

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (10): 2264.doi: 10.7503/cjcu20200246
陈淡宜, 张福梅, 何丹, 张紫媚, 钟芬, 文思妙妙, 刘祈星( ), 周海峰(
), 周海峰( )
)
收稿日期:2020-05-03
出版日期:2020-10-10
发布日期:2020-06-12
通讯作者:
刘祈星
E-mail:qixingliu86@163.com;zhouhf@ctgu.edu.cn
作者简介:周海峰, 男, 博士, 教授, 主要从事不对称催化方面的研究. E-mail: 基金资助:
CHEN Danyi, ZHANG Fumei, HE Dan, ZHANG Zimei, ZHONG Fen, WEN Simiaomiao, LIU Qixing( ), ZHOU Haifeng(
), ZHOU Haifeng( )
)
Received:2020-05-03
Online:2020-10-10
Published:2020-06-12
Contact:
LIU Qixing
E-mail:qixingliu86@163.com;zhouhf@ctgu.edu.cn
Supported by:摘要:
以苯基/苯并噻唑甲酮为原料, 手性二胺钌络合物为催化剂, 甲酸钠为氢源, i?PrOH/H2O(体积比1:1)为溶剂, 在室温(30 ℃)条件下, 通过不对称转移氢化, 合成了一系列手性苯基/苯并噻唑甲醇类化合物, 其对映选择性(e.e.)值高达99%. 此外, 还探讨了其它苯基/氮杂环甲酮的不对称转移氢化. 该方法具有反应条件温和、 催化剂廉价易得及操作简便等优点.
中图分类号:
TrendMD:
陈淡宜, 张福梅, 何丹, 张紫媚, 钟芬, 文思妙妙, 刘祈星, 周海峰. 钌催化的手性苯基/苯并噻唑甲醇的转移氢化合成. 高等学校化学学报, 2020, 41(10): 2264.
CHEN Danyi, ZHANG Fumei, HE Dan, ZHANG Zimei, ZHONG Fen, WEN Simiaomiao, LIU Qixing, ZHOU Haifeng. Synthesis of Chiral Phenylbenzothiazole Methanol via Transfer Hydrogenation Catalyzed by Ruthenium Complexes†. Chem. J. Chinese Universities, 2020, 41(10): 2264.
| Compd. | Appearance | Yield(%) | m.p./℃ | Compd. | Appearance | Yield(%) | m.p./℃ |
|---|---|---|---|---|---|---|---|
| 2a | White solid | 89 | 110—113 | 2n | Yellow solid | 88 | 130—132 |
| 2b | White solid | 77 | 119—121 | 2o | White solid | 55 | 93—95 |
| 2c | White solid | 73 | 111—113 | 2p | White solid | 74 | 153—155 |
| 2d | White solid | 89 | 110—112 | 2q | Yellow solid | 81 | 123—125 |
| 2e | White solid | 75 | 63—65 | 2r | Yellow solid | 83 | 131—133 |
| 2f | Yellow solid | 78 | 114—116 | 2s | White solid | 75 | 86—88 |
| 2g | White solid | 81 | 108—110 | 2t | White solid | 85 | 75—77 |
| 2h | White solid | 69 | 115—117 | 2u | White solid | 84 | 136—138 |
| 2i | Yellow solid | 74 | 145—147 | 2v | White solid | 52 | 135—137 |
| 2j | Yellow solid | 70 | 126—128 | 2w | White solid | 74 | 151—153 |
| 2k | White solid | 82 | 125—127 | 2x | White solid | 76 | 72—74 |
| 2l | White solid | 83 | 100—102 | 2y | White solid | 77 | 123—125 |
| 2m | Yellow solid | 77 | 179—181 | 2z | White solid | 88 | 107—109 |
Table 1 Appearance, yields and melting point data of target compounds 2a—2z
| Compd. | Appearance | Yield(%) | m.p./℃ | Compd. | Appearance | Yield(%) | m.p./℃ |
|---|---|---|---|---|---|---|---|
| 2a | White solid | 89 | 110—113 | 2n | Yellow solid | 88 | 130—132 |
| 2b | White solid | 77 | 119—121 | 2o | White solid | 55 | 93—95 |
| 2c | White solid | 73 | 111—113 | 2p | White solid | 74 | 153—155 |
| 2d | White solid | 89 | 110—112 | 2q | Yellow solid | 81 | 123—125 |
| 2e | White solid | 75 | 63—65 | 2r | Yellow solid | 83 | 131—133 |
| 2f | Yellow solid | 78 | 114—116 | 2s | White solid | 75 | 86—88 |
| 2g | White solid | 81 | 108—110 | 2t | White solid | 85 | 75—77 |
| 2h | White solid | 69 | 115—117 | 2u | White solid | 84 | 136—138 |
| 2i | Yellow solid | 74 | 145—147 | 2v | White solid | 52 | 135—137 |
| 2j | Yellow solid | 70 | 126—128 | 2w | White solid | 74 | 151—153 |
| 2k | White solid | 82 | 125—127 | 2x | White solid | 76 | 72—74 |
| 2l | White solid | 83 | 100—102 | 2y | White solid | 77 | 123—125 |
| 2m | Yellow solid | 77 | 179—181 | 2z | White solid | 88 | 107—109 |
| Compd. | 1H NMR(400 Hz), δ | 13C NMR(100 Hz), δ |
|---|---|---|
| 2a | 8.00(d, J=8.0 Hz, 1H), 7.86(d, J=8.0 Hz, 1H), 7.61—7.59(m, 1H), 7.51—7.48(m, 1H), 7.42—7.37(m, 1H), 7.31—7.28(m, 2H), 7.24—7.22(m, 1H), 6.38(s, 1H), 4.72(s, 1H), 2.44(s, 3H) | 175.4, 152.6, 139.1, 136.1, 135.3, 130.9, 128.6, 126.9, 126.5, 126.1, 125.2, 123.1, 121.8, 71.4, 19.5 |
| 2b | 8.02(d, J=8.0 Hz, 1H), 7.87(d, J=8.0 Hz, 1H), 7.61(dd, J1=J2=7.6 Hz,1H), 7.50(dd, J1=J2=7.6 Hz,1H), 7.42—7.34(m, 2H), 7.20(dd, J1=J2=7.6 Hz,1H), 7.13(dd, J1=J2=7.6 Hz,1H), 6.51(s, 1H), 4.66(s, 1H) | 173.9, 161.4(d, J=246.4 Hz), 152.5, 135.3, 130.4(d, J=8.2 Hz), 128.4(d, J=3.7 Hz), 128.2(d, J=14.0 Hz), 126.2, 125.3, 124.7(d, J=3.4 Hz), 123.1, 121.8, 115.8(d, J=21.1 Hz), 68.2(d, J=3.8 Hz) |
| 2c | 8.03(d, J=8.4 Hz, 1H), 7.86(d, J=8.0 Hz, 1H), 7.70—7.68(m, 1H), 7.52—7.47(m, 1H), 7.46—7.43(m, 1H), 7.40(d, J=7.2 Hz, 1H), 7.36—7.30(m, 2H), 6.64(s, 1H), 4.83(s, 1H) | 173.6, 152.3, 138.6, 135.3, 132.9, 129.8, 129.7, 128.6, 127.4, 126.2, 125.3, 123.1, 121.8, 70.8 |
| 2d | 8.06(d, J=8.0 Hz, 1H), 7.87(d, J=8.0 Hz, 1H), 7.68—7.63(m, 2H), 7.54—7.49(m, 1H), 7.44—7.38(m, 2H), 7.28—7.24(m, 1H), 6.60(d, J=4.0 Hz, 1H), 4.57(d, J=4.4 Hz, 1H) | 173.3, 152.3, 140.2, 135.5, 133.0, 130.2, 128.9, 128.1, 126.2, 125.4, 123.2, 122.9, 121.8, 73.1 |
| 2e | 8.07(d, J=8.0 Hz, 1H), 7.87(d, J=8.0 Hz, 1H), 7.77(d, J=8.0 Hz, 2H), 7.64—7.59(m,1H), 7.55—7.49(m, 2H), 7.45—7.40(m, 1H), 6.58(d, J=3.6 Hz, 1H), 4.59(d, J=4.0 Hz, 1H) | 173.4, 152.2, 139.6, 135.6, 132.6, 129.7, 128.8, 128.2(q, J=30.2 Hz), 126.3, 125.9(q, J=5.5 Hz), 125.6(q, J=254.4 Hz), 125.4, 123.3, 121.8, 69.4(q, J=3.6 Hz) |
| 2f | 9.17(s, 1H), 7.99(d, J=8.0 Hz, 1H), 7.87(d, J=7.6 Hz, 1H), 7.53—7.48(m, 1H), 7.44—7.40(m, 2H), 7.31—7.24(m, 1H), 7.02—6.95(m, 2H), 6.40(s, 1H), 4.51(s, 1H) | 175.9, 154.7, 152.0, 134.5, 130.0, 126.7, 126.6, 126.5, 125.4, 122.7, 121.9, 120.7, 118.6, 72.1 |
| 2g | 8.02(d, J=8.0 Hz, 1H), 7.90(d, J=8.0 Hz, 1H), 7.57—7.50(m, 3H), 7.44—7.39(m, 1H), 7.14—7.10(m, 2H), 6.17(s, 1H), 4.04(s, 1H) | 174.6, 164.1(d, J=246.2 Hz), 152.6, 136.8, 135.3, 128.7(d, J=8.3 Hz), 126.3, 125.3, 123.1, 121.9, 116.0(d, J=21.6 Hz), 73.8 |
| 2h | 8.02(d, J=7.6 Hz, 1H), 7.89(d, J=7.6 Hz, 1H), 7.54—7.50(m, 3H), 7.44—7.39(m, 3H), 6.17(s, 1H), 3.98(s, 1H) | 174.2, 152.5, 139.4, 135.3, 134.6, 129.0, 128.1, 126.3, 125.4, 123.2, 121.9, 73.8 |
| 2i | 8.30(d, J=8.4 Hz, 2H), 8.04(d, J=8.0 Hz, 1H), 7.91(d, J=8.0 Hz, 1H), 7.82(d, J=8.4 Hz, 2H), 7.56—7.52(m, 1H), 7.46—7.42(m, 1H), 6.31(s, 1H), 4.21(s, 1H) | 173.0, 152.4, 147.9, 147.5, 135.1, 127.5, 126.5, 125.7, 124.0, 123.3, 122.0, 73.4 |
| 2j | 8.03(d, J=8.4 Hz, 1H), 7.89(d, J=8.0 Hz, 1H), 7.74—7.67(m, 4H), 7.55—7.50(m, 1H), 7.45—7.40(m, 1H), 6.26(s, 1H), 4.16(s, 1H) | 173.7, 152.5, 144.6, 135.2, 130.9(q, J=36.3 Hz), 127.0, 126.4, 125.9(q, J=3.8 Hz), 125.5, 125.4(q, J=271.8 Hz), 123.2, 121.9, 73.8 |
| 2k | 8.03(d, J=8.0 Hz, 1H), 7.87(d, J=8.0 Hz, 1H), 7.52—7.47(m, 1H), 7.46(d, J=8.0 Hz, 2H), 7.42—7.37(m, 1H), 7.23(d, J=8.0 Hz, 2H), 6.15(s, 1H), 3.97(s, 1H), 2.39(s, 3H) | 175.2, 152.7, 138.6, 138.1, 135.3, 129.6, 126.7, 126.1, 125.1, 123.1, 121.8, 74.3, 21.3 |
| 2l | 8.03(d, J=8.0 Hz, 1H), 7.88(d, J=8.0 Hz, 1H), 7.53—7.46(m, 3H), 7.43—7.38(m, 1H), 6.96(d, J=8.8 Hz, 2H), 6.14(s, 1H), 3.84(s, 3H), 3.80(s, 1H) | 175.2, 159.9, 152.7, 133.2, 133.2, 128.2, 126.1, 125.1, 123.1, 121.8, 114.2, 74.1, 55.3 |
| 2m | 9.45(s, 1H), 8.09(d, J=8.0 Hz, 1H), 7.91(d, J=8.0 Hz, 1H), 7.50—7.45(m, 1H), 7.42—7.38(m, 1H), 7.30(d, J=8.4 Hz, 2H), 6.82(d, J=4.0 Hz, 1H), 6.75(d, J=8.4 Hz, 2H), 5.95(d, J=4.4 Hz, 1H) | 178.6, 157.4, 153.5, 134.7, 133.2, 128.3, 126.4, 125.2, 122.9, 122.7, 115.5, 73.1 |
| 2n | 8.03(d, J=8.0 Hz, 1H), 7.88(d, J=8.0 Hz, 1H), 7.53(m, 3H), 7.16—7.12(m, 2H), 6.92(d, J=8.0 Hz, 1H), 6.16(s, 1H), 3.91(s, 1H), 3.84(s, 3H) | 174.7, 160.0, 152.6, 142.5, 135.4, 129.9, 126.2, 125.2, 123.1, 121.8, 119.0, 114.4, 112.1, 74.4, 55.3 |
| 2o | 8.01(d, J=8.4 Hz, 1H), 7.90—7.87(m, 2H), 7.76(d, J=8.0 Hz, 1H), 7.64(d, J=7.6 Hz, 1H), 7.55—7.49(m, 2H), 7.44—7.39(m, 1H), 6.26(d, J=1.6 Hz, 1H), 4.51(s, 1H) | 174.0, 152.4, 141.8, 135.2, 131.3(q, J=32.2 Hz), 130.1, 129.3, 126.4, 125.5, 125.4(q, J=3.6 Hz), 125.3(q, J=270.7 Hz), 123.5(q, J=4.0 Hz), 123.1, 121.9, 73.7 |
| Compd. | 1H NMR(400 Hz), δ | 13C NMR(100 Hz), δ |
| 2p | 8.49(s, 1H), 8.22(d, J=8.0 Hz, 1H), 8.01(d, J=8.0 Hz, 1H), 7.93(d, J=8.0 Hz, 1H), 7.89(d, J=8.0 Hz, 1H), 7.59(dd, J1=J2=7.6 Hz,1H), 7.54—7.50(m, 1H), 7.45—7.41(m, 1H), 6.31(d, J=2.4 Hz, 1H), 4.55(d, J=3.2 Hz, 1H) | 173.4, 152.4, 148.5, 142.8, 135.1, 132.8, 129.8, 126.5, 125.6, 123.5, 123.2, 122.0, 121.7, 73.2 |
| 2q | 8.04(d, J=8.0 Hz, 1H), 7.87(d, J=8.0 Hz, 1H), 7.75(d, J=2.8 Hz, 1H), 7.54—7.49(m, 1H), 7.44—7.40(m, 1H), 7.36(d, J=8.4 Hz, 1H), 7.32—7.29(m, 1H), 6.58(d, J=3.2 Hz, 1H), 5.15(d, J=3.6 Hz, 1H) | 172.7, 152.1, 140.2, 135.3, 133.5, 130.9, 130.8, 129.9, 128.6, 126.4, 125.6, 123.2, 121.9, 70.3 |
| 2r | 8.01(d, J=7.6 Hz, 1H), 7.91(d, J=8.0 Hz, 1H), 7.52(dt, J1=7.6 Hz, J2=1.2 Hz, 1H), 7.44—7.32(m, 2H), 7.00—6.93(m, 2H), 6.53(s, 1H), 4.26(s, 1H) | 173.1(d, J=2.8 Hz), 162.4(dd, J1=256.9 Hz, J2=7.5 Hz), 152.7, 135.3, 131.0(t, J=10.5 Hz), 126.2, 125.2, 123.2, 121.8, 117.2, 112.2(dd, J1=19.3 Hz, J2=5.7 Hz), 65.7(t, J=3.7 Hz) |
| 2s | 8.03(d, J=8.0 Hz, 1H), 7.89(d, J=8.0 Hz, 1H), 7.60—7.49(m, 2H), 7.44—7.40(m, 1H), 6.97—6.86(m, 2H), 6.45(s, 1H), 4.52(s, 1H) | 173.4, 164.4(dd, J1=248.8 Hz, J2=11.9 Hz), 161.5(dd, J1=247.2 Hz, J2=12.0 Hz), 152.4, 135.2, 129.5(dd, J1=9.7 Hz, J2=4.9 Hz), 126.3, 125.4, 124.4(d, J=17.0 Hz), 123.2, 121.8, 111.8(dd, J1=21.3 Hz, J2=3.7 Hz), 104.2(dd, J1=J2=25.2 Hz), 67.8(d, J=3.5 Hz) |
| 2t | 8.02(d, J=8.0 Hz, 1H), 7.87(d, J=8.0 Hz, 1H), 7.51—7.38(m, 3H), 6.73(d, J=8.0 Hz, 1H), 6.68(d, J=8.0 Hz, 1H) 6.41(s, 1H), 4.53(s, 1H), 3.82(s, 3H) | 174.5, 162.1(d, J=246.1 Hz), 161.2(d, J=11.0 Hz), 152.6, 135.3, 129.2(d, J=5.4 Hz), 126.2, 125.2, 123.1, 121.8, 120.4(d, J=13.5 Hz), 110.4(d, J=2.9 Hz), 101.9(d, J=24.9 Hz), 68.1, 55.6 |
| 2u | 9.25(s, 1H), 7.99(d, J=8.4 Hz, 1H), 7.93(d, J=8.4 Hz, 1H), 7.55—7.51(m, 1H), 7.46—7.42(m, 1H), 7.23(dd, J1=9.6 Hz, J2=2.4 Hz, 1H), 6.99—6.92(m, 2H), 6.42(s, 1H), 3.93(s, 1H) | 175.8, 158.3(d, J=237.4 Hz), 151.9, 150.2, 134.1, 128.8(d, J=7.1 Hz), 126.6, 125.5, 122.7, 122.0, 119.9(d, J=7.7 Hz), 116.2(d, J=22.9 Hz), 112.6(d, J=24.7 Hz), 70.8 |
| 2v | 8.04—8.00(m, 2H), 7.89—7.84(m, 4H), 7.63(dd, J1=8.8 Hz, J2=2.0 Hz, 1H), 7.55—7.51(m, 2H), 7.49—7.45(m, 1H), 7.40—7.36(m, 1H), 6.34(s, 1H), 4.66(s, 1H) | 175.2, 152.6, 138.3, 135.3, 133.4, 133.2, 128.8, 128.3, 127.8, 126.5, 126.4, 126.2, 126.0, 125.2, 124.3, 123.1, 121.8, 74.5 |
| 2w | 8.25—8.22(m, 1H), 8.02(d, J=8.0 Hz, 1H), 7.93—7.90(m, 2H), 7.82—7.79(m, 2H), 7.56—7.46(m, 4H), 7.38(dd, J1=J2=7.6 Hz, 1H), 6.86(d, J=3.6 Hz, 1H), 4.87(d, J=3.2 Hz, 1H) | 175.3, 152.5, 136.3, 135.5, 134.0, 130.7, 129.6, 128.9, 126.6, 126.2, 125.9, 125.4, 125.3, 125.2, 123.8, 123.1, 121.8, 72.2 |
| 2x | 7.58—7.38(m, 7H), 7.34—7.24(m, 2H), 6.58(s, 1H), 5.99(d, J=4.0 Hz, 1H), 2.76(d, J=4.0 Hz, 1H) | 158.5, 155.1, 140.3, 128.7, 128.4, 128.1, 126.8, 124.4, 122.9, 121.2, 111.4, 140.1, 70.7 |
| 2y | 7.75—7.70(m, 1H), 7.60—7.56(m, 2H), 7.53—7.49(m, 1H), 7.45—7.34(m, 5H), 6.09(d, J=4.8 Hz, 1H), 4.29(d, J=5.2 Hz, 1H) | 166.6, 151.0, 140.3, 138.9, 128.9, 128.8, 126.9, 125.3, 124.6, 120.2, 110.9, 70.6 |
| 2z | 7.68—7.66(m, 1H), 7.52—7.49(m, 2H), 7.43—7.33(m, 3H), 7.31—7.29(m, 1H), 6.07(s, 1H), 4.90(s, 1H) | 175.0, 142.2, 141.6, 128.7, 128.4, 126.6, 119.6, 73.6 |
Table 2 1H NMR and 13C NMR data of target compounds 2a—2z
| Compd. | 1H NMR(400 Hz), δ | 13C NMR(100 Hz), δ |
|---|---|---|
| 2a | 8.00(d, J=8.0 Hz, 1H), 7.86(d, J=8.0 Hz, 1H), 7.61—7.59(m, 1H), 7.51—7.48(m, 1H), 7.42—7.37(m, 1H), 7.31—7.28(m, 2H), 7.24—7.22(m, 1H), 6.38(s, 1H), 4.72(s, 1H), 2.44(s, 3H) | 175.4, 152.6, 139.1, 136.1, 135.3, 130.9, 128.6, 126.9, 126.5, 126.1, 125.2, 123.1, 121.8, 71.4, 19.5 |
| 2b | 8.02(d, J=8.0 Hz, 1H), 7.87(d, J=8.0 Hz, 1H), 7.61(dd, J1=J2=7.6 Hz,1H), 7.50(dd, J1=J2=7.6 Hz,1H), 7.42—7.34(m, 2H), 7.20(dd, J1=J2=7.6 Hz,1H), 7.13(dd, J1=J2=7.6 Hz,1H), 6.51(s, 1H), 4.66(s, 1H) | 173.9, 161.4(d, J=246.4 Hz), 152.5, 135.3, 130.4(d, J=8.2 Hz), 128.4(d, J=3.7 Hz), 128.2(d, J=14.0 Hz), 126.2, 125.3, 124.7(d, J=3.4 Hz), 123.1, 121.8, 115.8(d, J=21.1 Hz), 68.2(d, J=3.8 Hz) |
| 2c | 8.03(d, J=8.4 Hz, 1H), 7.86(d, J=8.0 Hz, 1H), 7.70—7.68(m, 1H), 7.52—7.47(m, 1H), 7.46—7.43(m, 1H), 7.40(d, J=7.2 Hz, 1H), 7.36—7.30(m, 2H), 6.64(s, 1H), 4.83(s, 1H) | 173.6, 152.3, 138.6, 135.3, 132.9, 129.8, 129.7, 128.6, 127.4, 126.2, 125.3, 123.1, 121.8, 70.8 |
| 2d | 8.06(d, J=8.0 Hz, 1H), 7.87(d, J=8.0 Hz, 1H), 7.68—7.63(m, 2H), 7.54—7.49(m, 1H), 7.44—7.38(m, 2H), 7.28—7.24(m, 1H), 6.60(d, J=4.0 Hz, 1H), 4.57(d, J=4.4 Hz, 1H) | 173.3, 152.3, 140.2, 135.5, 133.0, 130.2, 128.9, 128.1, 126.2, 125.4, 123.2, 122.9, 121.8, 73.1 |
| 2e | 8.07(d, J=8.0 Hz, 1H), 7.87(d, J=8.0 Hz, 1H), 7.77(d, J=8.0 Hz, 2H), 7.64—7.59(m,1H), 7.55—7.49(m, 2H), 7.45—7.40(m, 1H), 6.58(d, J=3.6 Hz, 1H), 4.59(d, J=4.0 Hz, 1H) | 173.4, 152.2, 139.6, 135.6, 132.6, 129.7, 128.8, 128.2(q, J=30.2 Hz), 126.3, 125.9(q, J=5.5 Hz), 125.6(q, J=254.4 Hz), 125.4, 123.3, 121.8, 69.4(q, J=3.6 Hz) |
| 2f | 9.17(s, 1H), 7.99(d, J=8.0 Hz, 1H), 7.87(d, J=7.6 Hz, 1H), 7.53—7.48(m, 1H), 7.44—7.40(m, 2H), 7.31—7.24(m, 1H), 7.02—6.95(m, 2H), 6.40(s, 1H), 4.51(s, 1H) | 175.9, 154.7, 152.0, 134.5, 130.0, 126.7, 126.6, 126.5, 125.4, 122.7, 121.9, 120.7, 118.6, 72.1 |
| 2g | 8.02(d, J=8.0 Hz, 1H), 7.90(d, J=8.0 Hz, 1H), 7.57—7.50(m, 3H), 7.44—7.39(m, 1H), 7.14—7.10(m, 2H), 6.17(s, 1H), 4.04(s, 1H) | 174.6, 164.1(d, J=246.2 Hz), 152.6, 136.8, 135.3, 128.7(d, J=8.3 Hz), 126.3, 125.3, 123.1, 121.9, 116.0(d, J=21.6 Hz), 73.8 |
| 2h | 8.02(d, J=7.6 Hz, 1H), 7.89(d, J=7.6 Hz, 1H), 7.54—7.50(m, 3H), 7.44—7.39(m, 3H), 6.17(s, 1H), 3.98(s, 1H) | 174.2, 152.5, 139.4, 135.3, 134.6, 129.0, 128.1, 126.3, 125.4, 123.2, 121.9, 73.8 |
| 2i | 8.30(d, J=8.4 Hz, 2H), 8.04(d, J=8.0 Hz, 1H), 7.91(d, J=8.0 Hz, 1H), 7.82(d, J=8.4 Hz, 2H), 7.56—7.52(m, 1H), 7.46—7.42(m, 1H), 6.31(s, 1H), 4.21(s, 1H) | 173.0, 152.4, 147.9, 147.5, 135.1, 127.5, 126.5, 125.7, 124.0, 123.3, 122.0, 73.4 |
| 2j | 8.03(d, J=8.4 Hz, 1H), 7.89(d, J=8.0 Hz, 1H), 7.74—7.67(m, 4H), 7.55—7.50(m, 1H), 7.45—7.40(m, 1H), 6.26(s, 1H), 4.16(s, 1H) | 173.7, 152.5, 144.6, 135.2, 130.9(q, J=36.3 Hz), 127.0, 126.4, 125.9(q, J=3.8 Hz), 125.5, 125.4(q, J=271.8 Hz), 123.2, 121.9, 73.8 |
| 2k | 8.03(d, J=8.0 Hz, 1H), 7.87(d, J=8.0 Hz, 1H), 7.52—7.47(m, 1H), 7.46(d, J=8.0 Hz, 2H), 7.42—7.37(m, 1H), 7.23(d, J=8.0 Hz, 2H), 6.15(s, 1H), 3.97(s, 1H), 2.39(s, 3H) | 175.2, 152.7, 138.6, 138.1, 135.3, 129.6, 126.7, 126.1, 125.1, 123.1, 121.8, 74.3, 21.3 |
| 2l | 8.03(d, J=8.0 Hz, 1H), 7.88(d, J=8.0 Hz, 1H), 7.53—7.46(m, 3H), 7.43—7.38(m, 1H), 6.96(d, J=8.8 Hz, 2H), 6.14(s, 1H), 3.84(s, 3H), 3.80(s, 1H) | 175.2, 159.9, 152.7, 133.2, 133.2, 128.2, 126.1, 125.1, 123.1, 121.8, 114.2, 74.1, 55.3 |
| 2m | 9.45(s, 1H), 8.09(d, J=8.0 Hz, 1H), 7.91(d, J=8.0 Hz, 1H), 7.50—7.45(m, 1H), 7.42—7.38(m, 1H), 7.30(d, J=8.4 Hz, 2H), 6.82(d, J=4.0 Hz, 1H), 6.75(d, J=8.4 Hz, 2H), 5.95(d, J=4.4 Hz, 1H) | 178.6, 157.4, 153.5, 134.7, 133.2, 128.3, 126.4, 125.2, 122.9, 122.7, 115.5, 73.1 |
| 2n | 8.03(d, J=8.0 Hz, 1H), 7.88(d, J=8.0 Hz, 1H), 7.53(m, 3H), 7.16—7.12(m, 2H), 6.92(d, J=8.0 Hz, 1H), 6.16(s, 1H), 3.91(s, 1H), 3.84(s, 3H) | 174.7, 160.0, 152.6, 142.5, 135.4, 129.9, 126.2, 125.2, 123.1, 121.8, 119.0, 114.4, 112.1, 74.4, 55.3 |
| 2o | 8.01(d, J=8.4 Hz, 1H), 7.90—7.87(m, 2H), 7.76(d, J=8.0 Hz, 1H), 7.64(d, J=7.6 Hz, 1H), 7.55—7.49(m, 2H), 7.44—7.39(m, 1H), 6.26(d, J=1.6 Hz, 1H), 4.51(s, 1H) | 174.0, 152.4, 141.8, 135.2, 131.3(q, J=32.2 Hz), 130.1, 129.3, 126.4, 125.5, 125.4(q, J=3.6 Hz), 125.3(q, J=270.7 Hz), 123.5(q, J=4.0 Hz), 123.1, 121.9, 73.7 |
| Compd. | 1H NMR(400 Hz), δ | 13C NMR(100 Hz), δ |
| 2p | 8.49(s, 1H), 8.22(d, J=8.0 Hz, 1H), 8.01(d, J=8.0 Hz, 1H), 7.93(d, J=8.0 Hz, 1H), 7.89(d, J=8.0 Hz, 1H), 7.59(dd, J1=J2=7.6 Hz,1H), 7.54—7.50(m, 1H), 7.45—7.41(m, 1H), 6.31(d, J=2.4 Hz, 1H), 4.55(d, J=3.2 Hz, 1H) | 173.4, 152.4, 148.5, 142.8, 135.1, 132.8, 129.8, 126.5, 125.6, 123.5, 123.2, 122.0, 121.7, 73.2 |
| 2q | 8.04(d, J=8.0 Hz, 1H), 7.87(d, J=8.0 Hz, 1H), 7.75(d, J=2.8 Hz, 1H), 7.54—7.49(m, 1H), 7.44—7.40(m, 1H), 7.36(d, J=8.4 Hz, 1H), 7.32—7.29(m, 1H), 6.58(d, J=3.2 Hz, 1H), 5.15(d, J=3.6 Hz, 1H) | 172.7, 152.1, 140.2, 135.3, 133.5, 130.9, 130.8, 129.9, 128.6, 126.4, 125.6, 123.2, 121.9, 70.3 |
| 2r | 8.01(d, J=7.6 Hz, 1H), 7.91(d, J=8.0 Hz, 1H), 7.52(dt, J1=7.6 Hz, J2=1.2 Hz, 1H), 7.44—7.32(m, 2H), 7.00—6.93(m, 2H), 6.53(s, 1H), 4.26(s, 1H) | 173.1(d, J=2.8 Hz), 162.4(dd, J1=256.9 Hz, J2=7.5 Hz), 152.7, 135.3, 131.0(t, J=10.5 Hz), 126.2, 125.2, 123.2, 121.8, 117.2, 112.2(dd, J1=19.3 Hz, J2=5.7 Hz), 65.7(t, J=3.7 Hz) |
| 2s | 8.03(d, J=8.0 Hz, 1H), 7.89(d, J=8.0 Hz, 1H), 7.60—7.49(m, 2H), 7.44—7.40(m, 1H), 6.97—6.86(m, 2H), 6.45(s, 1H), 4.52(s, 1H) | 173.4, 164.4(dd, J1=248.8 Hz, J2=11.9 Hz), 161.5(dd, J1=247.2 Hz, J2=12.0 Hz), 152.4, 135.2, 129.5(dd, J1=9.7 Hz, J2=4.9 Hz), 126.3, 125.4, 124.4(d, J=17.0 Hz), 123.2, 121.8, 111.8(dd, J1=21.3 Hz, J2=3.7 Hz), 104.2(dd, J1=J2=25.2 Hz), 67.8(d, J=3.5 Hz) |
| 2t | 8.02(d, J=8.0 Hz, 1H), 7.87(d, J=8.0 Hz, 1H), 7.51—7.38(m, 3H), 6.73(d, J=8.0 Hz, 1H), 6.68(d, J=8.0 Hz, 1H) 6.41(s, 1H), 4.53(s, 1H), 3.82(s, 3H) | 174.5, 162.1(d, J=246.1 Hz), 161.2(d, J=11.0 Hz), 152.6, 135.3, 129.2(d, J=5.4 Hz), 126.2, 125.2, 123.1, 121.8, 120.4(d, J=13.5 Hz), 110.4(d, J=2.9 Hz), 101.9(d, J=24.9 Hz), 68.1, 55.6 |
| 2u | 9.25(s, 1H), 7.99(d, J=8.4 Hz, 1H), 7.93(d, J=8.4 Hz, 1H), 7.55—7.51(m, 1H), 7.46—7.42(m, 1H), 7.23(dd, J1=9.6 Hz, J2=2.4 Hz, 1H), 6.99—6.92(m, 2H), 6.42(s, 1H), 3.93(s, 1H) | 175.8, 158.3(d, J=237.4 Hz), 151.9, 150.2, 134.1, 128.8(d, J=7.1 Hz), 126.6, 125.5, 122.7, 122.0, 119.9(d, J=7.7 Hz), 116.2(d, J=22.9 Hz), 112.6(d, J=24.7 Hz), 70.8 |
| 2v | 8.04—8.00(m, 2H), 7.89—7.84(m, 4H), 7.63(dd, J1=8.8 Hz, J2=2.0 Hz, 1H), 7.55—7.51(m, 2H), 7.49—7.45(m, 1H), 7.40—7.36(m, 1H), 6.34(s, 1H), 4.66(s, 1H) | 175.2, 152.6, 138.3, 135.3, 133.4, 133.2, 128.8, 128.3, 127.8, 126.5, 126.4, 126.2, 126.0, 125.2, 124.3, 123.1, 121.8, 74.5 |
| 2w | 8.25—8.22(m, 1H), 8.02(d, J=8.0 Hz, 1H), 7.93—7.90(m, 2H), 7.82—7.79(m, 2H), 7.56—7.46(m, 4H), 7.38(dd, J1=J2=7.6 Hz, 1H), 6.86(d, J=3.6 Hz, 1H), 4.87(d, J=3.2 Hz, 1H) | 175.3, 152.5, 136.3, 135.5, 134.0, 130.7, 129.6, 128.9, 126.6, 126.2, 125.9, 125.4, 125.3, 125.2, 123.8, 123.1, 121.8, 72.2 |
| 2x | 7.58—7.38(m, 7H), 7.34—7.24(m, 2H), 6.58(s, 1H), 5.99(d, J=4.0 Hz, 1H), 2.76(d, J=4.0 Hz, 1H) | 158.5, 155.1, 140.3, 128.7, 128.4, 128.1, 126.8, 124.4, 122.9, 121.2, 111.4, 140.1, 70.7 |
| 2y | 7.75—7.70(m, 1H), 7.60—7.56(m, 2H), 7.53—7.49(m, 1H), 7.45—7.34(m, 5H), 6.09(d, J=4.8 Hz, 1H), 4.29(d, J=5.2 Hz, 1H) | 166.6, 151.0, 140.3, 138.9, 128.9, 128.8, 126.9, 125.3, 124.6, 120.2, 110.9, 70.6 |
| 2z | 7.68—7.66(m, 1H), 7.52—7.49(m, 2H), 7.43—7.33(m, 3H), 7.31—7.29(m, 1H), 6.07(s, 1H), 4.90(s, 1H) | 175.0, 142.2, 141.6, 128.7, 128.4, 126.6, 119.6, 73.6 |
| Compd. | Product | Yieldb(%) | e.e.c(%) | Compd. | Product | Yieldb(%) | e.e.c(%) |
|---|---|---|---|---|---|---|---|
| 2a | 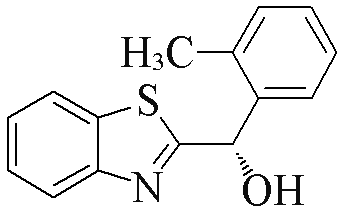 | 89 | 99 | 2l | 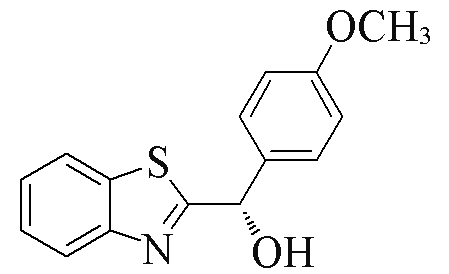 | 83 | 61 |
| 2b | 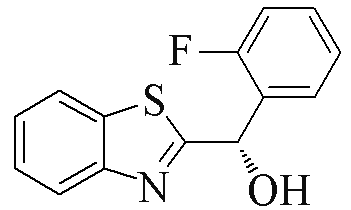 | 77 | 90 | 2m | 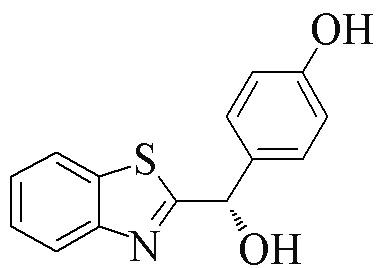 | 77 | 60 |
| 2c | 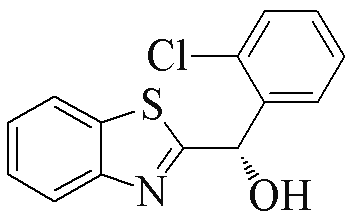 | 73 | 98 | 2n | 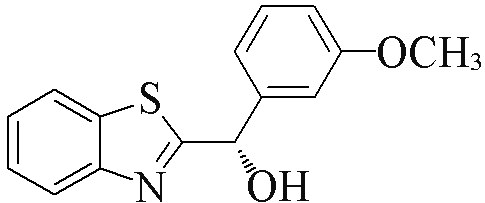 | 88 | 58 |
| 2d | 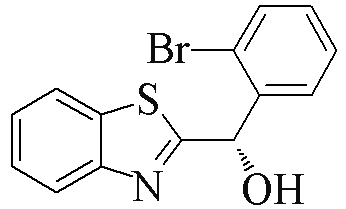 | 89 | 99 | 2o | 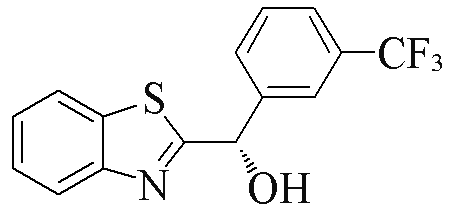 | 55 | 81 |
| 2e | 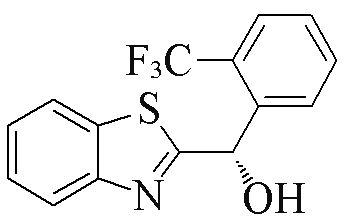 | 75 | 99 | 2p | 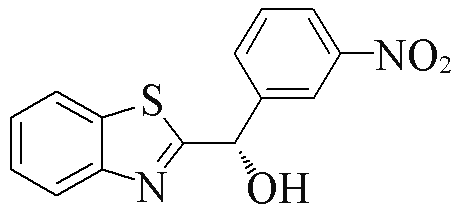 | 74 | 85 |
| 2f | 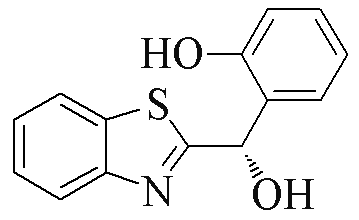 | 78 | 31 | 2q | 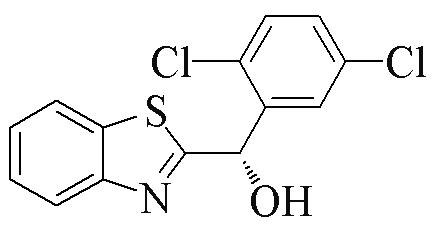 | 81 | 97 |
| 2g | 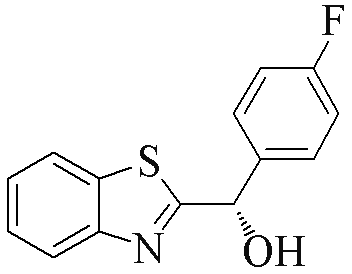 | 81 | 78 | 2r | 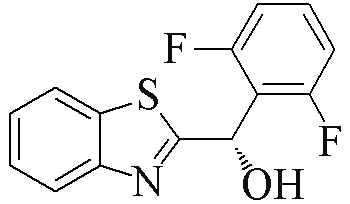 | 83 | 89 |
| 2h | 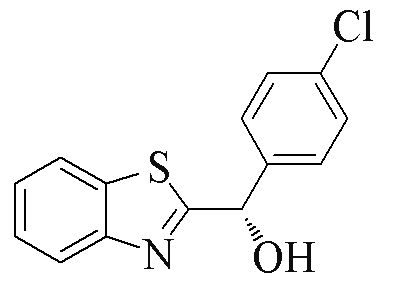 | 69 | 77 | 2s | 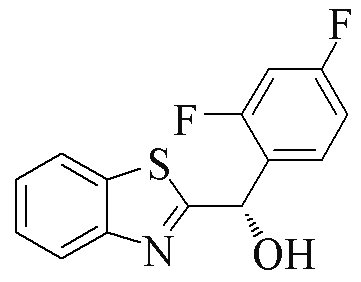 | 75 | 95 |
| 2i | 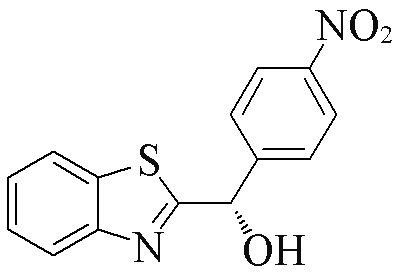 | 74 | 86 | 2t | 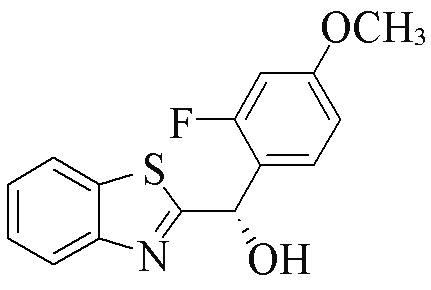 | 85 | 91 |
| 2j | 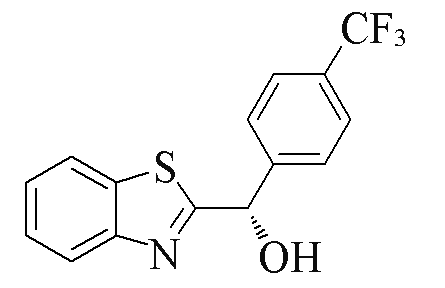 | 70 | 70 | 2u | 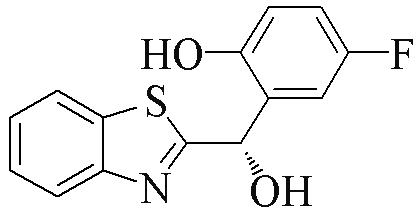 | 84 | 59 |
| 2k | 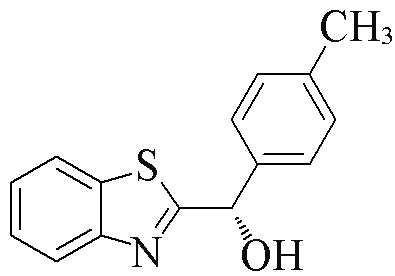 | 82 | 50 | 2v | 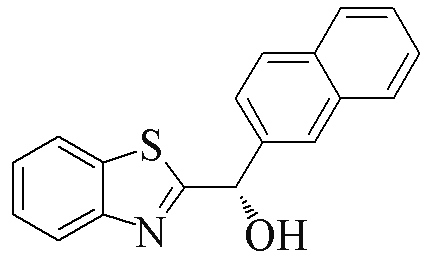 | 52 | 55 |
| Compd. | Product | Yieldb(%) | e.e.c(%) | Compd. | Product | Yieldb(%) | e.e.c(%) |
| 2w | 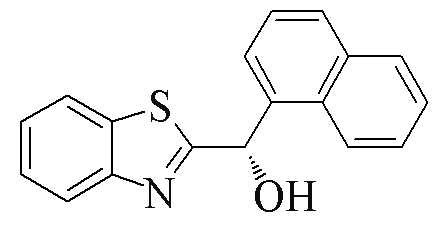 | 74 | 94 | 2y | 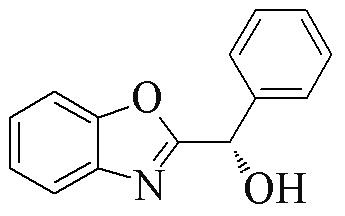 | 77 | 0 |
| 2x | 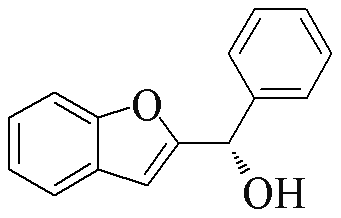 | 76 | 52 | 2z | 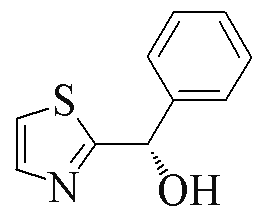 | 88 | 9 |
Table 4 Substrate scope for the productsa
| Compd. | Product | Yieldb(%) | e.e.c(%) | Compd. | Product | Yieldb(%) | e.e.c(%) |
|---|---|---|---|---|---|---|---|
| 2a |  | 89 | 99 | 2l |  | 83 | 61 |
| 2b |  | 77 | 90 | 2m |  | 77 | 60 |
| 2c |  | 73 | 98 | 2n |  | 88 | 58 |
| 2d |  | 89 | 99 | 2o |  | 55 | 81 |
| 2e |  | 75 | 99 | 2p |  | 74 | 85 |
| 2f |  | 78 | 31 | 2q |  | 81 | 97 |
| 2g |  | 81 | 78 | 2r |  | 83 | 89 |
| 2h |  | 69 | 77 | 2s |  | 75 | 95 |
| 2i |  | 74 | 86 | 2t |  | 85 | 91 |
| 2j |  | 70 | 70 | 2u |  | 84 | 59 |
| 2k |  | 82 | 50 | 2v |  | 52 | 55 |
| Compd. | Product | Yieldb(%) | e.e.c(%) | Compd. | Product | Yieldb(%) | e.e.c(%) |
| 2w |  | 74 | 94 | 2y |  | 77 | 0 |
| 2x |  | 76 | 52 | 2z |  | 88 | 9 |
| 1 | Yang Z. Y., Cheng Y. Y., Zhou L., Chin. J. Syn. Chem., 2020, 28(1),84—90(杨志勇, 成园园, 周亮. 合成化学, 2020, 28(1), 84—90) |
| 2 | Kida T., Fujii A., Sakai O., Iemura M., Atsumi I., Wada T., Sakaki H., Exp. Eye Res., 2010, 91(1), 85—91 |
| 3 | Hellbom E., Med. Hypotheses,2006, 66(4), 689—690 |
| 4 | Rizzetto M., Ciancio A., Lancet Infect. Dis., 2015, 15(10), 1119—1120 |
| 5 | Slaughter S. R., Hearns⁃Stokes R., van der Vlugt T., Joffe H. V., New Engl. J. Med., 2014, 370(12), 1081—1083 |
| 6 | Xu G. C., Wang Y., Tang M. H., Zhou J. Y., Zhao J., Han R. Z., Ni Y., ACS Catal.,2018, 8(9), 8336—8345 |
| 7 | Liu B. B., Qu G., Li J. K., Fan W. C., Ma J. A., Xu Y., Nie Y., Sun Z. T., Adv. Synth. Catal.,2019, 361(13), 3182—3190 |
| 8 | Liu Y. L., Lin X. T., Adv. Synth. Catal., 2019, 361(5), 876—918 |
| 9 | Xie X. M., Lu B., Li W. F., Zhang Z. G., Coordin. Chem. Rev.,2018, 355, 39—53 |
| 10 | Zassinovich G., Mestroni G., Gladiali S., Chem. Rev.,1992, 92(5), 1051—1069 |
| 11 | Hashiguchi S., Fujii A., Takehara J., Ikariya T., Noyori R., J. Am. Chem. Soc., 1995, 117(28), 7562—7563 |
| 12 | Zhao J. F., Dou H. J., Zhou Y. H., Qu J. P., Chem. J. Chinese Universities, 2011, 32(10), 2331—2334(赵金凤, 窦海建, 周宇涵, 曲景平. 高等学校化学学报, 2011, 32(10), 2331—2334) |
| 13 | Wang B. G., Zhou H. F., Lu G. R., Liu Q. X., Jiang X. L., Org. Lett.,2017, 19(8), 2094—2097 |
| 14 | Liu Q. X., Wang C. Q., Zhou H. F., Wang B. G., Lv J. L., Cao L., Fu Y. G., Org. Lett.,2018, 20(4), 971—974 |
| 15 | Zhang K. L., Yang Y. P., Liu H., Liu Q. X., Lv J. L., Zhou H. F., Adv. Synth. Catal.,2019, 361(17), 4106—4110 |
| 16 | Cui P., Liu Q. X., Wang J., Liu H., Zhou H. F., Green Chem.,2019, 21(3), 634—639 |
| 17 | Liu S. S., Cui P., Wang J., Zhou H. F., Liu Q. X., Lv J. L., Org. Biomol. Chem.,2019, 17(2), 264—267 |
| 18 | Liu S. S., Liu H., Zhou H. F.,Liu Q. X., Lv J. L., Org. Lett.,2018, 20(4), 1110—1113 |
| 19 | Liu H., Liu S. S., Zhou H. F.,Liu Q. X., Wang C. Q., RSC Adv.,2018, 8(27), 14829—14832 |
| 20 | Sui Y. Z., Zhang X. C., Wu J. W., Li S. J., Zhou J. N., Li M., Fang W. J., Chan A. S. C., Wu J., Chem. Eur. J.,2012, 18(24), 7486—7492 |
| 21 | Tao X. M., Li W. F., Ma X., Li X. M., Fan W. Z., Xie X. M., Ayad T., Ratovelomanana⁃Vidal V., Zhang Z. G., J. Org. Chem.,2012, 77(1), 612—616 |
| 22 | Yang H. L., Huo N. N., Yang P., Pei H., Lv H., Zhang X. M., Org. Lett.,2015, 17(17), 4144—4147 |
| 23 | Chen F. M., He D. X., Chen L., Chang X. Y., Wang D. Z., Xu C., Xing X. Y., ACS Catal.,2019, 9(6), 5562—5566 |
| 24 | Nian S. F., Ling F., Chen J. C., Wang Z., Shen H. W., Yi X., Yang Y. F., She Y. B., Zhong W. H., Org. Lett.,2019, 21(14), 5392—2396 |
| 25 | Chen C. Y., Reamer R. A., Chilenski J. R., McWilliams C. J., Org. Lett., 2003, 5(26), 5039—5042 |
| 26 | Lebedev Y., Polishchuk I., Maity B., Guerreiro M. D. V., Cavallo L., Rueping M., J. Am. Chem. Soc., 2019, 141(49), 19415—19423 |
| [1] | 黄秋红, 李文军, 李鑫. 有机催化靛红衍生酮亚胺与噁唑酮的不对称Mannich型加成反应[J]. 高等学校化学学报, 2022, 43(8): 20220131. |
| [2] | 赵莹, 乔玲, 赵国锋, 陈莉. 含苹果酸酯的石蒜碱衍生物的合成及生物活性[J]. 高等学校化学学报, 2021, 42(9): 2789. |
| [3] | 董旭, 封博谞, 陈莉, 李鑫. 叔胺硫脲催化的2,5-二羟基-1,4-二噻烷与氮叔丁氧羰基醛亚胺的不对称[3+2]环化反应[J]. 高等学校化学学报, 2018, 39(8): 1707. |
| [4] | 曹笃霞, 方奇, 王东, 薛刚, 于文涛, 刘志强, 周玉芳. 上转换荧光化合物DMSSB与CSSB的合成、结构与光物理性质[J]. 高等学校化学学报, 2004, 25(1): 76. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||