

Chem. J. Chinese Universities ›› 2022, Vol. 43 ›› Issue (3): 20210689.doi: 10.7503/cjcu20210689
• Review • Previous Articles Next Articles
ZHANG Xiaoyu1, XUE Dongping1, DU Yu1, JIANG Su1, WEI Yifan1, YAN Wenfu2( ), XIA Huicong1, ZHANG Jianan1(
), XIA Huicong1, ZHANG Jianan1( )
)
Received:2021-09-26
Online:2022-03-10
Published:2021-11-22
Contact:
YAN Wenfu,ZHANG Jianan
E-mail:yanw@jlu.edu.cn;zjn@zzu.edu.cn
Supported by:CLC Number:
TrendMD:
ZHANG Xiaoyu, XUE Dongping, DU Yu, JIANG Su, WEI Yifan, YAN Wenfu, XIA Huicong, ZHANG Jianan. MOF-derived Carbon-based Electrocatalysts Confinement Catalyst on O2 Reduction and CO2 Reduction Reactions[J]. Chem. J. Chinese Universities, 2022, 43(3): 20210689.
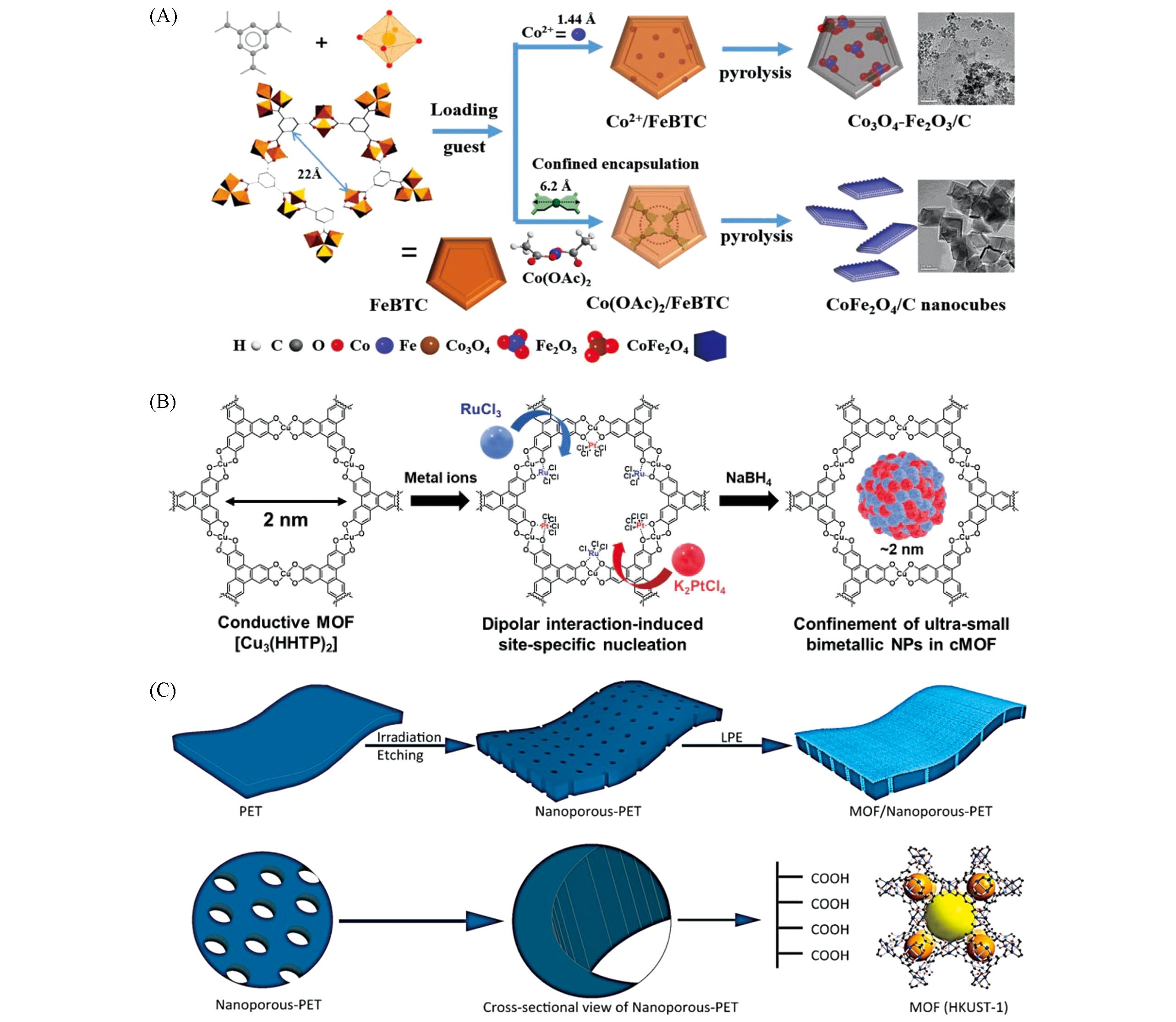
Fig.1 Confinement engneering on catalytic activity(A) Schematic illustration of assembly MOFs-derived bimetallic spinel oxides CoFe2O4 nanocubes through the combination of exchange-coordination and pyrolysis[49]. Copyright 2020, Wiley-VCH. (B) Schematic illustration for the preparation of PtRu@cMOF[53]. Copyright 2021, Wiley-VCH. (C) Schematic illustration of the formation of nanochannels in the polymer membrane and subsequent confinement of the porous HKUST-1 metal-organic framework[55]. Copyright 2020, American Chemical Society.
| Catalyst | Species of metals | Electrolyte | E1/2/V (vs. RHE) | Limiting current density/(mA· cm-2) | Eonset/V (vs. RHE) | Durability | Ref. |
|---|---|---|---|---|---|---|---|
| Co?N?GA | Nanoparticles | 0.1 mol/L KOH | — | 6 | 0.9 | 10000 s | [ |
| Fe?NC | Nanoparticles | 0.1 mol/L KOH | 0.877 | 5.82 | 0.963 | 20000 s | [ |
| 20Mn?NC?second | Single atom | 0.5 mol/L H2SO4 | 0.80 | ca. 3.9 | — | 100 h | [ |
| C?FeHZ8@g?C3N4?950 | Single atom | 0.1 mol/L HClO4 | 0.78 | ca. 5.5 | — | 8000 s | [ |
| Fe SAC/N?C | Single atom | 0.1 mol/L KOH | 0.89 | ca. 5.5 | — | 4000 s | [ |
| Fe?N?C?P/N,P?C | Single atom | 0.1 mol/L HClO4 | 0.80 | 6 | 1.06 | — | [ |
| 6%Fe?N?S CNN | Single atom | 0.1 mol/L KOH | 0.91 | ca. 5.6 | — | 12 h | [ |
| FeNi0.25?NC | Single atom | 0.1 mol/L HClO4 | 0.79 | — | — | — | [ |
| Co(mIm)?NC | Single atom | 0.5 mol/L H2SO4 | 0.82 | ca. 4 | 0.93 | 50 h | [ |
| Co SAs/N?C(900) | Single atom | 0.1 mol/L KOH | 0.881 | ca. 5.6 | 0.982 | — | [ |
| CoOx@PNC | Cluster | 0.1 mol/L KOH | 0.88 | ca. 6.5 | 0.98 | 200 h | [ |
| FeNC?S?FexC/Fe | Cluster | 0.1 mol/L HClO4 | 0.821 | 5.75 | 1.05 | — | [ |
| BTC?Co?O?Cu?BTA | Cluster | 0.1 mol/L NaOH | 0.95 | ca. 6 | 1.06 | — | [ |
| Cu@Fe?N?C | Nanoparticles | 0.1 mol/L KOH | 0.892 | ca. 5.52 | 1.01 | 20000 s | [ |
| Co?ZnO@NC/CNT?700 | Nanoparticles | 0.1 mol/L KOH | 0.86 | ca. 5.98 | 0.9 | 25000 s | [ |
Table 1 Summary of previously reported MOF-derived carbon-based catalysts and their application in ORR
| Catalyst | Species of metals | Electrolyte | E1/2/V (vs. RHE) | Limiting current density/(mA· cm-2) | Eonset/V (vs. RHE) | Durability | Ref. |
|---|---|---|---|---|---|---|---|
| Co?N?GA | Nanoparticles | 0.1 mol/L KOH | — | 6 | 0.9 | 10000 s | [ |
| Fe?NC | Nanoparticles | 0.1 mol/L KOH | 0.877 | 5.82 | 0.963 | 20000 s | [ |
| 20Mn?NC?second | Single atom | 0.5 mol/L H2SO4 | 0.80 | ca. 3.9 | — | 100 h | [ |
| C?FeHZ8@g?C3N4?950 | Single atom | 0.1 mol/L HClO4 | 0.78 | ca. 5.5 | — | 8000 s | [ |
| Fe SAC/N?C | Single atom | 0.1 mol/L KOH | 0.89 | ca. 5.5 | — | 4000 s | [ |
| Fe?N?C?P/N,P?C | Single atom | 0.1 mol/L HClO4 | 0.80 | 6 | 1.06 | — | [ |
| 6%Fe?N?S CNN | Single atom | 0.1 mol/L KOH | 0.91 | ca. 5.6 | — | 12 h | [ |
| FeNi0.25?NC | Single atom | 0.1 mol/L HClO4 | 0.79 | — | — | — | [ |
| Co(mIm)?NC | Single atom | 0.5 mol/L H2SO4 | 0.82 | ca. 4 | 0.93 | 50 h | [ |
| Co SAs/N?C(900) | Single atom | 0.1 mol/L KOH | 0.881 | ca. 5.6 | 0.982 | — | [ |
| CoOx@PNC | Cluster | 0.1 mol/L KOH | 0.88 | ca. 6.5 | 0.98 | 200 h | [ |
| FeNC?S?FexC/Fe | Cluster | 0.1 mol/L HClO4 | 0.821 | 5.75 | 1.05 | — | [ |
| BTC?Co?O?Cu?BTA | Cluster | 0.1 mol/L NaOH | 0.95 | ca. 6 | 1.06 | — | [ |
| Cu@Fe?N?C | Nanoparticles | 0.1 mol/L KOH | 0.892 | ca. 5.52 | 1.01 | 20000 s | [ |
| Co?ZnO@NC/CNT?700 | Nanoparticles | 0.1 mol/L KOH | 0.86 | ca. 5.98 | 0.9 | 25000 s | [ |
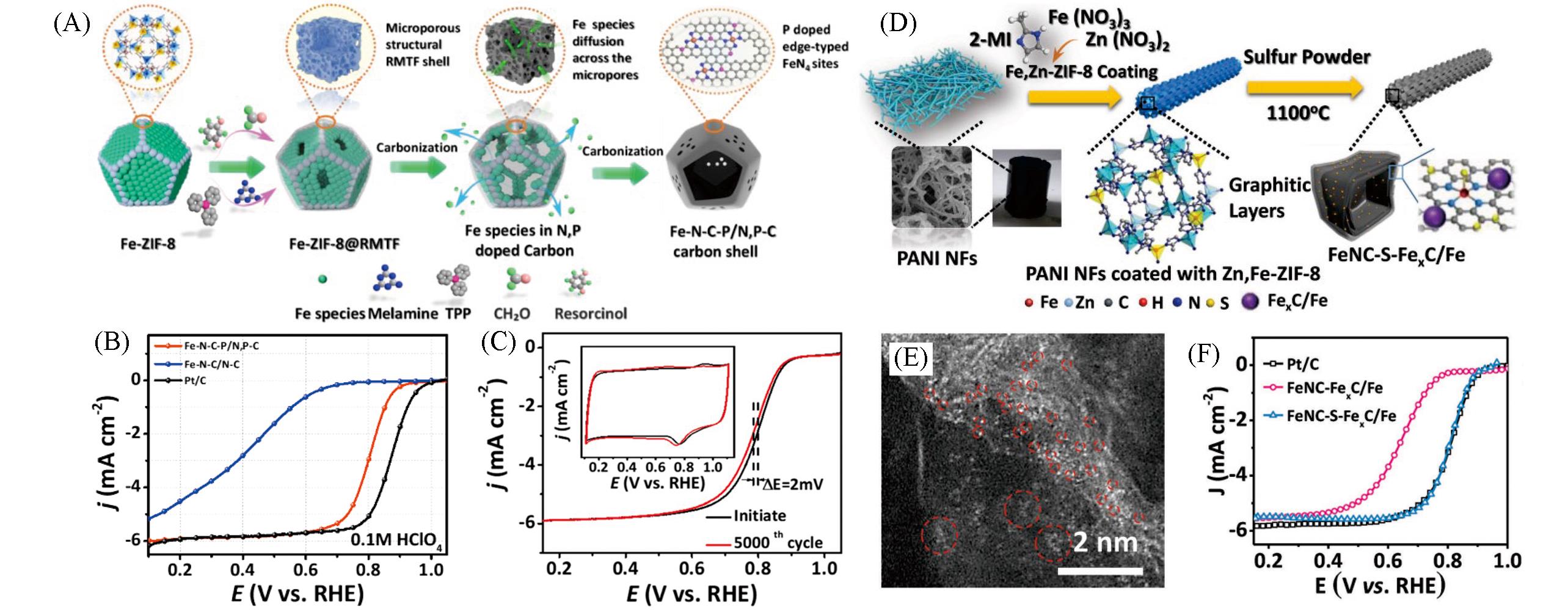
Fig.3 MOF?derived atomically dispersion metal carbon?based materials for confinement electrocatalytic ORR(A) Schematic of the preparation of the Fe-N-C-P/N,P-C; (B) LSV curves of ORR in O2-saturated 0.1 mol/L HClO4 at 1600 r/min for Fe-N-C-P/N,P-C, Fe-N-C/N-C and Pt/C; (C) ORR polarization LSV and CV curves of Fe-N-C-P/N,P-C measurement before and after 5000 potential cycles at the scan rate of 50 mV/s[62]. Copyright 2021, American Chemical Society; (D) synthesis scheme of the Fe-NC-S-Fe x C/Fe catalyst; (E) HAADF-STEM image of Fe-NC-S-Fe x C/Fe; (F) LSV curves of ORR in O2-saturated 0.1 mol/L HClO4 at 1600 r/min for different catalysts[68]. Copyright 2018, Wiley-VCH.
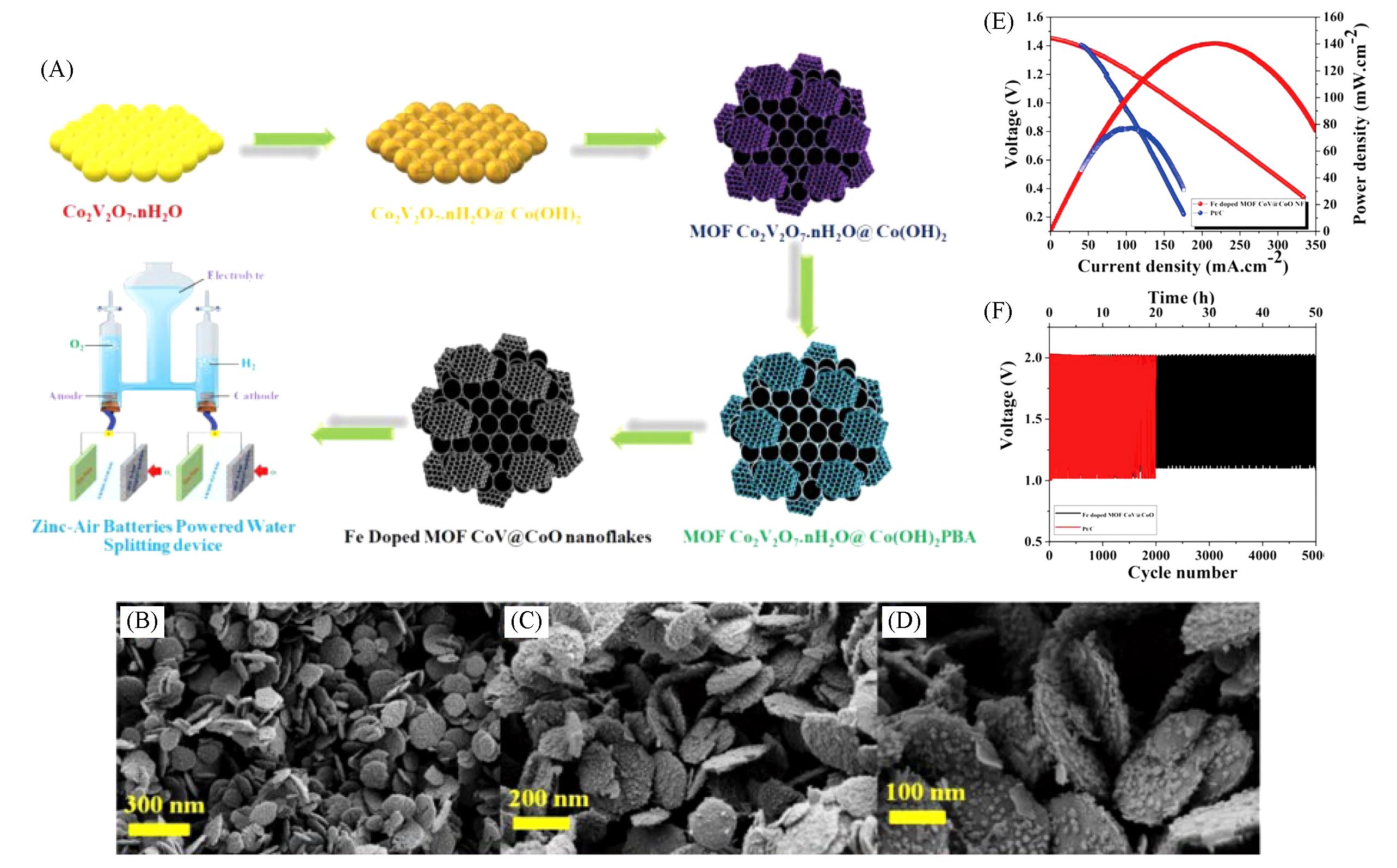
Fig.4 MOF?derived metal nanoparticles carbon?based materials for confinement electrocatalytic ORR[82](A) The schematic illustration of synthetic strategy of Fe doped MOF CoV@CoO nanoflakes and self-powered zinc-air battery water splitting applications; (B—D) different magnifications FESEM images of Fe doped MOF CoV@CoO nanoflakes; (E) discharge pola-rization curves and related power densities of Fe doped MOF CoV@CoO nanoflakes and Pt/C/IrO2 catalyst; (F) galvanostatic charge and discharge cycling curve at 10 mA/cm2 for Fe doped MOF CoV@CoO nanoflakes and commercial Pt/C/IrO2 catalyst. Copyright 2021, Elsevier.
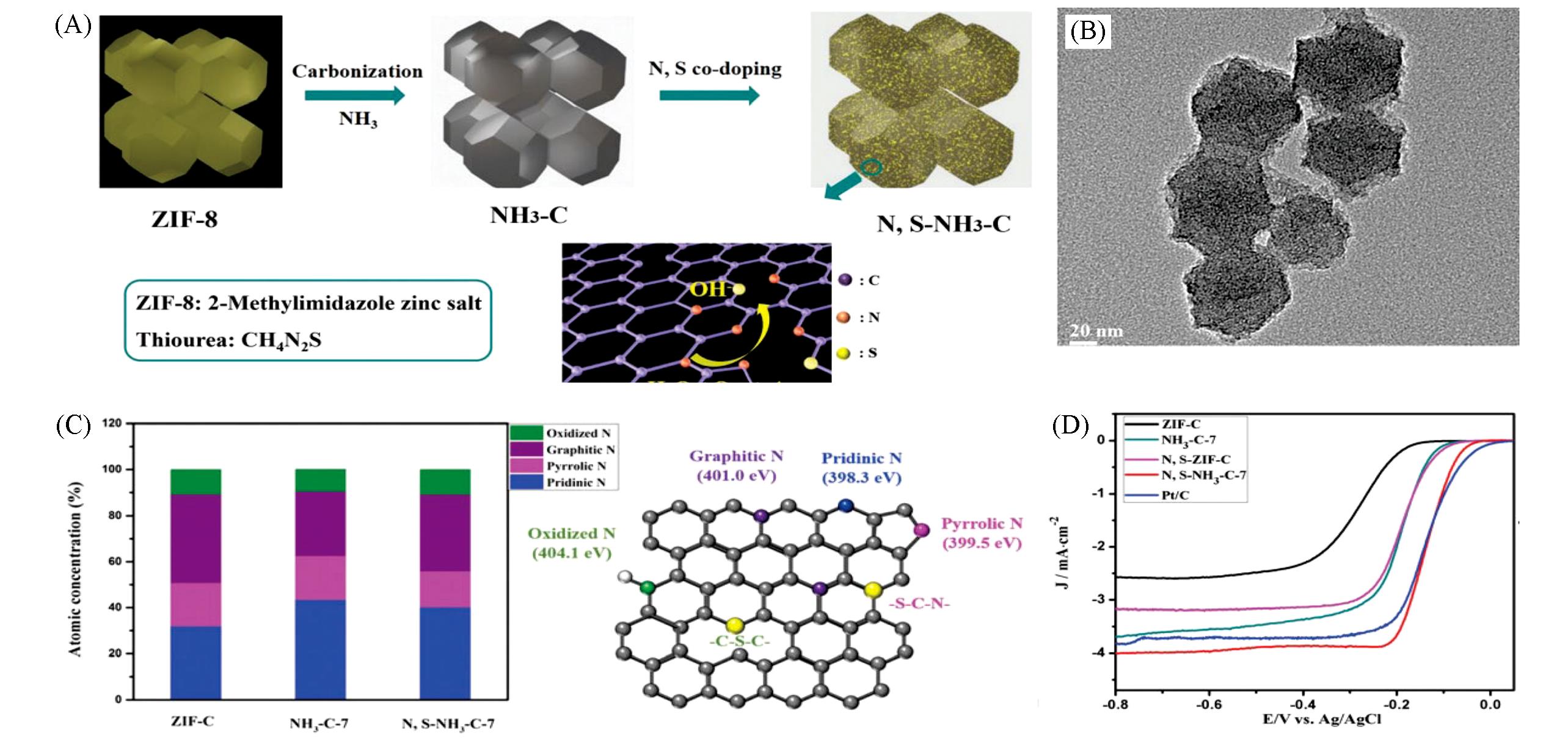
Fig.5 MOF?derived nonmetallic carbon?based materials for confinement electrocatalytic ORR[84](A) Schematic illustration of the fabrication of the N,S-co-doped nanocarbon as the electrocatalyst toward ORR; (B) TEM image of N,S-NH3-C-7; (C) bar diagrams representing the atomic concentration of four kinds of nitrogen species(left); atomic structure of the N,S-doped nanocarbon with chemical bonding configurations of nitrogen and sulfur dopants(right); (D) linear sweep voltammograms(LSVs) of ZIF-C(black), NH3-C-7(blue), N,S-NH3-C-7(red). Copyright 2017, Royal Society of Chemistry.
| Catalysis | Species of metals | Electrolyte | Product and FE (%) | Current density/(mA·cm-2) | E/V (vs. RHE) | Durability/h | Ref. |
|---|---|---|---|---|---|---|---|
| DHPC | Single atom | 0.5 mol/L KHCO3 | CO@99.5 | jCOca. -5 | -0.5 | — | [ |
| DPC?NH3?950 | Single atom | 0.1 mol/L KHCO3 | CO@95.2 | 2.84 | -0.5 | 24 | [ |
| Ni SAs/N?C | Single atom | 0.5 mol/L KHCO3 | CO@71.9 | 10.48 | -1.0 | 60 | [ |
| Co?N2 | Single atom | 0.5 mol/L KHCO3 | CO@94 | 18.1 | -0.63 | — | [ |
| Ni1?N?C | Single atom | 0.5 mol/L KHCO3 | CO@96.8 | jCOca. 27 | -0.8 | 10 | [ |
| Ni/Fe?NC | Single atom | 0.5 mol/L KHCO3 | CO@98 | 9.5 | -0.70 | >30 | [ |
| InCuO?0.92 | Nanoparticles | 0.5 mol/L KHCO3 | CO@92.1 | 11.2 | -0.8 | 24 | [ |
| PcCu?O8?Zn/CNT | Nanoparticles | 0.1 mol/L KHCO3 | CO@88 | — | -0.7 | >10 | [ |
| m?Cu NPs | Nanoparticles | 0.1 mol/L KHCO3 | CH4@>50 | 10.9 | -1.4 | — | [ |
Table 2 Summary of previously reported MOF-derived carbon-based catalysts and their application in CO2RR
| Catalysis | Species of metals | Electrolyte | Product and FE (%) | Current density/(mA·cm-2) | E/V (vs. RHE) | Durability/h | Ref. |
|---|---|---|---|---|---|---|---|
| DHPC | Single atom | 0.5 mol/L KHCO3 | CO@99.5 | jCOca. -5 | -0.5 | — | [ |
| DPC?NH3?950 | Single atom | 0.1 mol/L KHCO3 | CO@95.2 | 2.84 | -0.5 | 24 | [ |
| Ni SAs/N?C | Single atom | 0.5 mol/L KHCO3 | CO@71.9 | 10.48 | -1.0 | 60 | [ |
| Co?N2 | Single atom | 0.5 mol/L KHCO3 | CO@94 | 18.1 | -0.63 | — | [ |
| Ni1?N?C | Single atom | 0.5 mol/L KHCO3 | CO@96.8 | jCOca. 27 | -0.8 | 10 | [ |
| Ni/Fe?NC | Single atom | 0.5 mol/L KHCO3 | CO@98 | 9.5 | -0.70 | >30 | [ |
| InCuO?0.92 | Nanoparticles | 0.5 mol/L KHCO3 | CO@92.1 | 11.2 | -0.8 | 24 | [ |
| PcCu?O8?Zn/CNT | Nanoparticles | 0.1 mol/L KHCO3 | CO@88 | — | -0.7 | >10 | [ |
| m?Cu NPs | Nanoparticles | 0.1 mol/L KHCO3 | CH4@>50 | 10.9 | -1.4 | — | [ |
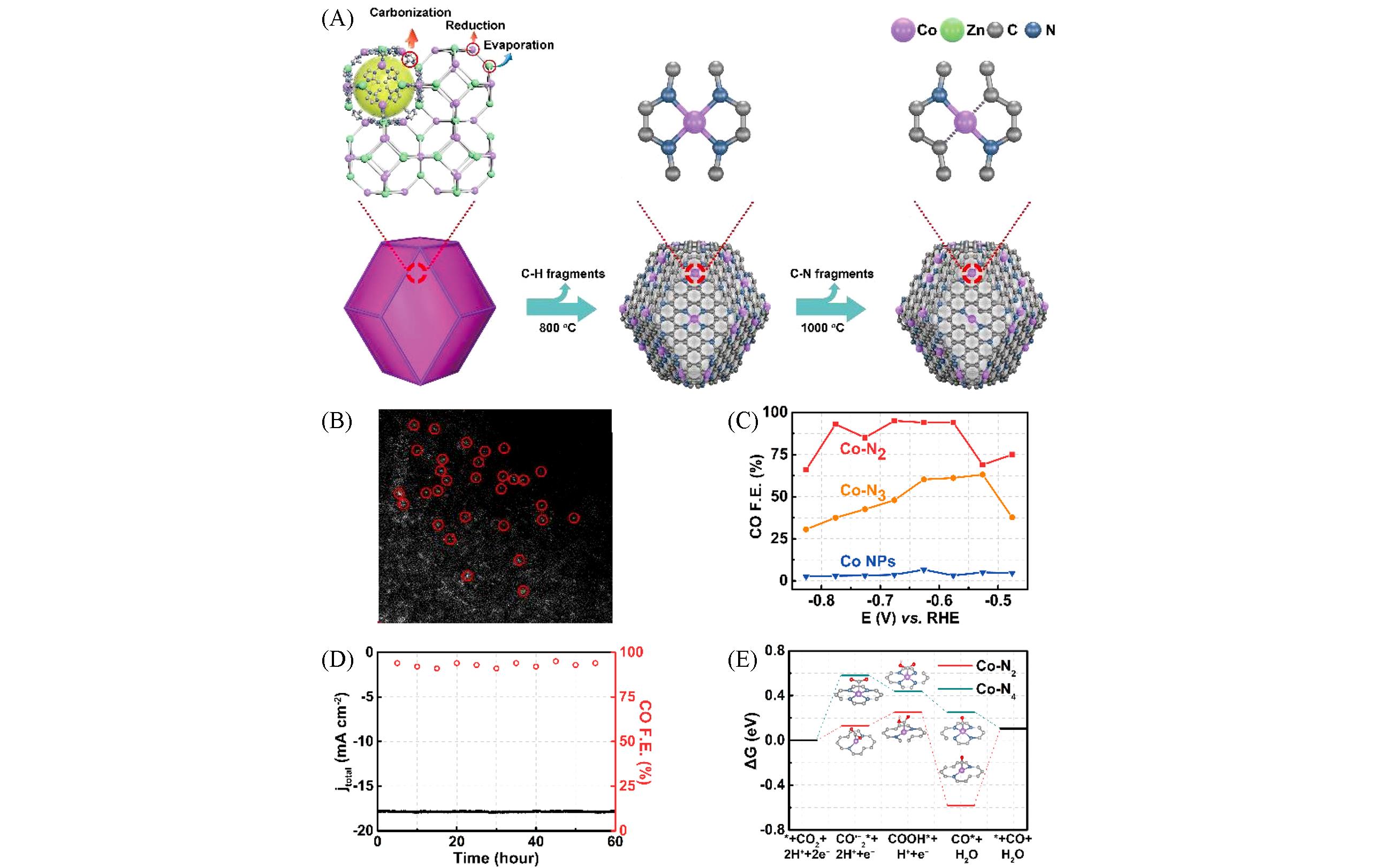
Fig.7 MOF?derived atomically dispersion metal carbon?based materials for confinement electrocatalytic CO2RR[96](A) Schematic formation process of Co-N4 and Co-N2; (B) magnified HAADF-STEM images of Co-N2 shows the atomic dispersion of Co atoms; (C) CO Faradaic efficiencies at different applied potentials; (D) catalytic stability test at -0.63 V for 60 h; (E) calculated Gibbs free energy diagrams for CO2 electroreduction to CO on Co-N2 and Co-N4. Copyright 2018, Wiley-VCH.
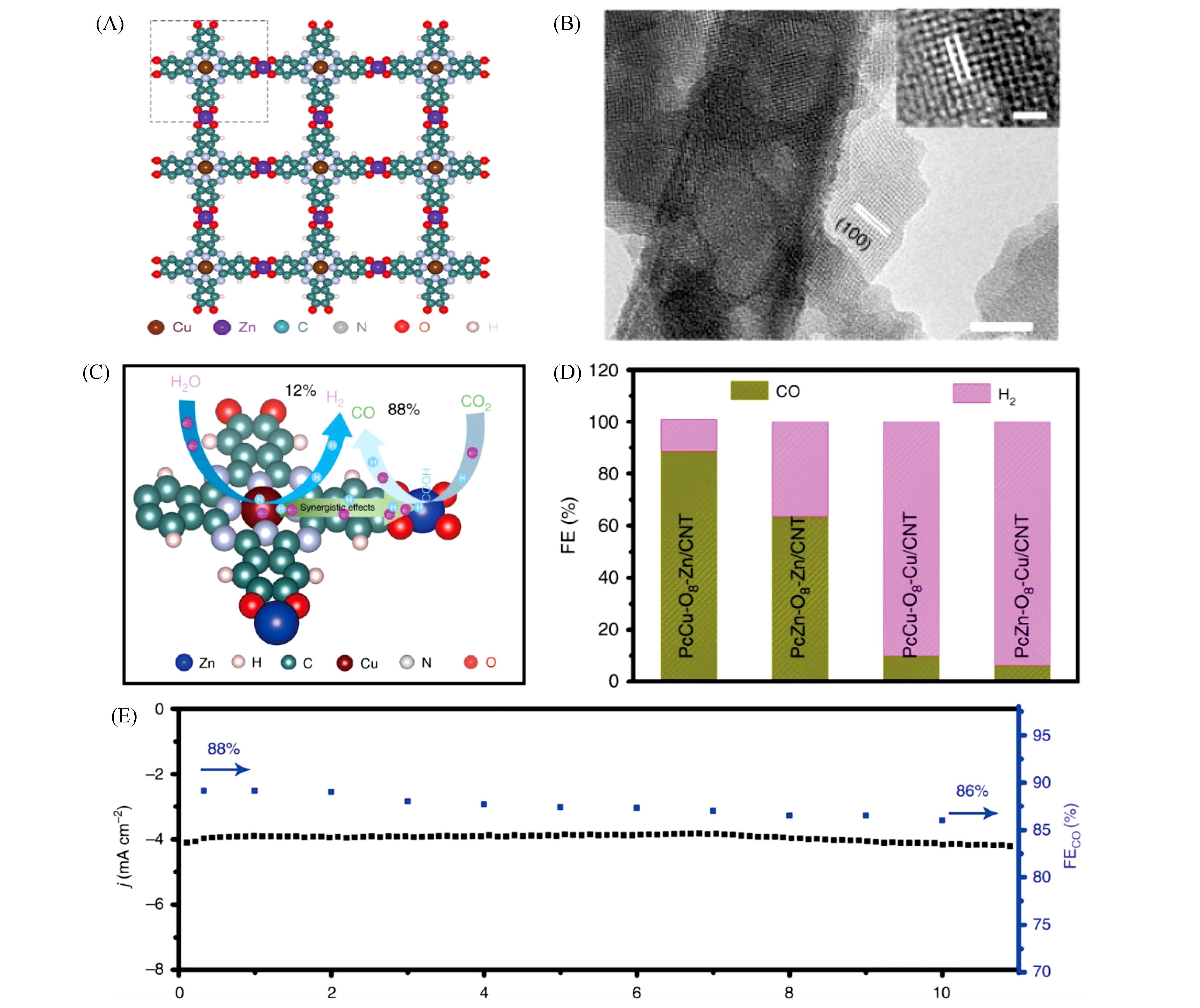
Fig.8 MOF?derived metal nanoparticles carbon?based materials for confinement electrocatalytic CO2RR[100](A) Schematic structure of PcCu-O8-Zn (the dashed rectangular indicates the unit cell); (B) HRTEM image of PcCu-O8-Zn sample. Scale bar: 20?nm (inset: 5?nm); (C) schematic HER and CO2RR reaction process of PcCu-O8-Zn; (D) Faradaic efficiency of CO and H2 for PcCu-O8-Zn/CNT, PcCu-O8-Cu/CNT, PcZn-O8-Zn/CNT and PcZn-O8-Cu/CNT at -0.7?V(vs. RHE); (E) amperometry (i?t) stability and the according Faradaic efficiency for CO of PcCu-O8-Zn/CNT at -0.7?V(vs. RHE) in CO2-saturated 0.1 mol/L KHCO3.Copyright 2020, Springer Nature.
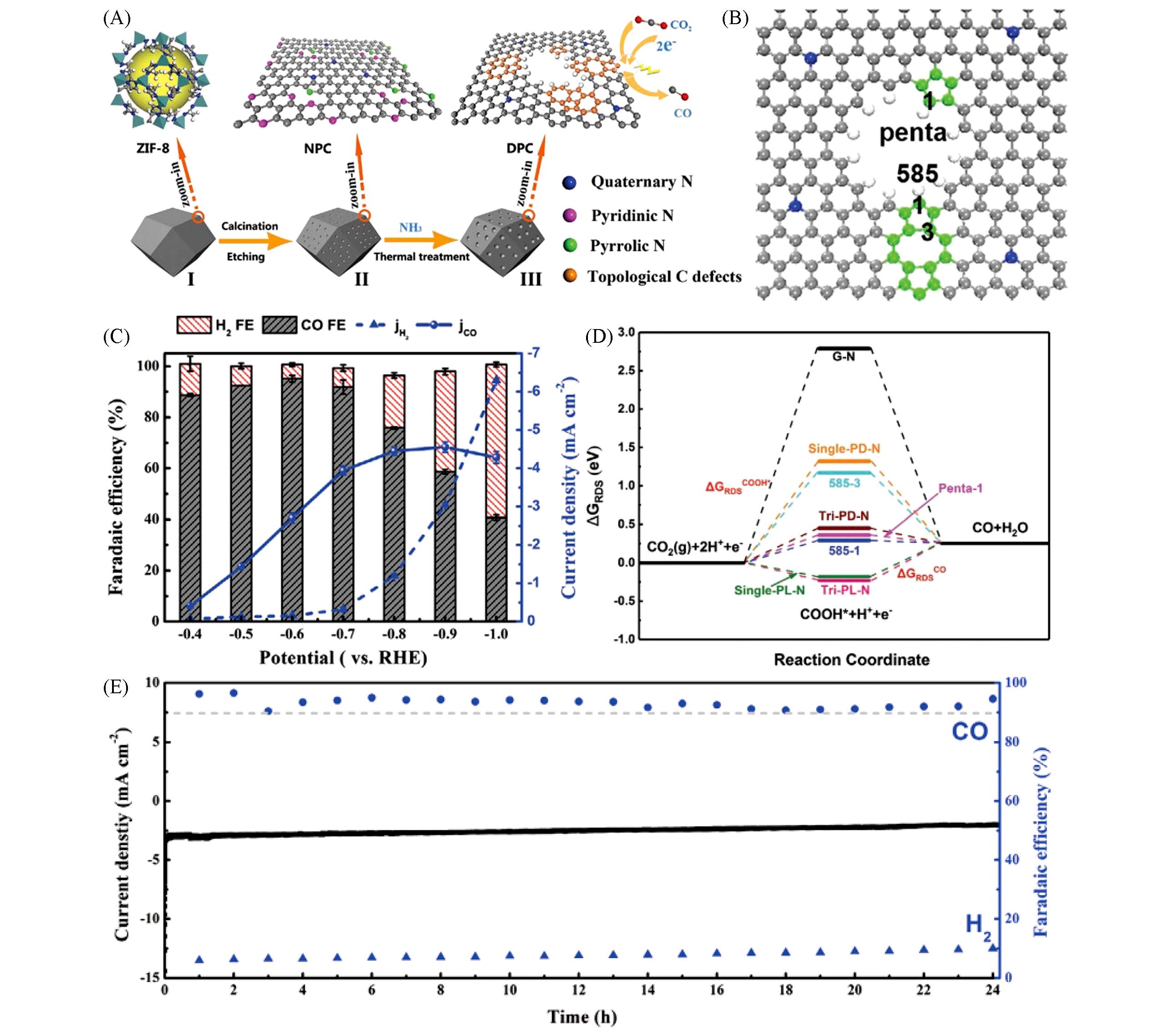
Fig.9 MOF?derived nonmetallic carbon?based materials for confinement electrocatalytic CO2RR[94](A) Schematic illustration of the synthetic route and the corresponding models of ZIF-8 precursor(I), 3D N-enriched porous carbon particles(II), and 3D topologically defected porous carbon particles(III); (B) the partial charge distribution at defect sites to illustrate their high activitie, C, O, N, and H atoms are represented by gray, red, blue, and white spheres, the green atoms emphasized the penta and 585 defects; (C) Faradaic efficiencies of CO(gray) and H2(red) and the partial current of CO on DPC-NH3-950 under a range of applied potentials; (D) the calculated free-energy diagram for CO2RR at N-doped sites, penta-hole, and 585-1(3) sites, G-N, single/tri-PD-N, and single/tri-PL-N refer to graphite-N, and single or triple pyridinic-N and pyrrolic-N, respectively; (E) the CO2RR stability test of DPC-NH3-950 under the potential of -0.6 V(vs, RHE) for 24 h. Copyright 2020, Wiley-VCH.
| 46 | Ji S., Chen Y., Wang X., Zhang Z., Wang D., Li Y., Chem. Rev., 2020, 120(21), 11900—11955 |
| 47 | Liu J., Yang D., Zhou Y., Zhang G., Xing G., Liu Y., Ma Y., Terasaki O., Yang S., Chen L., Angew. Chem. Int. Ed., 2021, 60(26), 14473—14479 |
| 48 | He T., Chen S., Ni B., Gong Y., Wu Z., Song L., Gu L., Hu W., Wang X., Angew. Chem. Int. Ed., 2018, 57(13), 3493—3498 |
| 49 | Qin L., Xu Z., Zheng Y., Li C., Mao J., Zhang G., Adv. Funct. Mater., 2020, 30(14), 1910257 |
| 50 | Yi J. D., Si D. H., Xie R., Yin Q., Zhang M. D., Wu Q., Chai G. L., Huang Y. B., Cao R., Angew. Chem. Int. Ed., 2021, 60(31), 17108—17114 |
| 51 | Dang Q., Huang H., Li L., Lyu X., Zhong S., Yu Y., Xu D., Chem. Mater., 2021, 33(14), 5690—5699 |
| 52 | Chen C., Chen H. Z., Qiao H. Y., Wang K., Xu L., Zhang N., Chem. J. Chinese Universities, 2016, 37(4), 723—727(陈超, 陈恒泽, 乔慧颖, 王凯, 徐力, 张宁. 高等学校化学学报, 2016, 37(4), 723—727) |
| 53 | Park C., Koo W. T., Chong S., Shin H., Kim Y. H., Cho H. J., Jang J. S., Kim D. H., Lee J., Park S., Ko J., Kim J., Kim I. D., Adv. Mater., 2021, 2101216 |
| 54 | Wang T. T., Kou Z. K., Mu S. C., Liu J. P., He D. P., Amiinu I. S., Meng W., Zhou K., Lou Z. X., Cheamchuen S., Verpoort F., Adv. Funct. Mater., 2018, 28(5), 1705048 |
| 55 | Usman M., Ali M., Al⁃Maythalony B. A., Ghanem A. S., Saadi O. W., Ali M., Jafar Mazumder M. A., Abdel⁃Azeim S., Habib M. A., Yamani Z. H., Ensinger W., ACS Appl. Mater. Inter., 2020, 12(44), 49992—50001 |
| 56 | Wroblowa H. S., Yen Chi P., Razumney G., J. Electroanal. Chem. Interfacial Electrochem., 1976, 69(2), 195—201 |
| 57 | Choi C. H., Lim H. K., Chung M. W., Chon G., Ranjbar Sahraie N., Altin A., Sougrati M. T., Stievano L., Oh H. S., Park E. S., Luo F., Strasser P., Dražić G., Mayrhofer K. J. J., Kim H., Jaouen F., Energy Environ. Sci., 2018, 11(11), 3176—3182 |
| 58 | Wang H. F., Chen L., Pang H., Kaskel S., Xu Q., Chem. Soc. Rev., 2020, 49(5), 1414—1448 |
| 59 | Ren Q., Wang H., Lu X. F., Tong Y. X., Li G. R., Adv. Sci., (Weinh), 2018, 5(3), 1700515 |
| 60 | Deng Y., Chi B., Tian X., Cui Z., Liu E., Jia Q., Fan W., Wang G., Dang D., Li M., Zang K., Luo J., Hu Y., Liao S., Sun X., Mukerjee S., J. Mater. Chem. A, 2019, 7(9), 5020—5030 |
| 61 | Lin Y., Liu P., Velasco E., Yao G., Tian Z., Zhang L., Chen L., Adv. Mater., 2019, 31(18), 1—9 |
| 62 | Yin H., Yuan P., Lu B. A., Xia H., Guo K., Yang G., Qu G., Xue D., Hu Y., Cheng J., Mu S., Zhang J. N., ACS Catal., 2021, 11(20), 12754—12762 |
| 63 | Jin H., Zhou H., He D., Wang Z., Wu Q., Liang Q., Liu S., Mu S., Appl. Catal. B: Environ., 2019, 250, 143—149 |
| 64 | Liu J., Fan C., Liu G., Jiang L., Appl. Surf. Sci., 2021, 538, 148017 |
| 65 | Xie X., He C., Li B., He Y., Cullen D. A., Wegener E. C., Kropf A. J., Martinez U., Cheng Y., Engelhard M. H., Bowden M. E., Song M., Lemmon T., Li X. S., Nie Z., Liu J., Myers D. J., Zelenay P., Wang G., Wu G., Ramani V., Shao Y., Nat. Catal., 2020, 3(12), 1044—1054 |
| 66 | Yin P., Yao T., Wu Y., Zheng L., Lin Y., Liu W., Ju H., Zhu J., Hong X., Deng Z., Zhou G., Wei S., Li Y., Angew. Chem. Int. Ed., 2016, 55(36), 10800—10805 |
| 67 | Tan Y., Zhu W., Zhang Z., Wu W., Chen R., Mu S., Lv H., Cheng N., Nano Energy, 2021, 83, 105813 |
| 68 | Qiao Y., Yuan P., Hu Y., Zhang J., Mu S., Zhou J., Li H., Xia H., He J., Xu Q., Adv. Mater., 2018, 30(46), 1804504 |
| 69 | Sanad M. F., Puente Santiago A. R., Tolba S. A., Ahsan M. A., Fernandez⁃Delgado O., Shawky Adly M., Hashem E. M., Mahrous Abodouh M., El⁃Shall M. S., Sreenivasan S. T., Allam N. K., Echegoyen L., J. Am. Chem. Soc., 2021, 143(10), 4064—4073 |
| 70 | Wang Z., Jin H., Meng T., Liao K., Meng W., Yang J., He D., Xiong Y., Mu S., Adv. Funct. Mater., 2018, 28(39), 1802596 |
| 71 | Zhao X., He X., Chen B., Yin F., Li G., Appl. Surf. Sci., 2019, 487, 1049—1057 |
| 72 | Fu X., Zamani P., Choi J. Y., Hassan F. M., Jiang G., Higgins D. C., Zhang Y., Hoque M. A., Chen Z., Adv. Mater., 2017, 29(7), 1604456 |
| 73 | Huynh M., Ozel T., Liu C., Lau E. C., Nocera D. G., Chem. Sci., 2017, 8(7), 4779—4794 |
| 74 | Zhang H., Chung H. T., Cullen D. A., Wagner S., Kramm U. I., More K. L., Zelenay P., Wu G., Energy Environ. Sci., 2019, 12(8), 2548—2558 |
| 75 | Martinez U., Komini Babu S., Holby E. F., Chung H. T., Yin X., Zelenay P., Adv. Mater., 2019, 31(31), 1—20 |
| 76 | Holby E. F., Wang G., Zelenay P., ACS Catal., 2020, 10(24), 14527—14539 |
| 77 | Chen Z., Liao X., Sun C., Zhao K., Ye D., Li J., Wu G., Fang J., Zhao H., Zhang J., Appl. Catal. B: Environ., 2021, 288, 120021 |
| 78 | Wang Y., Gan R., Liu H., Dirican M., Wei C., Ma C., Shi J., Zhang X., J. Mater. Chem. A, 2021, 9(5), 2764—2774 |
| 79 | Shi Q., Liu Q., Ma Y., Fang Z., Liang Z., Shao G., Tang B., Yang W., Qin L., Fang X., Adv. Energy Mater., 2020, 10(10), 1903854 |
| 80 | Liu X., Amiinu I. S., Liu S., Cheng K., Mu S., Nanoscale, 2016, 8(27), 13311—13320 |
| 81 | Morris R. H., Coord. Chem. Rev., 2017, 350, 105—116 |
| 82 | Muthurasu A., Tiwari A. P., Chhetri K., Dahal B., Kim H. Y., Nano Energy, 2021, 88, 106238 |
| 83 | Zhu Y., Zhang Z., Li W., Lei Z., Cheng N., Tan Y., Mu S., Sun X., ACS Sustain. Chem. Eng., 2019, 7(21), 17855—17862 |
| 84 | Song Z., Liu W., Cheng N., Norouzi Banis M., Li X., Sun Q., Xiao B., Liu Y., Lushington A., Li R., Liu L., Sun X., Mater. Horizons, 2017, 4(5), 900—907 |
| 85 | Yang Q., Xiao Z., Kong D., Zhang T., Duan X., Zhou S., Niu Y., Shen Y., Sun H., Wang S., Zhi L., Nano Energy, 2019, 66, 104096 |
| 86 | Zheng T., Jiang J., Wang J., Hu S., Ding W., Wei Z., Acta Phys. Chim. Sin., 2021, 37(11), 101—113 |
| 87 | Zhang Z., Ma C., Tu Y., Si R., Wei J., Zhang S., Wang Z., Li J. F., Wang Y., Deng D., Nano Research, 2019, 12(9), 2313—2317 |
| 88 | Lee S., Kim D., Lee J., Angew. Chem. Int. Ed., 2015, 54(49), 14701—14705 |
| 89 | Birdja Y. Y., Pérez⁃Gallent E., Figueiredo M. C., Göttle A. J., Calle⁃Vallejo F., Koper M. T. M., Nat. Energy, 2019, 4(9), 732—745 |
| 90 | Benson E. E., Kubiak C. P., Sathrum A. J., Smieja J. M., Chem. Soc. Rev., 2009, 38(1), 89—99 |
| 91 | Zhang Y. J., Sethuraman V., Michalsky R., Peterson A. A., ACS Catal., 2014, 4(10), 3742—3748 |
| 92 | Hori Y., Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry, Ed.: Vayenas C. G., White R. E., Gamboa⁃Aldeco M. E., Springer, New York, 2008, 89—189 |
| 93 | Wu Q., Gao J., Feng J., Liu Q., Zhou Y., Zhang S., Nie M., Liu Y., Zhao J., Liu F., Zhong J., Kang Z., J. Mater. Chem. A, 2020, 8(3), 1205—1211 |
| 94 | Dong Y., Zhang Q., Tian Z., Li B., Yan W., Wang S., Jiang K., Su J., Oloman C. W., Gyenge E. L., Ge R., Lu Z., Ji X., Chen L., Adv. Mater., 2020, 32(28), 1—10 |
| 95 | Zhao C., Dai X., Yao T., Chen W., Wang X., Wang J., Yang J., Wei S., Wu Y., Li Y., J. Am. Chem. Soc., 2017, 139(24), 8078—8081 |
| 96 | Wang X., Chen Z., Zhao X., Yao T., Chen W., You R., Zhao C., Wu G., Wang J., Huang W., Yang J., Hong X., Wei S., Wu Y., Li Y., Angew. Chem. Int. Ed., 2018, 57(7), 1944—1948 |
| 97 | Jiao L., Yang W., Wan G., Zhang R., Zheng X., Zhou H., Yu S. H., Jiang H. L., Angew. Chem. Int. Ed., 2020, 59(46), 20589—20595 |
| 98 | Ren W., Tan X., Yang W., Jia C., Xu S., Wang K., Smith S. C., Zhao C., Angew. Chem. Int. Ed., 2019, 58(21), 6972—6976 |
| 99 | Guo W., Sun X., Chen C., Yang D., Lu L., Yang Y., Han B., Green Chem., 2019, 21(3), 503—508 |
| 100 | Zhong H., Ghorbani⁃Asl M., Ly K. H., Zhang J., Ge J., Wang M., Liao Z., Makarov D., Zschech E., Brunner E., Weidinger I. M., Zhang J., Krasheninnikov A. V., Kaskel S., Dong R., Feng X., Nat. Commun., 2020, 11(1), 1409 |
| 101 | Kim M. K., Kim H. J., Lim H., Kwon Y., Jeong H. M., Electrochim. Acta, 2019, 306, 28—34 |
| 102 | Wang A., Li J., Zhang T., Nature Reviews Chemistry, 2018, 2(6), 65—81 |
| 103 | Yang X. F., Wang A., Qiao B., Li J., Liu J., Zhang T., Acc. Chem. Res., 2013, 46(8), 1740—1748 |
| 104 | Liu L., Corma A., Chem. Rev., 2018, 118(10), 4981—5079 |
| 105 | Qin R., Liu P., Fu G., Zheng N., Small Methods, 2018, 2(1), 1700286 |
| 106 | Chen Y., Ji S., Zhao S., Chen W., Dong J., Cheong W. C., Shen R., Wen X., Zheng L., Rykov A. I., Cai S., Tang H., Zhuang Z., Chen C., Peng Q., Wang D., Li Y., Nat. Commun., 2018, 9(1), 5422 |
| 107 | Wei S., Wang Y., Chen W., Li Z., Cheong W. C., Zhang Q., Gong Y., Gu L., Chen C., Wang D., Peng Q., Li Y., Chem. Sci., 2019, 11(3), 786—790 |
| 108 | Yoo M., Yu Y. S., Ha H., Lee S., Choi J. S., Oh S., Kang E., Choi H., An H., Lee K. S., Park J. Y., Celestre R., Marcus M. A., Nowrouzi K., Taube D., Shapiro D. A., Jung W., Kim C., Kim H. Y., Energy Environ. Sci., 2020, 13(4), 1231—1239 |
| 109 | Mohd Adli N., Shan W., Hwang S., Samarakoon W., Karakalos S., Li Y., Cullen D. A., Su D., Feng Z., Wang G., Wu G., Angew. Chem. Int. Ed., 2021, 60(2), 1022—1032 |
| 110 | Nam D. H., De Luna P., Rosas⁃Hernández A., Thevenon A., Li F., Agapie T., Peters J. C., Shekhah O., Eddaoudi M., Sargent E. H., Nat. Mater., 2020, 19(3), 266—276 |
| 111 | Jampaiah D., Damma D., Chalkidis A., Venkataswamy P., Bhargava S. K., Reddy B. M., Catal. Today, 2020, 356, 519—526 |
| 112 | Zhang X., Xie X. L., Xiong L. K., Peng Y., Chem. J. Chinese Universities, 2021, 42(9), 2824—2831(张想, 谢旭岚, 熊力堃, 彭扬. 高等学校化学学报, 2021, 42(9), 2824—2831) |
| 113 | Dong H., Zhang L., Li L., Deng W., Hu C., Zhao Z. J., Gong J., Small, 2019, 15(17), 1900289 |
| 114 | Jiao Y., Zheng Y., Jaroniec M., Qiao S. Z., J. Am. Chem. Soc., 2014, 136(11), 4394—4403 |
| 115 | Zhang L., Niu J., Li M., Xia Z., J. Phys. Chem. C, 2014, 118(7), 3545—3553 |
| 1 | Li W., Wang D., Zhang Y., Tao L., Wang T., Zou Y., Wang Y., Chen R., Wang S., Adv. Mater., 2020, 32(19), 1—20 |
| 2 | Tang L., Meng X., Deng D., Bao X., Adv. Mater., 2019, 31(50), 1—16 |
| 116 | Pei Z., Li H., Huang Y., Xue Q., Huang Y., Zhu M., Wang Z., Zhi C., Energy Environ. Sci., 2017, 10(3), 742—749 |
| 117 | Pei Z., Meng Q., Wei L., Fan J., Chen Y., Zhi C., Energy Storage Mater., 2020, 28, 55—63 |
| 118 | Zhao J., Lai H., Lyu Z., Jiang Y., Xie K., Wang X., Wu Q., Yang L., Jin Z., Ma Y., Liu J., Hu Z., Adv. Mater., 2015, 27(23), 3541—3545 |
| 3 | Banham D., Kishimoto T., Zhou Y., Sato T., Bai K., Ozaki J. I., Imashiro Y., Ye S., Sci. Adv., 2018, 4(3), 1—8 |
| 4 | Lin L., Liu T., Xiao J., Li H., Wei P., Gao D., Nan B., Si R., Wang G., Bao X., Angew. Chem. Int. Ed., 2020, 59(50), 22408—22413 |
| 119 | Jia Y., Zhang L., Zhuang L., Liu H., Yan X., Wang X., Liu J., Wang J., Zheng Y., Xiao Z., Taran E., Chen J., Yang D., Zhu Z., Wang S., Dai L., Yao X., Nat. Catal., 2019, 2(8), 688—695 |
| 120 | Wang Q., Lei Y., Wang D., Li Y., Energy Environ. Sci., 2019, 12(6), 1730—1750 |
| 5 | Lee J. W., Torres Pineda I., Lee J. H., Kang Y. T., Appl. Energy, 2016, 178, 164—176 |
| 6 | Jiao L., Seow J. Y. R., Skinner W. S., Wang Z. U., Jiang H. L., Mater. Today, 2019, 27, 43—68 |
| 7 | Nam D. H., Bushuyev O. S., Li J., De Luna P., Seifitokaldani A., Dinh C. T., Garcia de Arquer F. P., Wang Y., Liang Z., Proppe A. H., Tan C. S., Todorovic P., Shekhah O., Gabardo C. M., Jo J. W., Choi J., Choi M. J., Baek S. W., Kim J., Sinton D., Kelley S. O., Eddaoudi M., Sargent E. H., J. Am. Chem. Soc., 2018, 140(36), 11378—11386 |
| 8 | Li X., You S., Du J., Dai Y., Chen H., Cai Z., Ren N., Zou J., J. Mater. Chem. A, 2019, 7(45), 25853—25864 |
| 9 | Song Z., Zhang L., Doyle‐Davis K., Fu X., Luo J. L., Sun X., Adv. Energy Mater., 2020, 10(38), 2001561 |
| 10 | Srinivas K., Lu Y., Chen Y., Zhang W., Yang D., ACS Sustain. Chem. Eng., 2020, 8(9), 3820—3831 |
| 11 | Sankar S. S., Ede S. R., Anantharaj S., Karthick K., Sangeetha K., Kundu S., Catal. Sci. Technol., 2019, 9(8), 1847—1856 |
| 12 | Xing Z., Wang D., Meng T., Yang X., ACS Appl. Mater. Interfaces, 2020, 12(35), 39163—39169 |
| 13 | Cui X., Ren P., Ma C., Zhao J., Chen R., Chen S., Rajan N. P., Li H., Yu L., Tian Z., Deng D., Adv. Mater., 2020, 32(25), 1—7 |
| 14 | Yang M., Zhou Y. N., Cao Y. N., Tong Z., Dong B., Chai Y. M., Appl. Mater. Today, 2020, 20, 100692 |
| 15 | Zhang J., Liu C., Zhang B., Small Methods, 2019, 3(9), 1800481 |
| 16 | Zhu Y., Murali S., Cai W., Li X., Suk J. W., Potts J. R., Ruoff R. S., Adv. Mater., 2010, 22(35), 3906—3924 |
| 17 | Tang T., Jiang W. J., Liu X. Z., Deng J., Niu S., Wang B., Jin S. F., Zhang Q., Gu L., Hu J. S., Wan L. J., J. Am. Chem. Soc., 2020, 142(15), 7116—7127 |
| 18 | He C., Zhang Y., Zhang Y., Zhao L., Yuan L. P., Zhang J., Ma J., Hu J. S., Angew. Chem. Int. Ed., 2020, 59(12), 4914—4919 |
| 19 | Zhang J., Jiang W. J., Niu S., Zhang H., Liu J., Li H., Huang G. F., Jiang L., Huang W. Q., Hu J. S., Hu W., Adv. Mater., 2020, 32(11), 1—15 |
| 20 | Choi I., Jung Y. E., Yoo S. J., Kim J. Y., Kim H. J., Lee C. Y., Jang J. H., J. Electrochem. Sci. Te., 2017, 8(1), 61—68 |
| 21 | Furukawa H., Cordova K. E., O'Keeffe M., Yaghi O. M., Science, 2013, 341(6149), 1230444 |
| 22 | Deng H., Grunder S., Cordova K. E., Valente C., Furukawa H., Hmadeh M., Gandara F., Whalley A. C., Liu Z., Asahina S., Kazumori H., O'Keeffe M., Terasaki O., Stoddart J. F., Yaghi O. M., Science, 2012, 336(6084), 1018—1023 |
| 23 | Gascon J., Corma A., Kapteijn F., Llabrés i Xamena F. X., ACS Catal., 2013, 4(2), 361—378 |
| 24 | Vilhelmsen L. B., Walton K. S., Sholl D. S., J. Am. Chem. Soc., 2012, 134(30), 12807—12816 |
| 25 | Ma Y., Peng H., Liu J., Wang Y., Hao X., Feng X., Khan S. U., Tan H., Li Y., Inorg. Chem., 2018, 57(7), 4109—4116 |
| 26 | Zhu Q. L., Xu Q., Chem. Soc. Rev., 2014, 43(16), 5468—5512 |
| 27 | Jiang J., Yaghi O. M., Chem. Rev., 2015, 115(14), 6966—6997 |
| 28 | Kobayashi H., Mitsuka Y., Kitagawa H., Inorg. Chem., 2016, 55(15), 7301—7310 |
| 29 | Yang Q., Xu Q., Jiang H. L., Chem. Soc. Rev., 2017, 46(15), 4774—4808 |
| 30 | McCarthy B. D., Beiler A. M., Johnson B. A., Liseev T., Castner A. T., Ott S., Coord. Chem. Rev., 2020, 406, 213137 |
| 31 | Wen J., Li Y., Gao J., Chem. Res. Chinese Universities, 2020, 36(4), 662—679 |
| 32 | Lei J., Zeng M., Fu L., Chem. Res. Chinese Universities, 2020, 36(4), 504—510 |
| 33 | Shi X. F., Zhu J., Bai T. Y., Fu Z. X., Zhang J. J., Bu X. H., Chem. J. Chinese Universities, 2022, 43(1), 20210613(史潇凡, 朱 剑, 白田宇, 付子萱, 张冀杰, 卜显和. 高等学校化学学报, 2022, 43(1), 20210613) |
| 34 | Jin E., Song K. X., Cui L. L., Chem. J. Chinese Universities, 2020, 41(6), 1362—1369(金娥, 宋开绪, 崔丽莉. 高等学校化学学报,2020, 41(6), 1362—1369) |
| 35 | Han X., Ling X., Wang Y., Ma T., Zhong C., Hu W., Deng Y., Angew. Chem. Int. Ed., 2019, 58(16), 5359—5364 |
| 36 | He Y., Shi Q., Shan W., Li X., Kropf A. J., Wegener E. C., Wright J., Karakalos S., Su D., Cullen D. A., Wang G., Myers D. J., Wu G., Angew. Chem. Int. Ed., 2021, 60(17), 9516—9526 |
| 37 | Fang S., Zhu X., Liu X., Gu J., Liu W., Wang D., Zhang W., Lin Y., Lu J., Wei S., Li Y., Yao T., Nat. Commun., 2020, 11(1), 1029 |
| 38 | Zhou H., He D., Saana A. I., Yang J., Wang Z., Zhang J., Liang Q., Yuan S., Zhu J., Mu S., Nanoscale, 2018, 10(13), 6147—6154 |
| 39 | Zhu Z., Yang Y., Guan Y., Xue J., Cui L., J. Mater. Chem. A, 2016, 4(40), 15536—15545 |
| 40 | Wang Y., Pan Y., Zhu L., Yu H., Duan B., Wang R., Zhang Z., Qiu S., Carbon, 2019, 146, 671—679 |
| 41 | Jia Y., Chen J., Yao X., Mater. Chem. Front., 2018, 2(7), 1250—1268 |
| 42 | Jia Y., Zhang L., Du A., Gao G., Chen J., Yan X., Brown C. L., Yao X., Adv. Mater., 2016, 28(43), 9532—9538 |
| 43 | Wang J., Huang Z., Liu W., Chang C., Tang H., Li Z., Chen W., Jia C., Yao T., Wei S., Wu Y., Li Y., J. Am. Chem. Soc., 2017, 139(48), 17281—17284 |
| 44 | Chen Y., Ji S., Wang Y., Dong J., Chen W., Li Z., Shen R., Zheng L., Zhuang Z., Wang D., Li Y., Angew. Chem. Int. Ed., 2017, 56(24), 6937—6941 |
| 45 | Li J., Chen M., Cullen D. A., Hwang S., Wang M., Li B., Liu K., Karakalos S., Lucero M., Zhang H., Lei C., Xu H., Sterbinsky G. E., Feng Z., Su D., More K. L., Wang G., Wang Z., Wu G., Nat. Catal., 2018, 1(12), 935—945 |
| [1] | FAN Jianling, TANG Hao, QIN Fengjuan, XU Wenjing, GU Hongfei, PEI Jiajing, CEHN Wenxing. Nitrogen Doped Ultra-thin Carbon Nanosheet Composited Platinum-ruthenium Single Atom Alloy Catalyst for Promoting Electrochemical Hydrogen Evolution Process [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220366. |
| [2] | CHENG Qian, YANG Bolong, WU Wenyi, XIANG Zhonghua. S-doped Fe-N-C as Catalysts for Highly Reactive Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220341. |
| [3] | CHU Yuyi, LAN Chang, LUO Ergui, LIU Changpeng, GE Junjie, XING Wei. Single-atom Cerium Sites Designed for Durable Oxygen Reduction Reaction Catalyst with Weak Fenton Effect [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220294. |
| [4] | LI Yulong, XIE Fating, GUAN Yan, LIU Jiali, ZHANG Guiqun, YAO Chao, YANG Tong, YANG Yunhui, HU Rong. A Ratiometric Electrochemical Sensor Based on Silver Ion Interaction with DNA for the Detection of Silver Ion [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220202. |
| [5] | DING Yang, WANG Wanhui, BAO Ming. Recent Progress in Porous Framework-immobilized Molecular Catalysts for CO2 Hydrogenation to Formic Acid [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220309. |
| [6] | JIANG Hongbin, DAI Wenchen, ZHANG Rao, XU Xiaochen, CHEN Jie, YANG Guang, YANG Fenglin. Research on Co3O4/UiO-66@α-Al2O3 Ceramic Membrane Separation and Catalytic Spraying Industry VOCs Waste Gas [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220025. |
| [7] | LI Jiafu, ZHANG Kai, WANG Ning, SUN Qiming. Research Progress of Zeolite-encaged Single-atom Metal Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220032. |
| [8] | GU Yu, XI Baojuan, LI Jiangxiao, XIONG Shenglin. Structure Regulation of Single-atom Catalysts in Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220036. |
| [9] | JIN Xiangyuan, ZHANG Libing, SUN Xiaofu, HAN Buxing. Electrocatalytic CO2 Reduction over Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220035. |
| [10] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [11] | ZHOU Ying, HE Peinan, FENG Haisong, ZHANG Xin. Optimal Distribution of Active Sites of CO2 Reduction Reaction Catalyzed by Diatomic Site M-N-C [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210640. |
| [12] | HE Yujing, LI Jiale, WANG Dongyang, WANG Fuling, XIAO Zuoxu, CHEN Yanli. Zinc-based Activated Fe/Co/N Doped Biomass Carbon Electrocatalysts with High Oxygen Reduction Activity [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220475. |
| [13] | WU Yaqiang, LIU Siming, JIN Shunjin, YAN Yongqing, WANG Zhao, CHEN Lihua, SU Baolian. Synthesis of Zn-Doped NiCoP Catalyst with Porous Double-layer Nanoarray Structure and Its Electrocatalytic Properties for Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2021, 42(8): 2483. |
| [14] | CHANG Shuqing, XIN Xu, HUANG Yaqi, ZHANG Xincong, FU Yanghe, ZHU Weidong, ZHANG Fumin, LI Xiaona. Pyroelectrically-induced Catalytic Performance of Zr-based MOF Under Cold-hot Alternation [J]. Chem. J. Chinese Universities, 2021, 42(8): 2558. |
| [15] | YANG Tao, YAO Huiying, LI Qing, HAO Wei, CHI Lifeng, ZHU Jia. Density Functional Theoretical Studies on the Promising Electrocatalyst of M-BHT(M=Co or Cu) for CO2 Reduction to CH4 [J]. Chem. J. Chinese Universities, 2021, 42(4): 1268. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||