

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (5): 1091.doi: 10.7503/cjcu20190630
收稿日期:2019-12-04
出版日期:2020-05-10
发布日期:2020-03-06
通讯作者:
刘颖雅
E-mail:liu@dlut.edu.cn
基金资助:
LIU Hengshuo,YU Zhiquan,SUN Zhichao,WANG Yao,LIU Yingya( ),WANG Anjie
),WANG Anjie
Received:2019-12-04
Online:2020-05-10
Published:2020-03-06
Contact:
Yingya LIU
E-mail:liu@dlut.edu.cn
Supported by:摘要:
制备了一种含有联吡啶位点的共价有机骨架(COF)材料TpBpy, 并通过配体上的联吡啶位点固载铜盐实现了功能化, 得到的Cu@TpBpy具有大量的不饱和铜配位位点和高表面积, 可用作苯硼酸与咪唑的Chan-Lam偶联反应的多相催化剂. 通过优化溶剂、 铜源、 碱及反应时间等反应条件, 发现使用质子极性溶剂MeOH时的反应产率最高, 而Cu(OAc)2@TpBpy是效果最佳的催化剂, 可溶性有机碱三乙胺(TEA)的促进效果最好. Cu(OAc)2@TpBpy在碱TEA的促进下于70 ℃催化咪唑与苯硼酸反应4 h后, 得到目标产物1-苯基咪唑的最大产率为66%. 在最优反应条件下进行了底物拓展, 结果表明, 取代基的位阻效应对催化体系影响显著, 其中对位取代基的4-氯苯硼酸的产率最高(62%).
中图分类号:
TrendMD:
刘恒烁,遇治权,孙志超,王瑶,刘颖雅,王安杰. COF固载铜盐催化苯硼酸与咪唑的Chan-Lam偶联反应. 高等学校化学学报, 2020, 41(5): 1091.
LIU Hengshuo,YU Zhiquan,SUN Zhichao,WANG Yao,LIU Yingya,WANG Anjie. Copper Salt Anchored on a Covalent Organic Framework as Heterogeneous Catalyst for Chan-Lam Coupling Reaction †. Chem. J. Chinese Universities, 2020, 41(5): 1091.
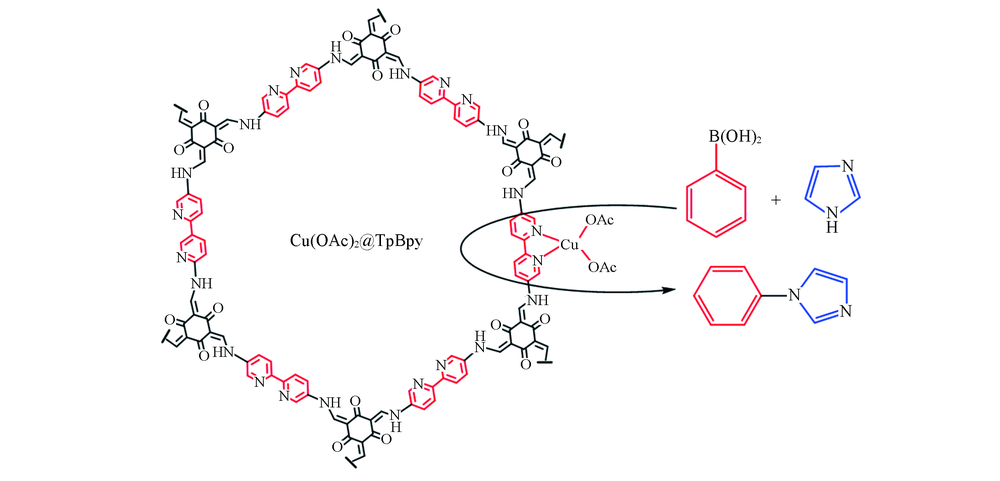
Scheme 2 Schematic reprentation of Cu(OAc)2 anchored on bipyridine sites of TpBpy to obtain Cu(OAc)2@TpBpy to catalyze the coupling reaction of imidazole and phenylboronic acid
| Entry | Cu source | Modification solvent |
|---|---|---|
| 1 | CuI | Acetonitrile |
| 2 | Cu(NO3)2·3H2O | Ethanol |
| 3 | CuCl2 | Ethanol |
| 4 | Cu2Cl2 | DMSO |
| 5 | Cu(OAc)2·H2O | Ethanol |
| 6 | CuSO4·5H2O | DMF |
| 7 | Cu(CF3SO3)2 | Acetonitrile |
Table 1 Corresponding solvents used for different copper sources
| Entry | Cu source | Modification solvent |
|---|---|---|
| 1 | CuI | Acetonitrile |
| 2 | Cu(NO3)2·3H2O | Ethanol |
| 3 | CuCl2 | Ethanol |
| 4 | Cu2Cl2 | DMSO |
| 5 | Cu(OAc)2·H2O | Ethanol |
| 6 | CuSO4·5H2O | DMF |
| 7 | Cu(CF3SO3)2 | Acetonitrile |
| Entry | Solvent | Catalyst | Base | GC yield(%) | Isolated yield(%) |
|---|---|---|---|---|---|
| 1b | EtOH | — | TEA | — | — |
| 2c | EtOH | TpBpy | TEA | — | — |
| 3d | EtOH | CuI@TpBpy | TEA | — | — |
| 4 | EtOH | CuI@TpBpy | TEA | 22 | |
| 5 | MeOH | CuI@TpBpy | TEA | 39 | 38 |
| 6 | MeOH | CuCl2@TpBpy | TEA | 23 | 21 |
| 7 | MeOH | Cu(NO3)2@TpBpy | TEA | 19 | |
| 8 | MeOH | Cu(CF3SO3)2@TpBpy | TEA | 61 | |
| 9 | MeOH | Cu2Cl2@TpBpy | TEA | 32 | |
| 10 | MeOH | CuSO4@TpBpy | TEA | 51 | |
| 11 | MeOH | Cu(OAc)2@TpBpy | TEA | 66 | |
| 12 | DMSO | Cu(OAc)2@TpBpy | TEA | 6 | |
| 13 | DMF | Cu(OAc)2@TpBpy | TEA | Trace | |
| 14 | 1,4-Dioxane | Cu(OAc)2@TpBpy | TEA | Trace | |
| 15 | DCM | Cu(OAc)2@TpBpy | TEA | — | |
| 16 | 1,2-Dichloroethane | Cu(OAc)2@TpBpy | TEA | — | |
| 17 | Toluene | Cu(OAc)2@TpBpy | TEA | Trace | |
| 18e | MeOH | Cu(OAc)2@TpBpy | — | 44 | |
| 19 | MeOH | Cu(OAc)2@TpBpy | Na2CO3 | 37 | |
| 20 | MeOH | Cu(OAc)2@TpBpy | K2CO3 | 18 | |
| 21 | MeOH | Cu(OAc)2@TpBpy | pyridine | 45 |
Table 2 Optimization of Chan-Lam coupling reaction conditionsa
| Entry | Solvent | Catalyst | Base | GC yield(%) | Isolated yield(%) |
|---|---|---|---|---|---|
| 1b | EtOH | — | TEA | — | — |
| 2c | EtOH | TpBpy | TEA | — | — |
| 3d | EtOH | CuI@TpBpy | TEA | — | — |
| 4 | EtOH | CuI@TpBpy | TEA | 22 | |
| 5 | MeOH | CuI@TpBpy | TEA | 39 | 38 |
| 6 | MeOH | CuCl2@TpBpy | TEA | 23 | 21 |
| 7 | MeOH | Cu(NO3)2@TpBpy | TEA | 19 | |
| 8 | MeOH | Cu(CF3SO3)2@TpBpy | TEA | 61 | |
| 9 | MeOH | Cu2Cl2@TpBpy | TEA | 32 | |
| 10 | MeOH | CuSO4@TpBpy | TEA | 51 | |
| 11 | MeOH | Cu(OAc)2@TpBpy | TEA | 66 | |
| 12 | DMSO | Cu(OAc)2@TpBpy | TEA | 6 | |
| 13 | DMF | Cu(OAc)2@TpBpy | TEA | Trace | |
| 14 | 1,4-Dioxane | Cu(OAc)2@TpBpy | TEA | Trace | |
| 15 | DCM | Cu(OAc)2@TpBpy | TEA | — | |
| 16 | 1,2-Dichloroethane | Cu(OAc)2@TpBpy | TEA | — | |
| 17 | Toluene | Cu(OAc)2@TpBpy | TEA | Trace | |
| 18e | MeOH | Cu(OAc)2@TpBpy | — | 44 | |
| 19 | MeOH | Cu(OAc)2@TpBpy | Na2CO3 | 37 | |
| 20 | MeOH | Cu(OAc)2@TpBpy | K2CO3 | 18 | |
| 21 | MeOH | Cu(OAc)2@TpBpy | pyridine | 45 |
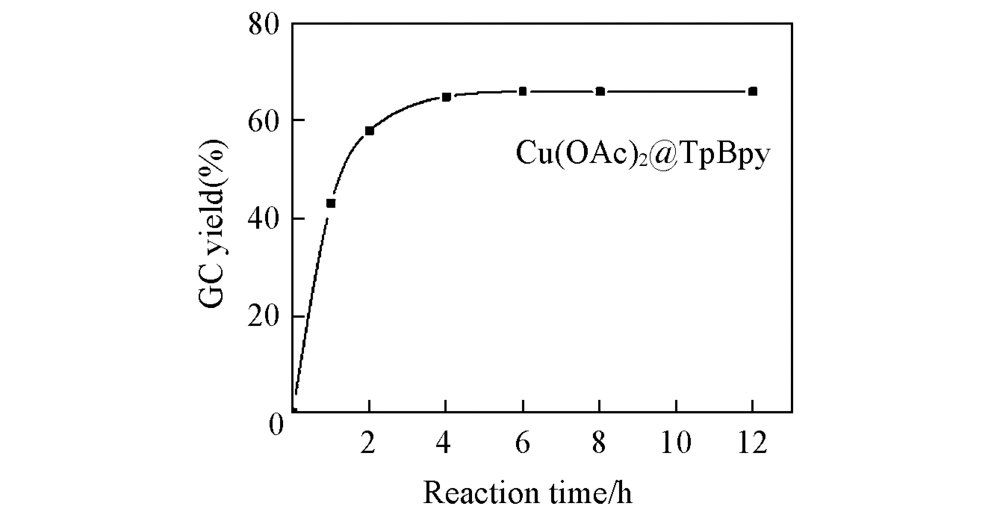
Fig.7 GC yield-time profile for the coupling reaction in methanol with Cu(OAc)2@TpBpy as catalyst Phenylboronic acid(0.82 mmol, 100 mg), imidazole(0.98 mmol), TEA(0.98 mmol), Cu(OAc)2@TpBpy(10%, molar fraction), MeOH 5 mL, 70 ℃.
| Entry | Solvent | Catalyst | Yieldb(%) |
|---|---|---|---|
| 1 | MeOH | CuI | 62 |
| 2 | MeOH | Cu(NO3)2·3H2O | 71 |
| 3 | MeOH | CuCl2 | 68 |
| 4 | MeOH | Cu2Cl2 | 68 |
| 5 | MeOH | Cu(OAc)2·H2O | 81 |
Table 3 Homogeneous reaction of imidazole and phenylboronic acida
| Entry | Solvent | Catalyst | Yieldb(%) |
|---|---|---|---|
| 1 | MeOH | CuI | 62 |
| 2 | MeOH | Cu(NO3)2·3H2O | 71 |
| 3 | MeOH | CuCl2 | 68 |
| 4 | MeOH | Cu2Cl2 | 68 |
| 5 | MeOH | Cu(OAc)2·H2O | 81 |
| Entry | Boronic acid | Amine | Product | Yield b(%) |
|---|---|---|---|---|
| 1 |  |  |  | 44 |
| 2 |  |  |  | 62 |
| 3 |  |  |  | 54 |
| 4 |  |  |  | 53 |
| 5 |  |  |  | Trace |
| 6 |  |  |  | Trace |
| 7 |  |  |  | 59 |
| 8 |  |  |  | 41 |
Table 4 Cu@TpBpy catalyzed N-arylation of imidazole with arylboronic acidsa
| Entry | Boronic acid | Amine | Product | Yield b(%) |
|---|---|---|---|---|
| 1 |  |  |  | 44 |
| 2 |  |  |  | 62 |
| 3 |  |  |  | 54 |
| 4 |  |  |  | 53 |
| 5 |  |  |  | Trace |
| 6 |  |  |  | Trace |
| 7 |  |  |  | 59 |
| 8 |  |  |  | 41 |
| [1] |
Pitzer J., Steiner K ., J. Biotechnol., 2016,235, 32—46
doi: 10.1016/j.jbiotec.2016.03.023 URL pmid: 26995609 |
| [2] |
Xu M., Zheng Z., Wang M., Kong L., Ao Y., Li Y., Org. Biomol. Chem, 2018,16(45), 8761—8768
doi: 10.1039/c8ob02419g URL pmid: 30402643 |
| [3] |
Jin X., Liu M. Y., Zhang D. F., Zhong X., Du K., Qian P., Gao H., Wei M. J., Pharmacol. Res., 2019,145, 104253—104253
doi: 10.1016/j.phrs.2019.104253 URL pmid: 31059788 |
| [4] | Kidwai M., Bansal V., Kumarb A., Mozumdarb S., Green Chem., 2007,9, 742—745 |
| [5] |
Rosen B. M., Quasdorf K. W., Wilson D. A., Zhang N., Resmerita A. M., Garg N. K., Percec V., Chem. Rev., 2011,111(3), 1346—1416
doi: 10.1021/cr100259t URL pmid: 21133429 |
| [6] | Shang R., Liu L., China Chem., 2011,54(11), 1670—1687 |
| [7] | Chan D. M. T., Monaco K. L., Wang R. P., Winters M. P., Tetrahedron Lett., 1998,39, 2933—2936 |
| [8] | Lam P. Y. S., Clarkt C. G., Saubernt S., Adamst J., Winters M. P., Chan D. M. T., Combst A., Tetrahedron Lett., 1998,39, 2941—2944 |
| [9] |
Munir I., Zahoor A. F., Rasool N., Naqvi S. A. R., Zia K. M., Ahmad R., Mol. Divers., 2019,23(1), 215—259
doi: 10.1007/s11030-018-9870-z URL pmid: 30159807 |
| [10] | Gajare S., Jagadale M., Naikwade A., Bansode P., Rashinkar G., Appl. Organomet. Chem, 2019,33(6), e4915 |
| [11] |
Kantam M. L., Roy M., Roy S., Sreedhar B., De R. L., Catal. Commun., 2008,9(13), 2226—2230
doi: 10.1158/1535-7163.MCT-13-1109 URL pmid: 24980946 |
| [12] | Islam S. M., Salam N., Mondal P., Roy A. S., Ghosh K., Tuhina K., J. Mol. Catal. A: Chem., 2014,387, 7—19 |
| [13] | Bukowska A., Bukowski W., Bester K., Hus K., Appl. Organomet. Chem, 2017,31(12), e3847 |
| [14] |
Dhakshinamoorthy A., Garcia H., Chem. Soc. Rev, 2014,43(16), 5750—5765
doi: 10.1039/c3cs60442j URL pmid: 24614959 |
| [15] |
Dhakshinamoorthy A., Asiri A. M., Garcia H., Chem. Soc. Rev., 2015,44(7), 1922—1947
doi: 10.1039/c4cs00254g URL pmid: 25608717 |
| [16] |
Arai T., Kawasaki N., Kanoh H ., Synlett., 2012,23(10), 1549—1553
doi: 10.1002/1097-4598(200010)23:10<1549::aid-mus11>3.0.co;2-0 URL pmid: 11003790 |
| [17] | Priyadarshini S., Amal Joseph P. J., Kantam M. L., Sreedhar B., Tetrahedron, 2013,69(31), 6409—6414 |
| [18] |
Ding S. Y., Wang W., Chem. Soc. Rev., 2013,42(2), 548—568
doi: 10.1039/c2cs35072f URL pmid: 23060270 |
| [19] | Wu M. X., Yang Y. W., Chinese Chem. Lett., 2017,28(6), 1135—1143 |
| [20] | Hu H., Yan Q., Ge R., Gao Y., Chinese J . Catal., 2018,39(7), 1167—1179 |
| [21] | Shinde D. B., Aiyappa H. B., Bhadra M., Biswal B. P., Wadge P., Kandambeth S., Garai B., Kundu T., Kurungot S., Banerjee R., J. Mater. Chem. A, 2016,4(7), 2682—2690 |
| [22] |
Ding S. Y., Gao J., Wang Q., Zhang Y., Song W. G., Su C. Y., Wang W., J. Am. Chem. Soc., 2011,133(49), 19816—19822
doi: 10.1021/ja206846p URL pmid: 22026454 |
| [23] |
Xu H., Gao J., Jiang D., Nat. Chem, 2015,7(11), 905—912
doi: 10.1038/nchem.2352 URL pmid: 26492011 |
| [24] | Han Y., Zhang M., Zhang Y. Q., Zhang Z. H., Green Chem., 2018,20(21), 4891—4900 |
| [25] | Puthiaraj P., Pitchumani K., Green Chem., 2014,16(9), 4223—4233 |
| [26] | Norini T., Guangbo W., Iuliia O., Nathalie D., Karen L., Rino M ., J. Catal., 2019,375, 242—248 |
| [27] | Nainmalai D., Palaniswamy S ., ChemCatChem, 2016,8, 1—9 |
| [28] |
Wu C. D., Li L., Shi L. X., Dalton Trans., 2009, (34), 6790—6794
doi: 10.1039/b823335g URL pmid: 19690690 |
| [29] |
Guan C., Feng Y., Zou G., Tang J ., Tetrahedron, 2017,73(49), 6906—6913
doi: 10.1016/j.tet.2017.10.043 URL |
| [30] |
Jia X., Peng P., Org. Biomol. Chem, 2018,16(46), 8984—8988
doi: 10.1039/c8ob02254b URL pmid: 30418460 |
| [31] | Li Z. H., Xue L. P., Wang L., Zhang S. T., Zhao B. T., Inorg. Chem. Commun., 2013,27, 119—121 |
| [32] | Islam S. M., Dey R. C., Roy A. S., Paul S., Mondal S., Transition Met. Chem., 2014,39(8), 961—969 |
| [33] |
Sharghi H., Sepehri S., Aberi M., Mol. Divers, 2017,21(4), 855—864
doi: 10.1007/s11030-017-9759-2 URL pmid: 28653129 |
| [34] |
James P. C., Min Z., Chi Z., Costanzo S., J. Org. Chem., 2001,66(23), 7892—7897
doi: 10.1021/jo010615u URL pmid: 11701055 |
| [35] |
Collman J. P., Zhong M., Organic Letters, 2000,2(9), 1233—1236
doi: 10.1021/ol000033j URL pmid: 10810715 |
| [36] |
King A. E., Brunold T. C., Stahl S. S., J. Am. Chem. Soc., 2009,131, 5044—5045
doi: 10.1021/ja9006657 URL pmid: 19309072 |
| [37] |
Vantourout J. C., Miras H. N., Isidro-Llobet A., Sproules S., Watson A. J., J. Am. Chem. Soc., 2017,139(13), 4769—4779
doi: 10.1021/jacs.6b12800 URL pmid: 28266843 |
| [38] | Khosravi A., Mokhtari J., Naimi-Jamal M. R., Tahmasebi S., Panahi L., RSC Adv., 2017,7(73), 46022—46027 |
| [1] | 丁杨, 王万辉, 包明. 多孔骨架固定分子催化剂催化CO2加氢制备甲酸研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220309. |
| [2] | 宋有为, 安江伟, 王征, 王旭慧, 权燕红, 任军, 赵金仙. Ag,Zn,Pd掺杂对铜基催化剂草酸二甲酯选择性加氢反应的影响[J]. 高等学校化学学报, 2022, 43(6): 20210842. |
| [3] | 李海勃, 肖长发, 江龙, 黄云, 淡宜. MCM-41分子筛负载氯化铝催化丙烯酸甲酯与1-辛烯共聚[J]. 高等学校化学学报, 2021, 42(9): 2974. |
| [4] | 王洋, 王思迪, 唐韶坤. 超临界二氧化碳中亚胺类共价有机骨架材料COF-LZU1的合成与表征[J]. 高等学校化学学报, 2020, 41(8): 1792. |
| [5] | 熊俊宇, 王姗姗, 许颜清, 胡长文. 原子级分散Fe-N-C温和条件下选择性氧化催化特性[J]. 高等学校化学学报, 2020, 41(6): 1262. |
| [6] | 王艳燕, 刘会贞, 韩布兴. 多相催化剂催化二氧化碳加氢合成甲醇的研究进展[J]. 高等学校化学学报, 2020, 41(11): 2393. |
| [7] | 刘小舟, 管新宇, 方千荣, 金永日. 室温离子液体法合成新型三维共价有机骨架材料[J]. 高等学校化学学报, 2019, 40(7): 1341. |
| [8] | 黄薇薇, 任家旺, 方千荣, VALTCHEV Valentin. 核壳结构分子筛β@IISERP-COF2小球的合成[J]. 高等学校化学学报, 2018, 39(6): 1127. |
| [9] | 汪小创, 张洁, 谢建伟. 水相铜/十二烷基取代酰肼-吡啶-N-氧化物催化咪唑的N-芳基化[J]. 高等学校化学学报, 2017, 38(7): 1178. |
| [10] | 杨明, 汤甲, 王静静, 范爽, 张欢, 陶柳实, 谭丽. Fe-MIL-101催化Paal-Knorr反应合成吡咯衍生物[J]. 高等学校化学学报, 2016, 37(1): 108. |
| [11] | 郑彦军, 李江, 卢俊金, 贾玉才, 李宝林. 磺化笼型介孔碳催化的1,1-二乙酸酯的选择性合成[J]. 高等学校化学学报, 2013, 34(12): 2738. |
| [12] | 裴翠颖 曲凤玉. 羟基官能化共价有机骨架化合物的设计合成及性质[J]. 高等学校化学学报, 2011, 32(6): 1234. |
| [13] | 王恒山, 粟武, 刘大学, 杨晓武, 达朝山, 王锐. 树脂固定化手性联萘酚-钛催化剂的合成及其催化特性研究[J]. 高等学校化学学报, 2000, 21(10): 1524. |
| [14] | 杨意泉, 林种玉, 车长针, 袁友珠, 林仁存, 董远群. 改进型铜基甲醇含成催化剂NC208的活性相谱学表征[J]. 高等学校化学学报, 1997, 18(1): 103. |
| [15] | 胡云行, 万惠霖, 蔡启瑞. 铜基甲醇合成催化剂TPR导数谱[J]. 高等学校化学学报, 1994, 15(10): 1550. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||