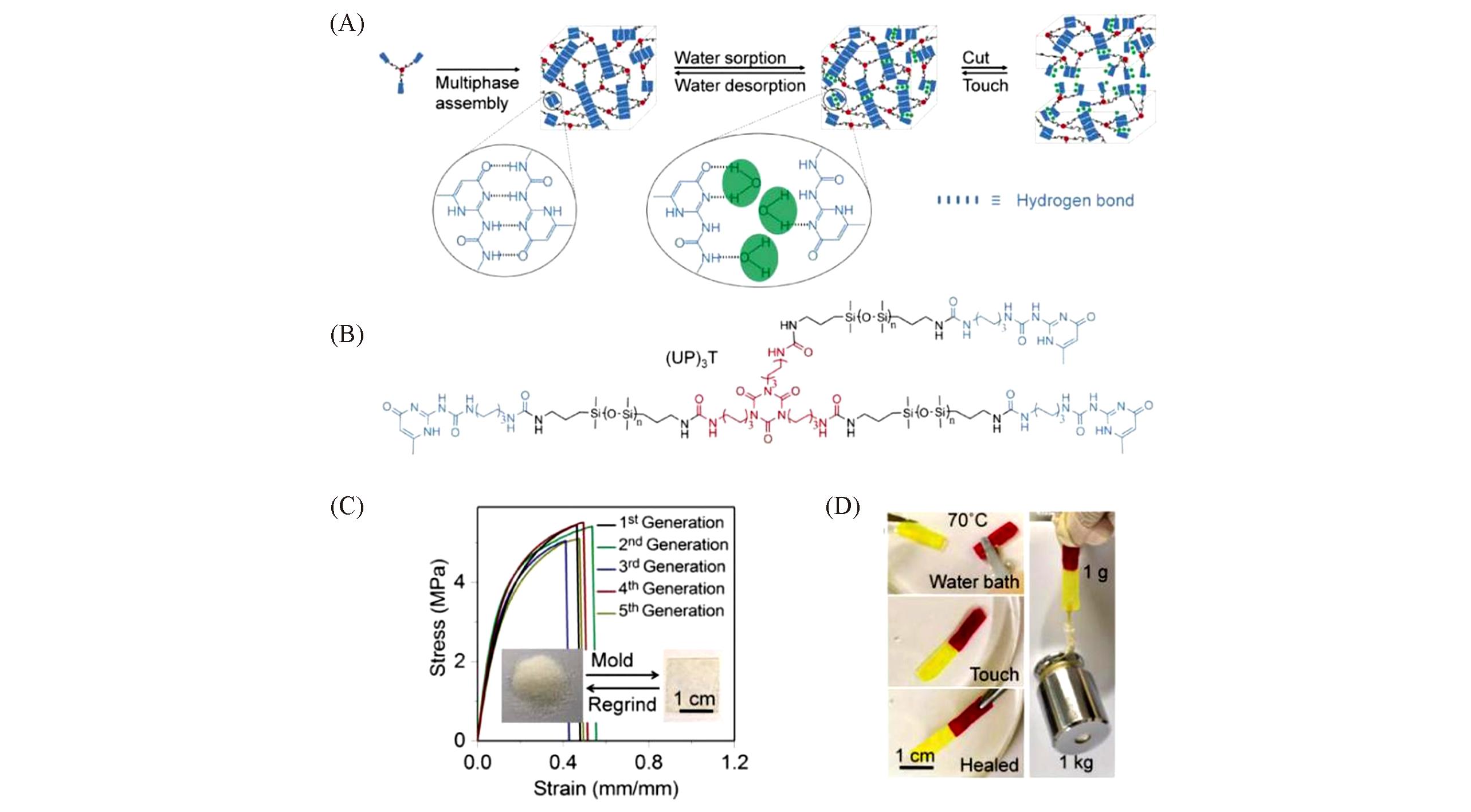
Fig.3 Multiphase design for healable siloxane oligomer (UP)3T[17](A) Self‐assembly of (UP)3T units into a 3D network via the dimerization of UPy(blue) motifs and the assembly of UPy stacks. UPy dimers dissociate and exchange with water molecules(green dots) upon water adsorption to release free UPy motifs at the interface. UPy dimers and stacks reform via touching two separate pieces together and finally heal by water desorption. (B) Molecular structure of siloxane oligomer (UP)3T. (C) Tensile stress-strain curves for the (UP)3T sample through five generations of molding from powder to film. (D) Two cutting pieces(stained red and yellow) were touch‐to‐heal in 70?℃ water bath for 5?min, and the healed sample(1?g weight) can withstand a 1?kg counterweight.Copyright 2018, Wiley-VCH.
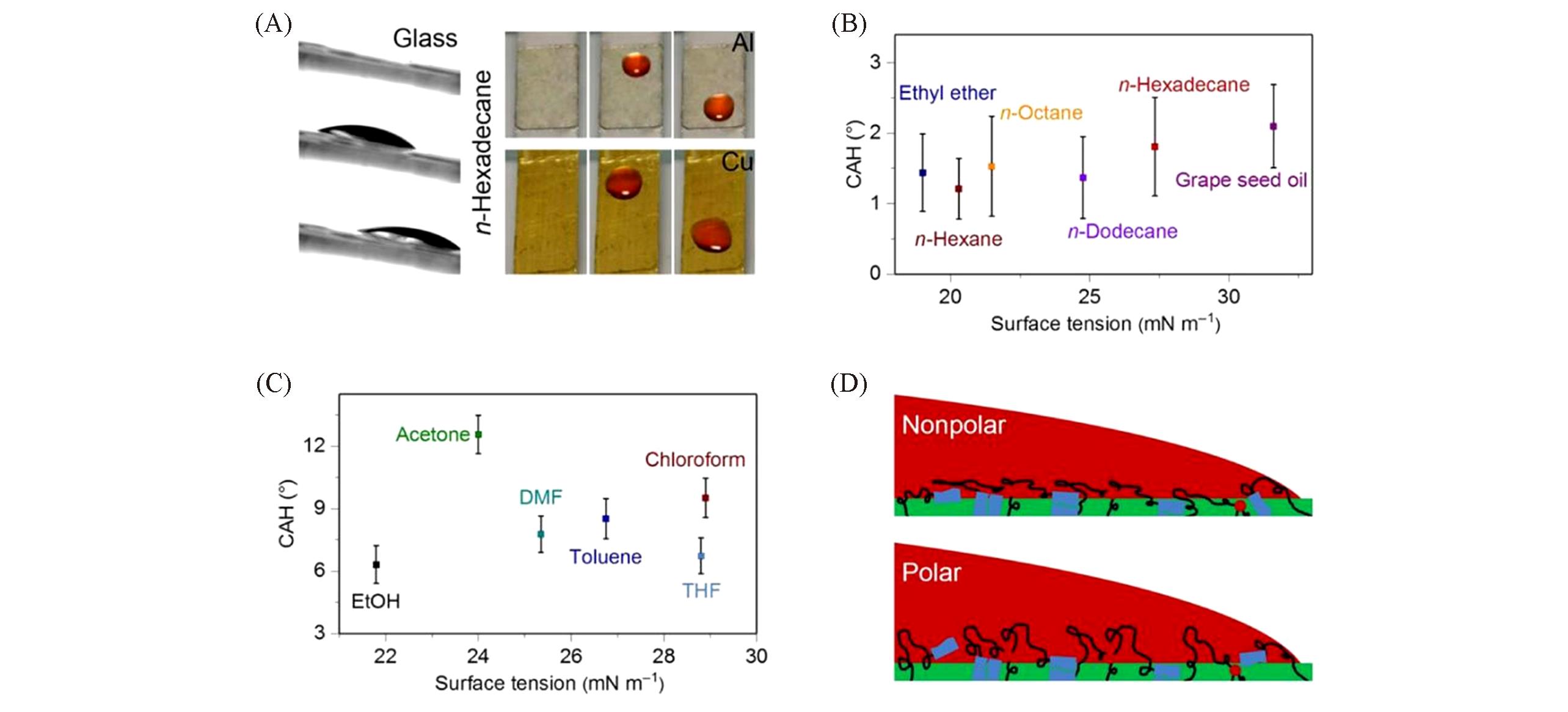
Fig.4 Wettability of the damage?healable, oil repellent supramolecular silicone coatings[18](A) The siloxane oligomer material-based coatings with small oil slide angles can be applied to various substrates such as glass, Al, and Cu-sheets, and 10-μL n-hexadecane drops can be repelled by all these coated surfaces. Credit: Liu Meijin, City University of Hong Kong. (B, C) Contact angle hysteresis(CAH) of various liquids on glass slides coated with siloxane oligomer materials. Nonpolar liquids can be repelled by the DOSS coating with a low CAH, while a couple of polar liquids can be repelled by the DOSS coating with a higher CAH. (D) Schematic illustrations of the interactions between the drops of nonpolar liquid or polar liquid and the DOSS coating, respectively. The interaction between the drop of nonpolar liquid and the DOSS coating is weak, as UPy motifs on the surface layer are shielded by the siloxane motifs, while the drop of polar liquid will strongly interact with the surface layer of the coating, particularly the dissociated UPy motifs, leading to higher CAHs.Copyright 2019, American Association for the Advancement of Science.
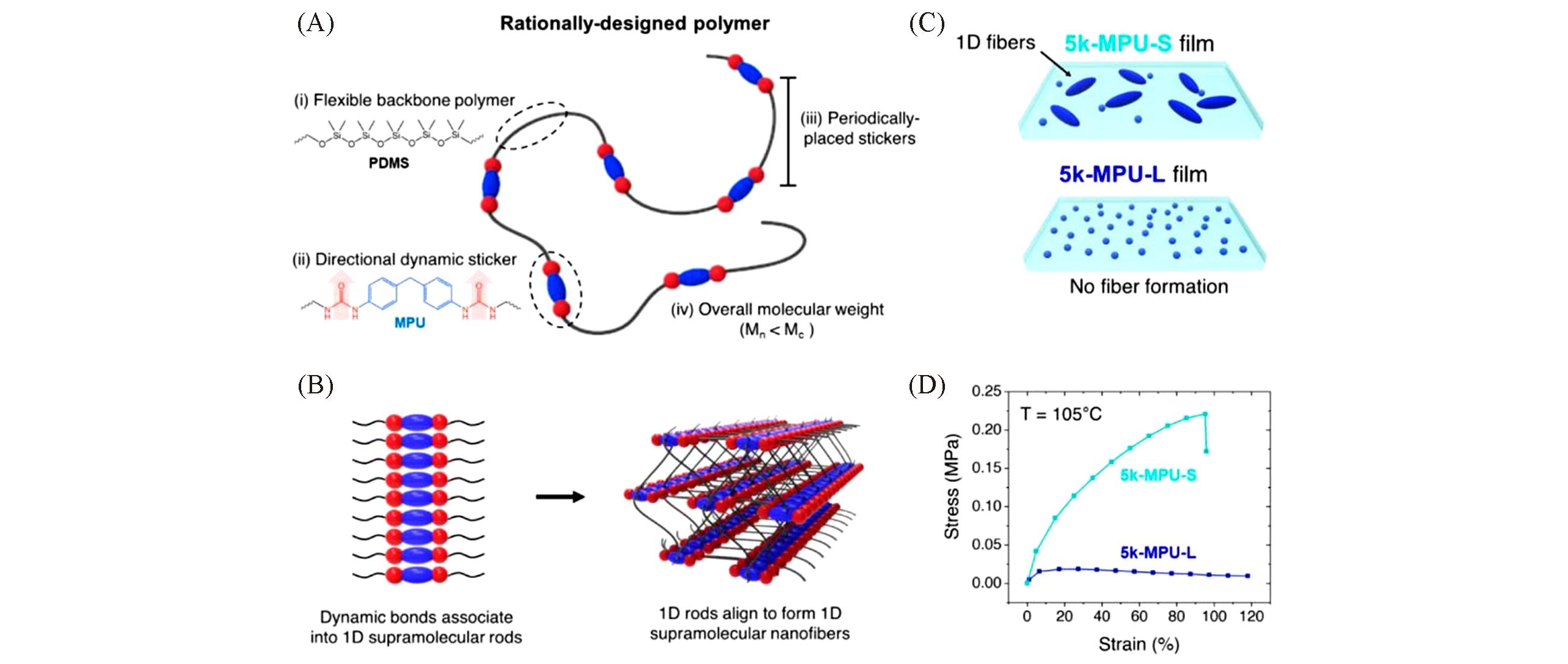
Fig.5 Rationally designed polymers self?assemble into supramolecular nanofibers[19](A) Periodic spacing of directional dynamic stickers along a flexible polymer backbone yields a rationally designed linear polymer chain that spontaneously assembles into supramolecular nanofibers. Importantly, the overall chain is under the critical entanglement molecular weight of the polymer backbone. (B) Formation of the supramolecular fiber begins with the association of dynamic stickers into 1D supramolecular rods, connected to many polymer backbones. These connecting polymer chains align the 1D rods into larger bundles, creating supramolecular nanofibers. (C) Controlling the overall molecular weight of the polymer allows to control the formation of the supramolecular nanofibers, which form in bulk films of the unentangled chains(5k-MPU-S) but not in bulk films of topolo-gically entangled chains(5k-MPU-L). (D) Stress-strain curves of 5k-MPU-S(light blue) and 5k-MPU-L(dark blue) showing improved mechanical properties of 5k-MPU-S at high temperature(105 ℃).Copyright 2020, American Chemical Society.
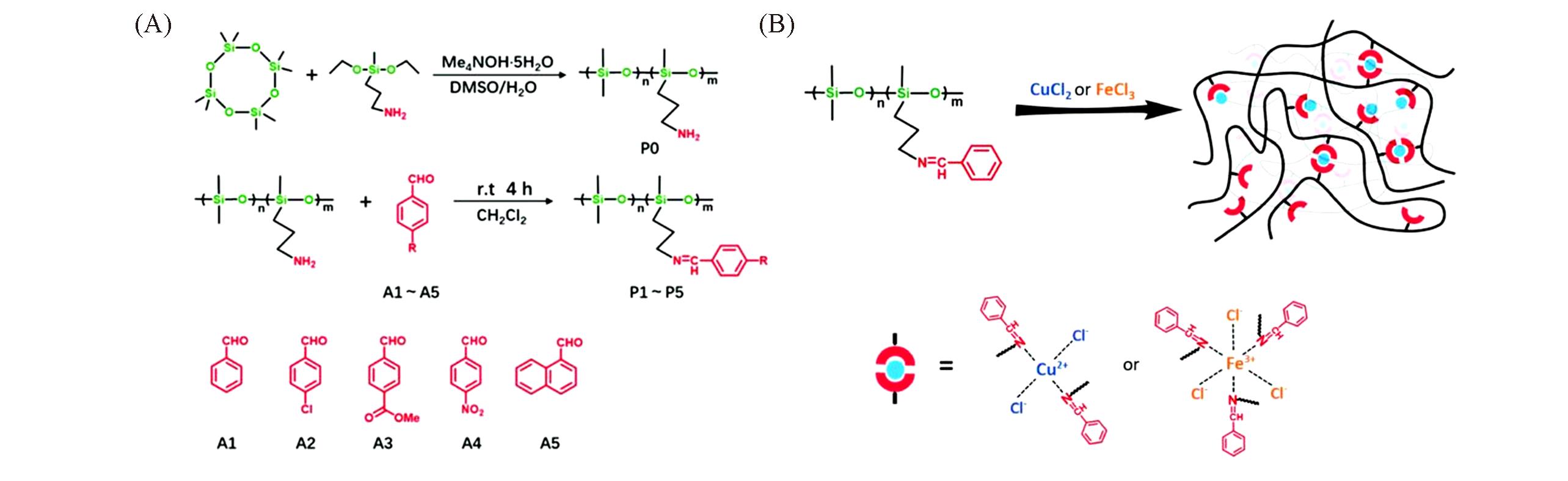
Fig.6 Coordination elastomer of imine?polysiloxane[28](A) Synthesis of poly[(aminopropyl)methylsiloxane-co-dimethylsiloxane](P0) and imine-functionalized polymers(P1—P5).(B) Schematic structures of supramolecular coordination elastomers based on imine-functionalized polysiloxanes.Copyright 2020, the Royal Society of Chemistry.
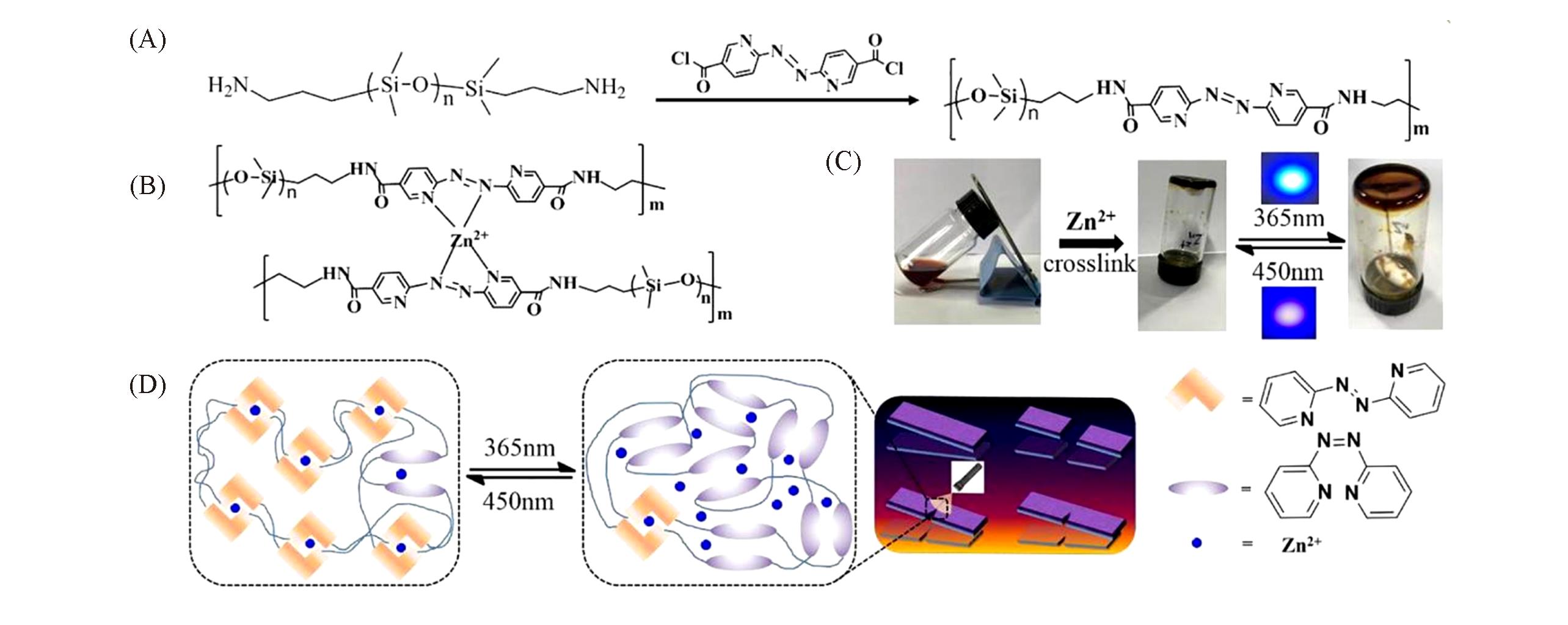
Fig.7 Photoresponsive coordination elastomer[32](A) The synthetic route of Abpy-PDMS; (B) structure of Zn(abpy)2-PDMS; (C) photographs of gelation formation of Abpy-PDMS toluene solution upon the addition of methanol solution of Zn(OTf)2; (D) schematic diagram of light-healing process.Copyright 2020, Elsevier.
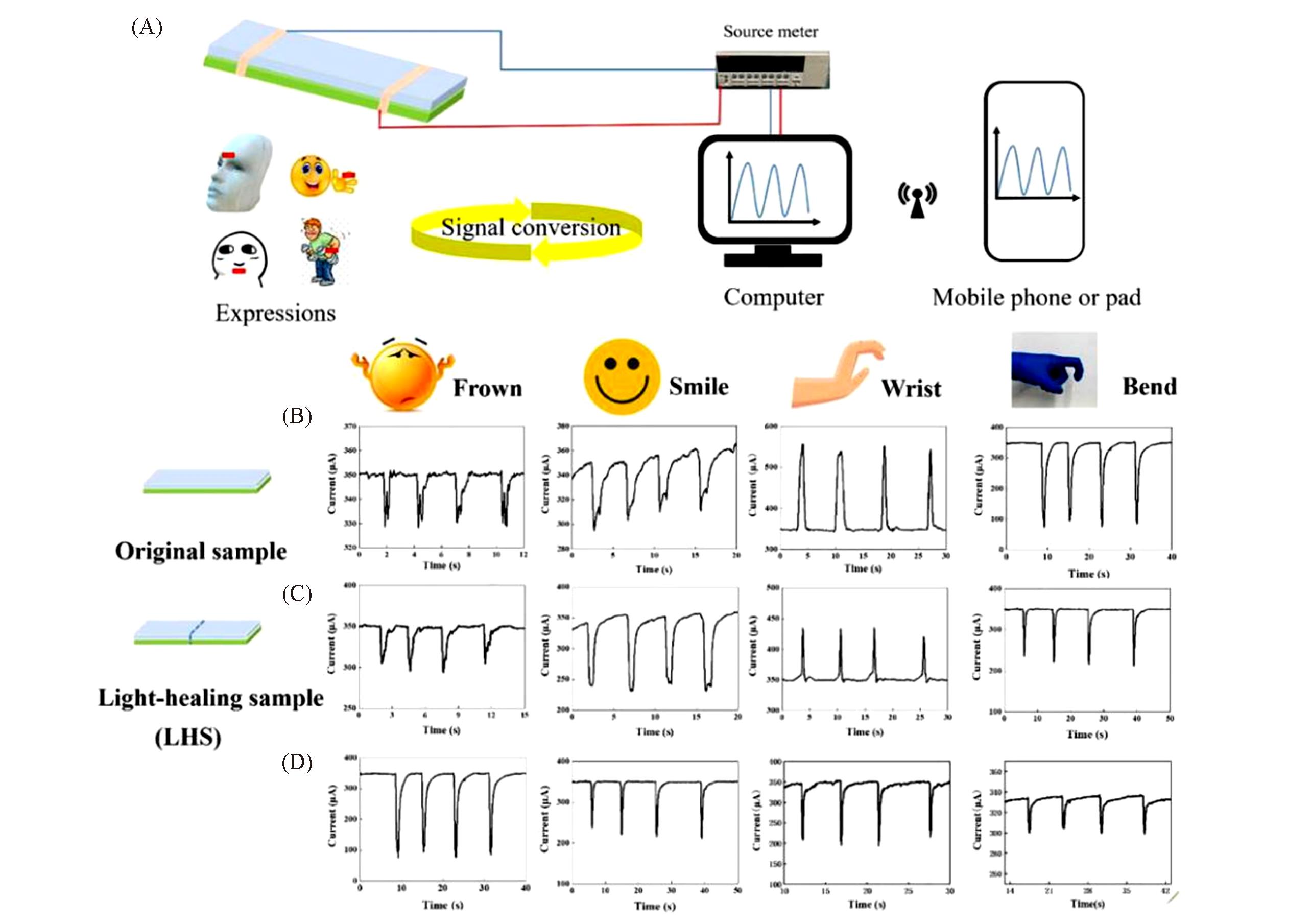
Fig.8 Sensing abilities of photoresponsive coordination elastomer[32](A) Sensing performance of our sensor in relative motion; (B, C) current responses of original specimen(B) and light-healing samples(C) for different physical movements; (D) sensing behavior of bend expression of several (0, 1, 3, 10) cut/healing times(from left to right).Copyright 2020, Elsevier.
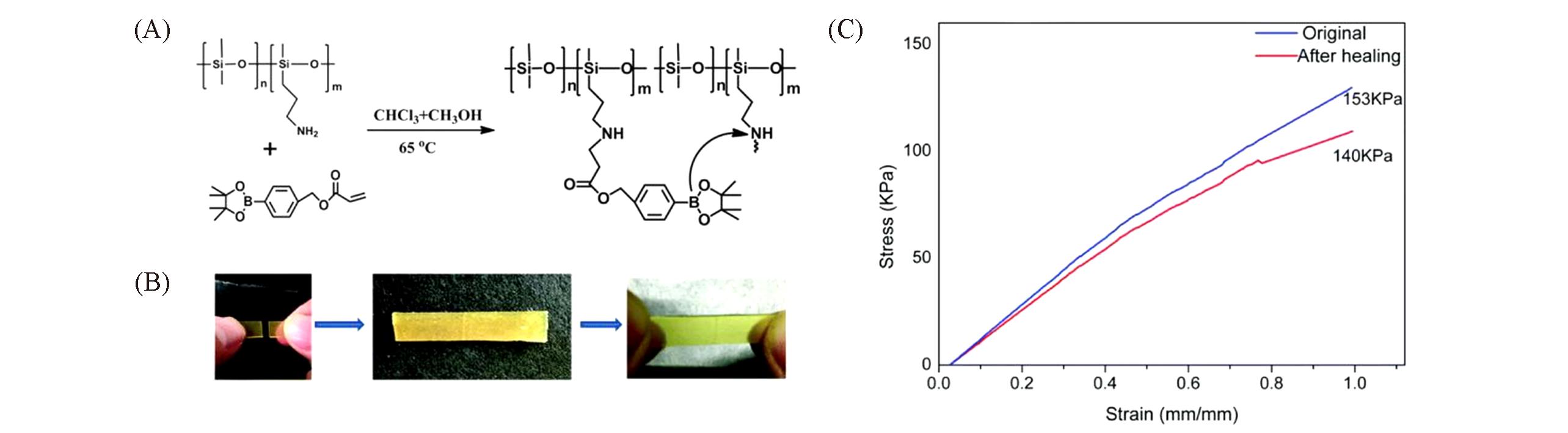
Fig.9 A readily self?healing and recyclable silicone elastomer via boron?nitrogen noncovalent crosslinking[44](A) The synthetic route of the elastomer; (B) self-healing behavior of the elastomer; (C) static tensile tests of the original elastomer and after healing under ambient conditions.Copyright 2018, the Royal Society of Chemistry.
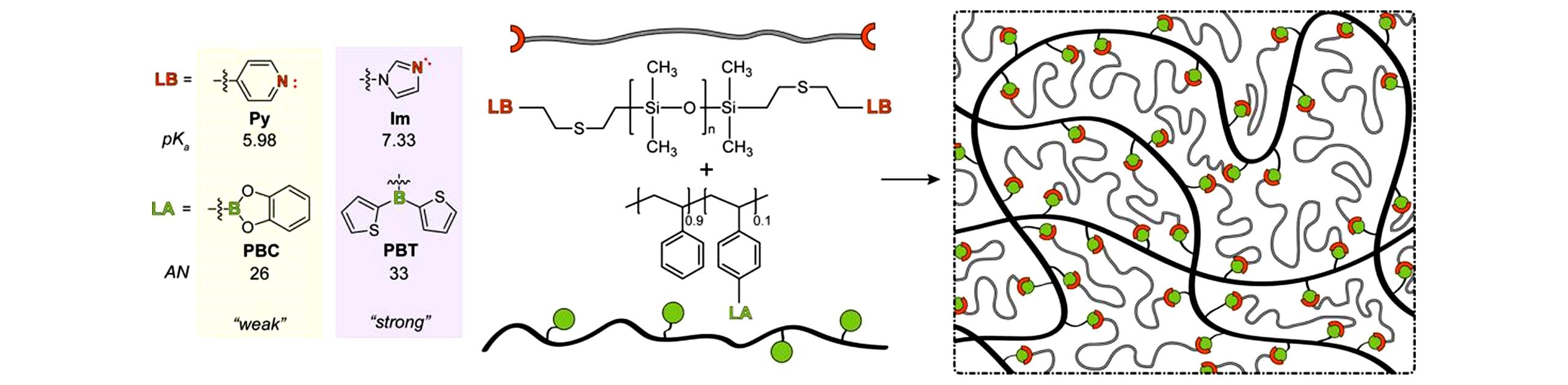
Fig.10 Schematic representation of transient polymer networks formed between Lewis acid?containing polystyrene(black lines) and Lewis base?terminated telechelic PDMS(grey lines)[45]Copyright 2019, American Chemical Society.
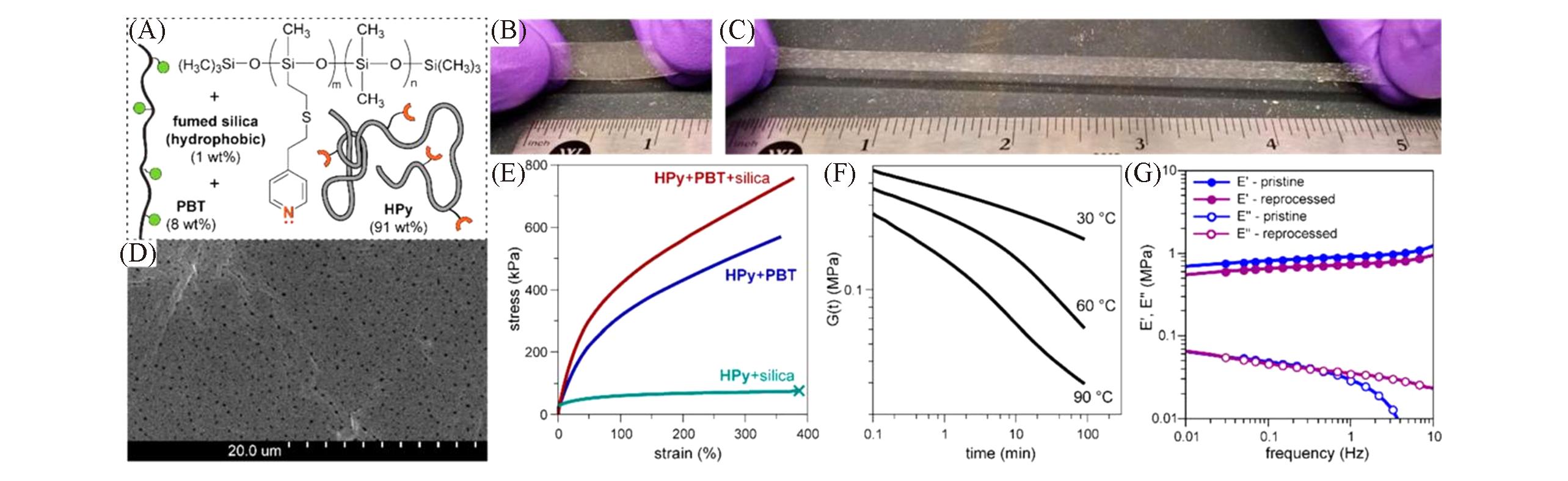
Fig.11 Doubly reinforced silicone rubber by the combined effect of the polystyrene and the silica filler[45](A) Composition of HPy+PBT+silica reinforced silicone rubber containing Lewis pair cross-links; (B, C) photographs of a rectangular specimen(0.5 mm thick) of (nano)composite silicone rubber at rest and under >400% strain(reference scale in inches); (D) scanning electron micrograph of the bulk rubber material HPy+PBT+silica showing the dispersion of the reinforcing silica nanoparticles(1%, mass fraction); (E) tensile testing of HPy+silica(1%, mass fraction) blend and cross-linked rubbers(elastomer specimens did not break under the testing conditions, 10 N/min). Dynamic mechanical characterization of HPy+PBT+silica(1%, mass fraction) in tensile mode: (F) stress relaxation(20% strain peak hold); (G) frequency sweeps(1% strain, 30 ℃).Copyright 2019, American Chemical Society.
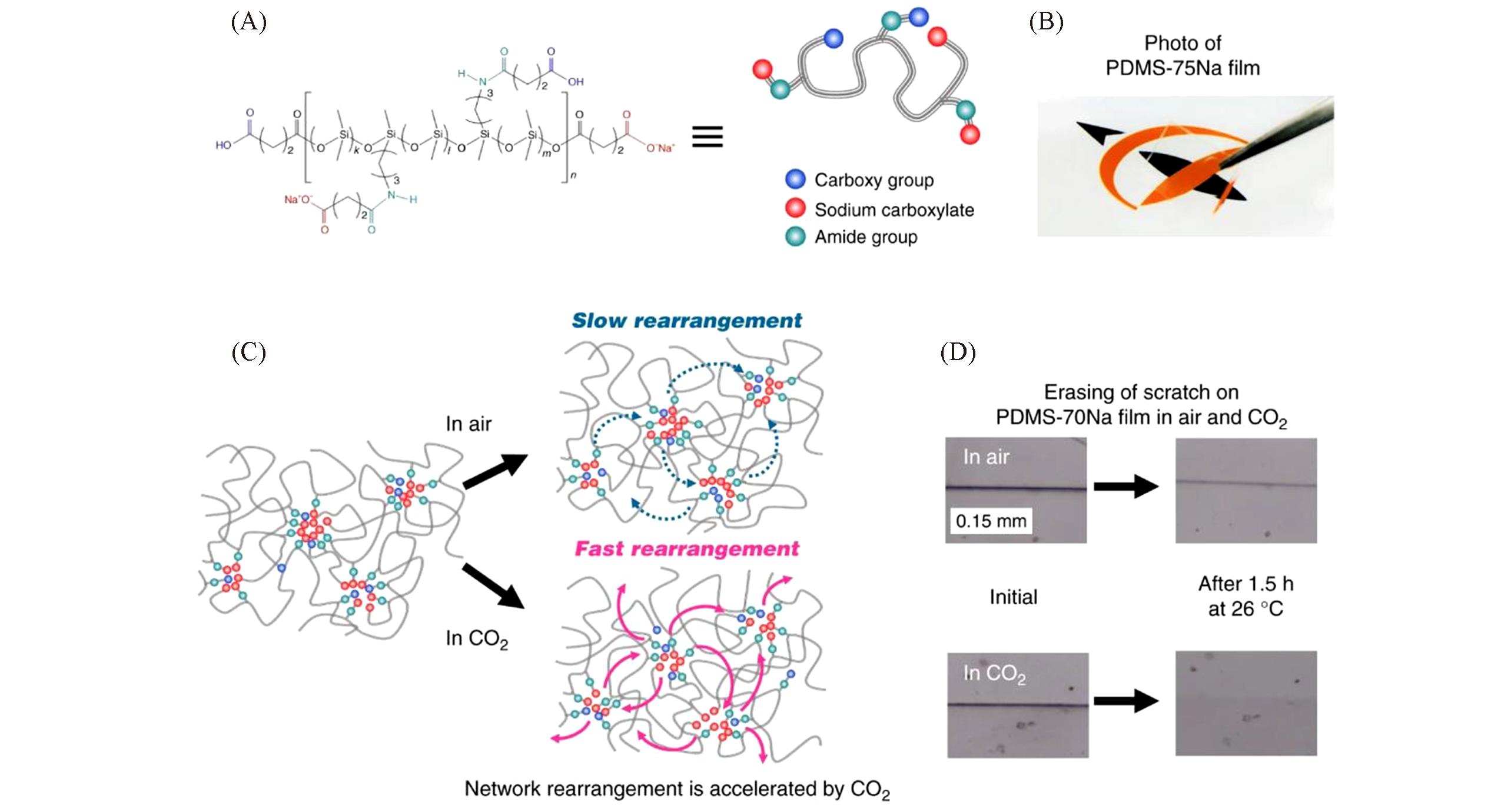
Fig.12 Molecular structure and network rearrangement chemistry of PDMS?xNa[46](A) Chemical structure and schematic illustration of PDMS-xNa. (B) Photograph of a transparent and colorless PDMS-75Na film. (C) Schematic illustration for slow and fast rearrangement of ionic crosslinks in air and CO2, respectively. Plasticization of ionic aggregate by CO2 gas provides rapid network rearrangement. (D) Optical microscopic images of a razor scratch on a PDMS-70Na film surface healed at 26?℃ for 1.5?h in air and in CO2.Copyright 2019, Springer Nature.
| 1 | Vidal F., Jakle F., Angew. Chem. Int. Ed., 2019, 58(18), 5846—5870 |
| 2 | Zhu D., Handschuh⁃Wang S., Zhou X., J. Mater. Chem. A,2017, 5(32), 16467—16497 |
| 3 | Eduok U., Faye O., Szpunar J., Prog. Org. Coat.,2017, 111, 124—163 |
| 4 | Wolf M. P., Salieb⁃Beugelaar G. B., Hunziker P., Prog. Polym. Sci.,2018, 83, 97—134 |
| 5 | Yilgör E., Yilgör I., Prog. Polym. Sci.,2014, 39(6), 1165—1195 |
| 6 | Amabilino D. B., Smith D. K., Steed J. W., Chem. Soc. Rev.,2017, 46(9), 2404—2420 |
| 7 | Savyasachi A. J., Kotova O., Shanmugaraju S., Bradberry S. J., Ó’Máille G. M., Gunnlaugsson T., Chem,2017, 3(5), 764—811 |
| 8 | Clemons T. D., Stupp S. I., Prog. Polym. Sci.,2020, 111, 101310 |
| 9 | Heinzmann C., Weder C., de Espinosa L. M., Chem. Soc. Rev.,2016, 45(2), 342—358 |
| 10 | Voorhaar L., Hoogenboom R., Chem. Soc. Rev.,2016, 45(14), 4013—4031 |
| 11 | Kajita T., Noro A., Matsushita Y., Polymer,2017, 128, 297—310 |
| 12 | Yılgör E., Yılgör İ., Polymer,2001, 42(19), 7953—7959 |
| 13 | Cheng L., Yu D., You J., Long T., Chen S., Zhou C., Prog. Chem., 2018, 30(12), 1852—1862(程龙, 于大江, 尤加健, 龙腾, 陈素素, 周传健. 化学进展, 2018, 30(12), 1852—1862) |
| 14 | Li C. H., Zuo J. L., Adv. Mater., 2020, 32(27), 1903762 |
| 15 | Yi B., Wang S., Hou C., Huang X., Cui J., Yao X., Chem. Eng. J.,2021, 405, 127023 |
| 16 | Meng Y., Xu W., Newman M. R., Benoit D. S. W., Anthamatten M., Adv. Funct. Mater.,2019, 29(38), 1903721 |
| 17 | Liu M., Liu P., Lu G., Xu Z., Yao X., Angew. Chem. Int. Ed.,2018, 57(35), 11242—11246 |
| 18 | Liu M., Wang Z., Liu P., Wang Z., Yao H., Yao X., Sci. Adv.,2019, 5(11), eaaw5643 |
| 19 | Cooper C. B., Kang J., Yin Y., Yu Z., Wu H. C., Nikzad S., Ochiai Y., Yan H., Cai W., Bao Z., J. Am. Chem. Soc.,2020, 142(39), 16814—16824 |
| 20 | Guo H., Han Y., Zhao W., Yang J., Zhang L., Nat. Commun.,2020, 11(1), 2037 |
| 21 | Cao P. F., Li B., Hong T., Townsend J., Qiang Z., Xing K., Vogiatzis K. D., Wang Y., Mays J. W., Sokolov A. P., Saito T., Adv. Funct. Mater.,2018, 28(22), 1800741 |
| 22 | Sirrine J. M., Schexnayder S. A., Dennis J. M., Long T. E., Polymer,2018, 154, 225—232 |
| 23 | Duan L., Lai J. C., Li C. H., Zuo J. L., ACS Appl. Mater. Interfaces,2020, 12(39), 44137—44146 |
| 24 | Cao J., Feng L., Feng S., New J. Chem.,2018, 42(3), 1973—1978 |
| 25 | Tazawa S., Shimojima A., Maeda T., Hotta A., J. Appl. Polym. Sci.,2018, 135(24), 45419 |
| 26 | Rambarran T., Bertrand A., Gonzaga F., Boisson F., Bernard J., Fleury E., Ganachaud F., Brook M. A., Chem. Commun.,2016, 52(40), 6681—6684 |
| 27 | Tan H., Lyu Q., Xie Z., Li M., Wang K., Wang K., Xiong B., Zhang L., Zhu J., Adv. Mater.,2019, 31(6), 1805496 |
| 28 | Hu H., Wang L., Wang L., Li L., Feng S., Polym. Chem., 2020, 11(48), 7721—7728 |
| 29 | Yi B., Liu P., Hou C., Cao C., Zhang J., Sun H., Yao X., ACS Appl. Mater. Interfaces,2019, 11(50), 47382—47389 |
| 30 | Cao C., Yi B., Zhang J., Hou C., Wang Z., Lu G., Huang X., Yao X., Chem. Eng. J.,2020, 392, 124834 |
| 31 | Wang X., Zhan S., Lu Z., Li J., Yang X., Qiao Y., Men Y., Sun J., Adv. Mater.,2020, 32(50), 2005759 |
| 32 | Peng B., Li H., Li Y., Lv Z., Wu M., Zhao C., Chem. Eng. J.,2020, 395, 125079 |
| 33 | Li C. H., Wang C., Keplinger C., Zuo J. L., Jin L., Sun Y., Zheng P., Cao Y., Lissel F., Linder C., You X. Z., Bao Z., Nat. Chem.,2016, 8(6), 618—624 |
| 34 | Lai J. C., Jia X. Y., Wang D. P., Deng Y. B., Zheng P., Li C. H., Zuo J. L., Bao Z., Nat. Commun.,2019, 10(1), 1164 |
| 35 | Lei Y., Huang Q., Shan S., Lin Y., Zhang A., New J. Chem.,2019, 43(44), 17441—17445 |
| 36 | Wu X., Luo R., Li Z., Wang J., Yang S., Chem. Eng. J.,2020, 398, 125593 |
| 37 | Wu X., Wang J., Huang J., Yang S., ACS Appl. Mater. Interfaces,2019, 11(7), 7387—7396 |
| 38 | Jia X. Y., Mei J. F., Lai J. C., Li C. H., You X. Z., Chem. Commun., 2015, 51(43), 8928—8930 |
| 39 | Lei Y., Huang W., Huang Q., Zhang A., New J. Chem., 2019, 43(1), 261—268 |
| 40 | Bai L., Qv P., Zheng J., J. Mater. Sci.,2020, 55(28), 14045—14057 |
| 41 | Sun H., Liu X., Liu S., Yu B., Ning N., Tian M., Zhang L., J. Mater. Chem. A,2020, 8(44), 23330—23343 |
| 42 | Shi J., Zhao N., Yan D., Song J., Fu W., Li Z., J. Mater. Chem. A, 2020, 8(12), 5943—5951 |
| 43 | Dodge L., Chen Y., Brook M. A., Chem. Eur. J.,2014, 20(30), 9349—9356 |
| 44 | Cao J., Han D., Lu H., Zhang P., Feng S., New J. Chem.,2018, 42(23), 18517—18520 |
| 45 | Vidal F., Gomezcoello J., Lalancette R. A., Jäkle F., J. Am. Chem. Soc., 2019, 141(40), 15963—15971 |
| 46 | Miwa Y., Taira K., Kurachi J., Udagawa T., Kutsumizu S., Nat. Commun.,2019, 10(1), 1828 |
| 47 | Bossion A., Olazabal I., Aguirresarobe R. H., Marina S., Martín J., Irusta L., Taton D., Sardon H., Polym. Chem.,2019, 10(21), 2723—2733 |
| 48 | Lu H., Feng S., J. Polym. Sci., Part A: Polym. Chem.,2017, 55(5), 903—911 |
| 49 | Fawcett A. S., Brook M. A., Macromolecules,2014, 47(5), 1656—1663 |
| 50 | Mei J. F., Jia X. Y., Lai J. C., Sun Y., Li C. H., Wu J. H., Cao Y., You X. Z., Bao Z., Macromol. Rapid. Commun.,2016, 37(20), 1667—1675 |
| [1] | 高慧玲, 曹珍珍, 顾芳, 王海军. 氢键型水凝胶自修复行为的Monte Carlo模拟[J]. 高等学校化学学报, 2022, 43(11): 20220482. |
| [2] | 杨伟明, 席澳千, 杨斌, 曾艳宁. 基于多重动态共价键的环氧类玻璃网络的制备与性能[J]. 高等学校化学学报, 2022, 43(11): 20220308. |
| [3] | 许文哲, 张皓. 超分子相互作用主导的纳米药物成核[J]. 高等学校化学学报, 2022, 43(10): 20220264. |
| [4] | 魏哲宇, 吴志康, 茹诗, 倪鲁彬, 魏永革. 多酸-环糊精超分子体系的研究进展[J]. 高等学校化学学报, 2022, 43(1): 20210665. |
| [5] | 李聪, 刘欢欢, 杨桂花, 田中建, 颜家强, 吉兴香, 韩文佳, 陈嘉川. 基于肟-氨基甲酸酯的超强自修复水性聚氨酯胶粘剂的制备及性能分析[J]. 高等学校化学学报, 2021, 42(8): 2651. |
| [6] | 刘冬生. 超分子作用构筑具有高光学不对称性的表面等离子纳米粒子手性组装体[J]. 高等学校化学学报, 2021, 42(6): 1619. |
| [7] | 孟妍, 王秀凤, 张莉, 刘鸣华. 不同取代位置的萘衍生两亲分子在气/液界面组装膜中的螺旋结构与圆偏振发光[J]. 高等学校化学学报, 2021, 42(4): 1253. |
| [8] | 宋文尧, 周张浪, 杨鑫莉, 陈岚, 葛广路. 介孔二氧化硅对映选择性吸附的手性印迹调控[J]. 高等学校化学学报, 2021, 42(10): 3144. |
| [9] | 郭文瑾, 王晓晗, 李想, 李洋, 孙俊奇. 基于金属配位键的自修复、 可重塑聚丁二烯橡胶的合成及性能[J]. 高等学校化学学报, 2020, 41(9): 2090. |
| [10] | 张丹维, 王辉, 黎占亭. 水溶性三维有序超分子和共价有机聚合物[J]. 高等学校化学学报, 2020, 41(6): 1139. |
| [11] | 刘亚冰,李明阳,田戈,阿拉腾沙嘎,裴桐鹤,聂婧思. 基于2-氨基吡啶的两个簇基超分子化合物的合成、 结构及催化性能[J]. 高等学校化学学报, 2020, 41(5): 995. |
| [12] | 盛叶明,程波,卢珣. 基于多重可逆作用的自修复聚氨酯弹性体的制备及性能[J]. 高等学校化学学报, 2020, 41(3): 572. |
| [13] | 彭与煜,王煜,于鑫垚,曾巨澜,肖忠良,曹忠. 基于单(6-巯基-6-去氧)-β-环糊精修饰金电极对L-半胱氨酸的快速灵敏检测[J]. 高等学校化学学报, 2020, 41(2): 268. |
| [14] | 王星火,汤钧,杨英威. 由聚合物门控的介孔二氧化硅基刺激响应性药物递送系统[J]. 高等学校化学学报, 2020, 41(1): 28. |
| [15] | 林木松, 彭磊, 张晟, 李丽, 付强, 侯君波. 电缆护套光屏蔽自愈合涂料的制备及性能[J]. 高等学校化学学报, 2019, 40(8): 1766. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||