

高等学校化学学报 ›› 2017, Vol. 38 ›› Issue (2): 206.doi: 10.7503/cjcu20160624
朱龙宝1, 陶玉贵1, 葛飞1, 李婉珍1, 刘义2( ), 堵国成3
), 堵国成3
收稿日期:2016-09-02
出版日期:2017-02-10
发布日期:2016-12-19
作者简介:联系人简介: 刘 义, 男, 博士, 副教授, 主要从事酶工程方面的研究. E-mail: 基金资助:
ZHU Longbao1, TAO Yugui1, GE Fei1, LI Wanzhen1, LIU Yi2,*( ), DU Guocheng3
), DU Guocheng3
Received:2016-09-02
Online:2017-02-10
Published:2016-12-19
Contact:
LIU Yi
E-mail:myputer@163.com
Supported by:摘要:
克隆Streptomyces maritimus来源的苯丙氨酸变位酶(SmPAM)基因, 并进行异源表达, 制备了重组SmPAM, 用于一步合成高附加值的β-芳香丙氨酸. 提取S. maritimus的基因组, 设计1对特异性引物, 采用PCR扩增编码SmPAM的结构基因pam, 与表达载体pET28a连接, 构建重组表达质粒pET28a-pam, 转入E.coli BL21中表达, 采用亲和层析柱分离纯化重组酶SmPAM. 结果表明, 克隆得到编码523个氨基酸长度的SmPAM基因pam, 并在大肠杆菌中实现了高效表达, 制得电泳纯的重组SmPAM. 该酶在最适温度30 ℃, pH=9.0条件下活力达到2.5 U/mg, 具有较高的热稳定性和pH稳定性, 在60~70 ℃下保持3 h未见活性下降, 在pH=9~11保持24 h, 残余酶活力达到98%. SmPAM具有较宽的底物谱, 可催化苯环上携带不同基团的3-芳香丙烯酸合成β-芳香丙氨酸, 当苯环上携带吸电子基团时催化反应更易完成, 其中2-硝基-β-苯丙氨酸的产率最高, 达到93%.
中图分类号:
TrendMD:
朱龙宝, 陶玉贵, 葛飞, 李婉珍, 刘义, 堵国成. Streptomyces maritimus苯丙氨酸变位酶的制备、表征及催化合成β-芳香丙氨酸. 高等学校化学学报, 2017, 38(2): 206.
ZHU Longbao, TAO Yugui, GE Fei, LI Wanzhen, LIU Yi, DU Guocheng. Production and Characterization of Phenylalanine Aminomutase from Streptomyces Maritimus and Synthesis of β-Arylalanine†. Chem. J. Chinese Universities, 2017, 38(2): 206.
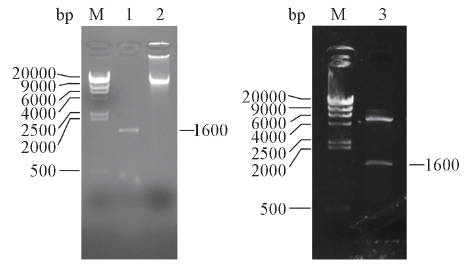
Fig.1 Agarose gel electrophoresis of gene DNA, PCR products and the digested pET28a-pamM: marker; lane 1: product of PCR; lane 2: genomeDNA; lane 3: the pET28a-pam was digested by EcoRⅠ and NdeⅠ.
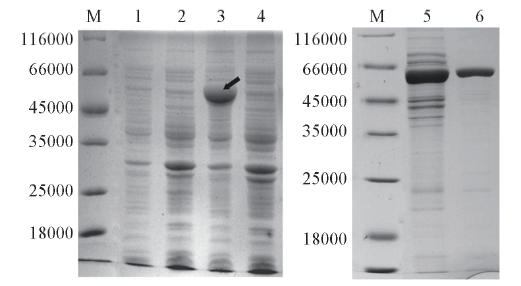
Fig.2 SDS-PAGE of recombinant enzyme expressed in E.coli BL21(DE3) and purification of the recombinant SmPAM using HisTrapTM/FFM: marker; lane 1: E.coli BL21; lane 2: cell extracts of E.coli BL21/pET28a-pam without induction by IPTG; lanes 3 and 5: the supernatant fraction after sonication of E.coli BL21/pET28a-pam induced by IPTG(the arrow in lane 3 represents the expressed SmPAM); lane 4: the precipitated fraction after sonication of E.coli BL21/pET28a-pam induced by IPTG; lane 6: the purified SmPAM by HisTrap TM/Faffinity chromatography column.
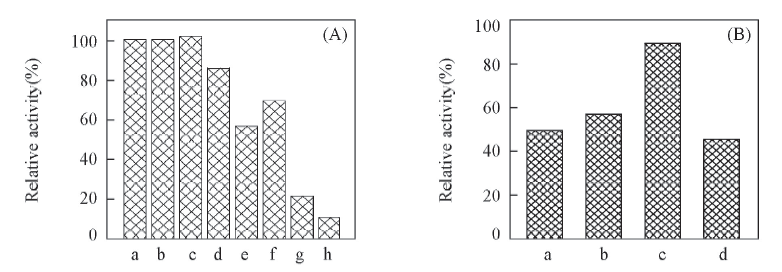
Fig.6 Effects of metal ions(A) and surfactants(B) on the activity of recombinant SmPAM(A) a. Na+; b. Mg2+; c. Ca2+; d. Fe2+; e. Cu2+; f. Zn2+; g. Mn2+; h. Co3+; (B) a. CTAB; b. Triton X-100; c. Tween 80; d. SDS.
| [1] | Kudo F., Miyanaga A ., Eguchi T., Nat. Prod. Rep., 2014, 31(8),1056—1073 |
| [2] | Grayson J. I., Roos J., Osswald S., Org. Process. Res. Dev., 2011, 15(5), 1201—1206 |
| [3] | Zhang X. H., Ye W. J., Wang K. L., TianY. S., Xiao X., Chem. Res. Chinese Universities,2015, 31(2), 203—207 |
| [4] | Ratnayake N. D., Theisen C., Walter T., Walker K. D., J. Biotechnol., 2016, 10(217), 12—21 |
| [5] | Liu J. Z., Xiong J. B., Zhao G. H., Liu Q., Jiao Q. C., Chem. J. Chinese Universities,2010, 31(11), 2234—2238 |
| (刘均忠, 熊吉滨, 赵根海, 刘茜, 焦庆才. 高等学校化学学报, 2010, 31(11), 2234—2238) | |
| [6] | Li D. C., Jia L., Wang X. F., Wei D. Z., Prep. Biochem. Biotechnol,2013, 43(2), 207—216 |
| [7] | Mathew S., Bea H., Nadarajan S. P., Chung T., Yun H., J. Biotechnol., 2015, 20(196—197), 1—8 |
| [8] | Mathew S., Jeong S. S., Chung T., Lee S. H., Yun H., Biotechnol. J., 2016, 11(1),185—190 |
| [9] | Zhang K., Liang X., He M., Wu J., Zhang Y., Xue W., Jin L., Yang S., Hu D., Molecules,2013, 18(6), 6142—6152 |
| [10] | Heberling M. M., Wu B., Bartsch S., Janssen D. B., Curr. Opin. Chem. Biol., 2013, 17(2), 250—260 |
| [11] | Lohman J. R., Shen B., Methods Enzymol., 2012, 516, 299—319 |
| [12] | Cooke H. A., Christianson C. V., Bruner S. D., Curr. Opin. Chem. Biol., 2009, 13(4), 460—468 |
| [13] | Walter T., King Z., Walker K. D.,Biochemistry,2016, 55(1), 1—4 |
| [14] | Weise N. J., Parmeggiani F., Ahmed S. T., Turner N. J., J. Am. Chem. Soc., 2015, 137(40), 12977—12983 |
| [15] | Lovelock S. L., Lloyd R. C., Turner N. J., Angew. Chem. Int. Ed. Engl., 2014, 53, 4652—4656 |
| [16] | Heberling M. M., Masman M. F., Bartsch S., Wybenga G. G., Dijkstra B. W., Marrink S. J., Janssen D. B., ACS Chem. Biol., 2015, 10(4), 989—997 |
| [17] | Zhu L. B., Cui W., Fang Y. Q., Liu Y., Gao X. X., Zhou Z. M., Biotechnol. Lett., 2013, 35, 751—756 |
| [18] | Parmeggiani F., Lovelock S. L., Weise N. J., Ahmed S. T., Turner N. J., Angew. Chem. Int. Ed. Engl., 2015, 54(15), 4608—4611 |
| [19] | Wu B., Szymanski W., de Wildeman S., Poelarends G. J., Feringa B. L., Janssen D. B., Adv. Synth. Catal., 2010, 352(9), 1409—1412 |
| [20] | Wu B., Szymanski W., Wybenga G. G., Heberling M. M., Stefaan de Wildeman S. B., Poelarends G. J., Dijkstra B. L., Bauke W., Janssen D. B., Angew. Chem. Int. Ed. Engl., 2012, 51(2), 482—486 |
| [21] | Feng L., Wanninayake U., Strom S., Geiger J., Walker K. D., Biochemistry,2011, 50(14), 2919—2930 |
| [22] | Wu B., Szymanski W., Wietzes P., de Wildeman S., Poelarends G. J., Feringa B. L., Janssen D. B., ChemBioChem,2009, 10(2), 338—344 |
| [1] | 唐玉静 胡敏 王霞 王启刚. 载酶纳米催化体系用于疾病诊疗研究进展[J]. 高等学校化学学报, 0, (): 20220640. |
| [2] | 常丽颖 凌鑫宇 陈和祺 王雪 刘涛. 基因编辑在线粒体疾病中的应用[J]. 高等学校化学学报, 2022, 43(Album-4): 20220363. |
| [3] | 曹舒杰, 李泓君, 管文丽, 任梦田, 周传政. 硫代磷酸酯寡聚核苷酸的立体控制合成研究进展[J]. 高等学校化学学报, 2022, 43(Album-4): 20220304. |
| [4] | 徐永斌 冯帅霞 陈洁 龚欢 施松善 王辉俊 王顺春. 红花均一多糖的结构表征及其抑制HepG2增殖活性[J]. 高等学校化学学报, 0, (): 20220600. |
| [5] | 仵宇帅, 尚颖旭, 蒋乔, 丁宝全. 可控自组装DNA折纸结构作为药物载体的研究进展[J]. 高等学校化学学报, 2022, 43(8): 20220179. |
| [6] | 刘文婷 刘柳宜 朱博琛 毛宗万. 核酸G-四链体的识别、复合物结构与细胞内探测的研究进展[J]. 高等学校化学学报, 0, (): 20220419. |
| [7] | 胡玉灿 曹朝辉 郑灵刚 沈俊涛 赵维 戴磊. CRISPR-Cas基因编辑技术在微生物组工程的应用[J]. 高等学校化学学报, 0, (): 20220362. |
| [8] | 方鑫 赵瑞奇 莫婧 王雅芬 翁小成. 检测核酸表观遗传修饰的测序方法[J]. 高等学校化学学报, 0, (): 20220342. |
| [9] | 张凯嵩 王少儒 张雨桐 田沺. 基于超分子化学的核酸表观遗传修饰研究[J]. 高等学校化学学报, 0, (): 20220335. |
| [10] | 朱凯, 利婕, 武潇逸, 胡薇薇, 吴冬梅, 虞诚潇, 葛志伟, 叶兴乾, 陈士国. 基于多孔石墨化碳柱-四极杆-飞行时间质谱解析甜菜果胶精细结构[J]. 高等学校化学学报, 2022, 43(6): 20220023. |
| [11] | 付俊, 吴美婵, 王书珍, 邵秀丽, 何峰. 基于标记法的福白菊精油抗真菌机理研究[J]. 高等学校化学学报, 2021, 42(12): 3657. |
| [12] | 赵卓, 王雪强. 核酸适体偶联药物的生物偶联构建技术与应用[J]. 高等学校化学学报, 2021, 42(11): 3367. |
| [13] | 陈旺, 胡代花, 刘格歌. 由醋酸去氢表雄酮合成熊去氧胆酸[J]. 高等学校化学学报, 2021, 42(9): 2782. |
| [14] | 胡皓程, 李文利, 张嘉宁, 刘宇博. 黑木耳寡糖的提取、 结构表征及生物活性[J]. 高等学校化学学报, 2021, 42(8): 2465. |
| [15] | 杨依然, 姚华, 闫江红, 孙志恒, 张余, 房雪晴, 李绪文, 金永日. 薤中新的甾体皂苷类化学成分[J]. 高等学校化学学报, 2021, 42(6): 1742. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||