

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (9): 1962.doi: 10.7503/cjcu20140209
• Physical Chemistry • Previous Articles Next Articles
WANG Qiang1, WANG Rui2,*( ), XU Haibo3
), XU Haibo3
Received:2014-03-13
Online:2014-09-10
Published:2019-08-01
Contact:
WANG Rui
E-mail:957599038@qq.com
Supported by:CLC Number:
TrendMD:
WANG Qiang, WANG Rui, XU Haibo. Preparation and Electrocatalytic Properties of Ir0.08Ti0.92O2 and Pt/Ir0.08Ti0.92
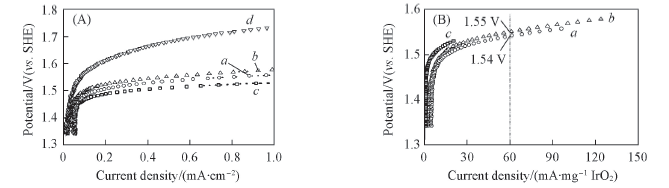
Fig.5 Anodic polarization curves relative to the apparent surface area(A) and the mass of IrO2(B) of Ir0.08Ti0.92O2(a), Pt/Ir0.08Ti0.92O2(b), IrO2(c) and Pt/C(d) in 0.5 mol/L H2SO4
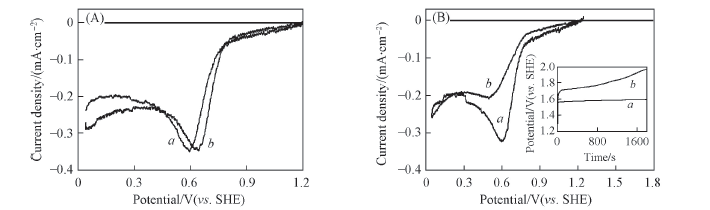
Fig.6 Linear sweep voltammetry curves of Pt/Ir0.08Ti0.92O2(a) and Pt/C(b) in 0.5 mol/L oxygen-aerated H2SO4 before(A) and after(B) oxygen revolution reaction(1 mA/cm2, 30 min) The inset of (B) shows the chronopotentiometry curves.
| [1] | Millera M., Bazylak A., J. Power Sources,2011, 196(2), 601—613 |
| [2] | Yuan X. X., Xia X. Y., Zeng X., Zhang H. J., Ma Z. F., Prog. Chem., 2010, 22(1), 19—31 |
| (原鲜霞, 夏小芸, 曾鑫, 张慧娟, 马紫峰. 化学进展, 2010, 22(1), 19—31) | |
| [3] | Yu H. M., Yi B. L., Scientia Sinica Chimica,2012, 42(4), 480—494 |
| (俞红梅, 衣宝廉. 中国科学: 化学, 2012, 42(4), 480—494) | |
| [4] | Yuan X. Z., Li H., Zhang S. S., Martin J., Wang H. J., J. Power Sources,2011, 196(22), 9107—9116 |
| [5] | Walsh F. C., Wills R. G. A., Electrochim. Acta,2010, 55(22), 6342—6351 |
| [6] | Antolini E., Gonzalez E. R., Solid State Ionics,2009, 180(9/10), 746—763 |
| [7] | Subban C., Zhou Q., Leonard B., Ranjan C., Edvenson H. M., Disalvo F. J., Munie S., Hunting J., Phil. Trans. R. Soc. A,2010, 368, 3243—3253 |
| [8] | Krstajic N. V., Vracar L. M., Radmilovic V. R., Neophytides S. G., Labou M., Jaksic J. M., Tunold R., Falaras P., Jaksic M. M., Surf. Sci., 2007, 601(9), 1949—1966 |
| [9] | Hou M., Yi B. L., J. Electrochem., 2012, 18(1), 1—13 |
| (侯明, 衣宝廉. 电化学, 2012, 18(1), 1—13) | |
| [10] | Millet P., Ngameni R., Grigoriev S. A., Fateev V. N., Int. J. Hydrogen Energ., 2011, 36(6), 4156—4163 |
| [11] | Aricò A. S., Siracusano S., Briguglio N., Baglio V., Di Blasi A., Antonucci V., J. Appl. Electrochem., 2013, 43(2), 107—118 |
| [12] | Siracusano S., Baglio V., D’Urso C., Antonucci V., Aricò A. S., Electrochim. Acta,2009, 54(26), 6292—6299 |
| [13] | Li G. F., Yu H. M., Wang X. Y., Yang D. L., Li Y. K., Shao Z. G., Yi B. L., J. Power Sources,2014, 249(1), 175—184 |
| [14] | Li G. F., Yu H. M., Wang X. Y., Sun S. C., Li Y. K., Shao Z. G., Yi B. L., Phys. Chem. Chem. Phys., 2013, 15(8), 2858—2866 |
| [15] | Sun R. X., Xu H. B., Wan N. F., Wang J., Chem. J. Chinese Universities,2007, 28(5), 904—908 |
| (孙仁兴, 徐海波, 万年坊, 王佳. 高等学校化学学报, 2007, 28(5), 904—908) | |
| [16] | Park S., Shao Y., Liu J., Wang Y., Energy Environ. Sci., 2012, 5(11), 9331—9344 |
| [17] | Gorlin Y., Jaramillo T. F., J. Am. Chem. Soc., 2010, 132(39), 13612—13614 |
| [18] | Zhang G., Shao Z. G., Lu W. T., Li G. F., Liu F. Q., Yi B. L., Electrochem. Commun., 2012, 22, 145—148 |
| [19] | Kong F. D., Zhang S., Yin G. P., Zhang N., Wang Z. B., Du C. Y., Electrochem. Commun., 2012, 14, 63—66 |
| [20] | Kong F. D., Zhang S., Yin G. P., Wang Z. B., Du C. Y., Chen G. Y., Zhang N., Int. J. Hydrogen Energ., 2012, 37, 59—67 |
| [21] | Kong F. D., Zhang S., Yin G. P., Zhang N., Wang Z. B., Du C. Y., J. Power Sources,2012, 210, 321—326 |
| [22] | Cruz J. C., Rivas S., Beltran D., Meas Y., Ornelas R., Osorio-Monreal G., Ortiz-Frade L., Ledesma-Garcia J., Arriaga L. G., Int. J. Hydrogen Energ., 2012, 37, 13522—13528 |
| [23] | Huang S. Y., Ganesan P., Jung H. Y., Popov B. N., J. Power Sources,2012, 198(1), 23—29 |
| [24] | Xu H. B., Lu Y. H., Li C. H., Hu J. Z., J. Appl. Electrochem., 2010, 40(4), 719—727 |
| [25] | Xu H. B., Lu Y. H., Wang J., Sun R. X., Nanometer Powder Catalyst and Its Preparation Method, US 12441880,2011-04-12 |
| [26] | Wang A. P., Xu H. B., Lu Y. H., Hu J. Z., Tian B. L., Dong H., Chin. J. Catal., 2009, 30(3), 179—181 |
| (王爱萍, 徐海波, 芦永红, 胡杰珍, 田丙伦, 董辉. 催化学报, 2009, 30(3), 179—181) | |
| [27] | Kong X. F., Shen J. Z., Lu T. H., Lu Y. H., Wang W., Fan X. Z., Xu H. B., Chem. J. Chinese Universities,2011, 32(2), 350—354 |
| (孔祥峰, 沈娟章, 陆天虹, 芦永红, 王伟, 范新庄, 徐海波. 高等学校化学学报, 2011, 32(2), 350—354) | |
| [28] | Siracusano S., Baglio V., Di Blasi A., Briguglio N., Stassi A., Ornelas R., Trifoni E., Antonucci V., Aricò A. S., Int. J. Hydrogen Energ., 2010, 35(11), 5558—5568 |
| [29] | Zhang Z.X., Zhao G. P., Luo X. J., Xu Y. H., Introduction to the Titanium Electrode, Metallurgical Ind. Press, Beijing, 2008, 369—371 |
| (张招贤, 赵国鹏, 罗小军, 徐永海. 钛电极学导论. 北京: 冶金工业出版社, 2008, 369—371) | |
| [30] | Pai Y. H., Tseng C. W., J. Power Sources,2012, 202(1), 28—34 |
| [1] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [2] | FAN Jianling, TANG Hao, QIN Fengjuan, XU Wenjing, GU Hongfei, PEI Jiajing, CEHN Wenxing. Nitrogen Doped Ultra-thin Carbon Nanosheet Composited Platinum-ruthenium Single Atom Alloy Catalyst for Promoting Electrochemical Hydrogen Evolution Process [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220366. |
| [3] | LIN Zhi, PENG Zhiming, HE Weiqing, SHEN Shaohua. Single-atom and Cluster Photocatalysis: Competition and Cooperation [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220312. |
| [4] | CHENG Qian, YANG Bolong, WU Wenyi, XIANG Zhonghua. S-doped Fe-N-C as Catalysts for Highly Reactive Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220341. |
| [5] | TENG Zhenyuan, ZHANG Qitao, SU Chenliang. Charge Separation and Surface Reaction Mechanisms for Polymeric Single-atom Photocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220325. |
| [6] | WANG Ruyue, WEI Hehe, HUANG Kai, WU Hui. Freezing Synthesis for Single Atom Materials [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220428. |
| [7] | YANG Jingyi, LI Qinghe, QIAO Botao. Synergistic Catalysis Between Ir Single Atoms and Nanoparticles for N2O Decomposition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220388. |
| [8] | LIN Gaoxin, WANG Jiacheng. Progress and Perspective on Molybdenum Disulfide with Single-atom Doping Toward Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220321. |
| [9] | REN Shijie, QIAO Sicong, LIU Chongjing, ZHANG Wenhua, SONG Li. Synchrotron Radiation X-Ray Absorption Spectroscopy Research Progress on Platinum Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220466. |
| [10] | CHU Yuyi, LAN Chang, LUO Ergui, LIU Changpeng, GE Junjie, XING Wei. Single-atom Cerium Sites Designed for Durable Oxygen Reduction Reaction Catalyst with Weak Fenton Effect [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220294. |
| [11] | QIU Xinsheng, WU Qin, SHI Daxin, ZHANG Yaoyuan, CHEN Kangcheng, LI Hansheng. Preparation and High Temperature Fuel Cell Performance of Ionic Crosslinked Sulfonated Polyimides for Proton Exchange Membranes [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220140. |
| [12] | WEI Chunhong, JIANG Qian, WANG Panpan, JIANG Chengfa, LIU Yuefeng. Atomic Scale Investigation of Pt Atoms/clusters Promoted Co-catalyzed Fischer-Tropsch Synthesis [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220074. |
| [13] | ZHANG Xinxin, XU Di, WANG Yanqiu, HONG Xinlin, LIU Guoliang, YANG Hengquan. Effect of Mn Promoter on CuFe-based Catalysts for CO2 Hydrogenation to Higher Alcohols [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220187. |
| [14] | ZHAO Runyao, JI Guipeng, LIU Zhimin. Efficient Electrocatalytic CO2 Reduction over Pyrrole Nitrogen-coordinated Single-atom Copper Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220272. |
| [15] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||