

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (1): 96.doi: 10.7503/cjcu20180589
• Physical Chemistry • Previous Articles Next Articles
MA Yucong1, FAN Baomin1,*( ), HAO Hua2, LÜ Jinyu1, FENG Yunhao3, YANG Biao1
), HAO Hua2, LÜ Jinyu1, FENG Yunhao3, YANG Biao1
Received:2018-08-23
Online:2019-01-10
Published:2018-12-20
Contact:
FAN Baomin
E-mail:fanbaomin@btbu.edu.cn
Supported by:CLC Number:
TrendMD:
MA Yucong,FAN Baomin,HAO Hua,LÜ Jinyu,FENG Yunhao,YANG Biao. Experimental and Theoretical Studies of Action Mechanism of an Octadecylamine-based Molecular Assembly on Mild Steel†[J]. Chem. J. Chinese Universities, 2019, 40(1): 96.
| pH | Conductivity/ (μS·cm-1) | Concentration of dissolved oxygen/(mg·L-1) | Concentration of Na+/ (μg·L-1) | Concentration of Cl-/ (μg·L-1) |
|---|---|---|---|---|
| 5.89 | 108.32 | 2.11 | 66.13 | 129.19 |
Table 1 Water parameters of the sampled condensate water at 25 ℃
| pH | Conductivity/ (μS·cm-1) | Concentration of dissolved oxygen/(mg·L-1) | Concentration of Na+/ (μg·L-1) | Concentration of Cl-/ (μg·L-1) |
|---|---|---|---|---|
| 5.89 | 108.32 | 2.11 | 66.13 | 129.19 |
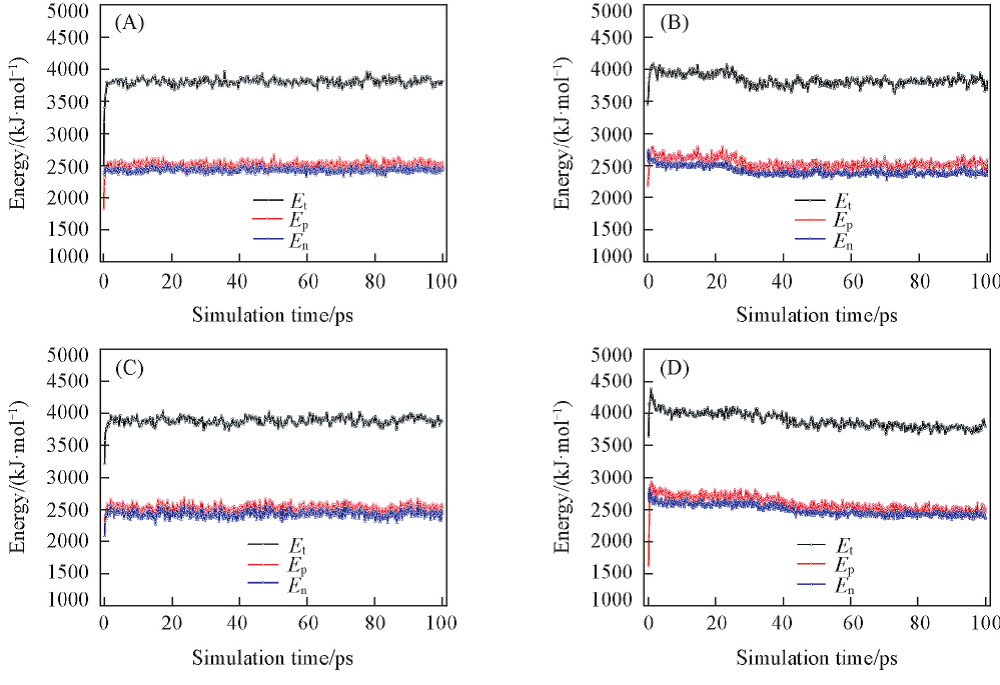
Fig.2 Calculated energies of CDDA for different configurations(A) Wide towards narrow; (B) wide towards wide; (C) narrow towards narrow; (D) narrow towards wide.
| Configuration | Et/(kJ·mol-1) | Ep/(kJ·mol-1) | En/(kJ·mol-1) |
|---|---|---|---|
| Wide towards narrow | 3820.59 | 2534.28 | 2444.70 |
| Wide towards wide | 3828.99 | 2562.72 | 2420.12 |
| Narrow towards narrow | 3830.92 | 2497.26 | 2464.99 |
| Narrow towards wide | 3815.06 | 2518.11 | 2402.59 |
Table 2 Equilibrium values of total energy(Et), potential energy(Ep) and non-bond energy(En) for different spatial configurations of CDDA
| Configuration | Et/(kJ·mol-1) | Ep/(kJ·mol-1) | En/(kJ·mol-1) |
|---|---|---|---|
| Wide towards narrow | 3820.59 | 2534.28 | 2444.70 |
| Wide towards wide | 3828.99 | 2562.72 | 2420.12 |
| Narrow towards narrow | 3830.92 | 2497.26 | 2464.99 |
| Narrow towards wide | 3815.06 | 2518.11 | 2402.59 |
| Temperature/℃ | c(CDDA)/(mol·L-1) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.3 | 0.6 | 1 | |
| 35 | 0.118 | 0.021(82.2) | 0.013(89.0) | 0.011(90.7) | 0.009(92.4) | 0.007(94.1) |
| 45 | 0.153 | 0.030(80.4) | 0.021(86.3) | 0.016(89.5) | 0.013(91.5) | 0.010(93.5) |
| 55 | 0.202 | 0.055(72.8) | 0.044(78.2) | 0.028(86.1) | 0.021(89.6) | 0.018(91.1) |
| 65 | 0.210 | 0.064(69.5) | 0.052(75.2) | 0.041(80.5) | 0.030(85.7) | 0.022(89.5) |
| 75 | 0.233 | 0.082(64.8) | 0.061(73.8) | 0.048(79.4) | 0.040(82.8) | 0.033(85.8) |
Table 3 Corrosion rates(g·m-2·h-1) of Q235 steel with various concentrations of CDDA in condensate water under preset temperatures along with the corresponding corrosion efficiencies(ηw, %) in the brackets
| Temperature/℃ | c(CDDA)/(mol·L-1) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.3 | 0.6 | 1 | |
| 35 | 0.118 | 0.021(82.2) | 0.013(89.0) | 0.011(90.7) | 0.009(92.4) | 0.007(94.1) |
| 45 | 0.153 | 0.030(80.4) | 0.021(86.3) | 0.016(89.5) | 0.013(91.5) | 0.010(93.5) |
| 55 | 0.202 | 0.055(72.8) | 0.044(78.2) | 0.028(86.1) | 0.021(89.6) | 0.018(91.1) |
| 65 | 0.210 | 0.064(69.5) | 0.052(75.2) | 0.041(80.5) | 0.030(85.7) | 0.022(89.5) |
| 75 | 0.233 | 0.082(64.8) | 0.061(73.8) | 0.048(79.4) | 0.040(82.8) | 0.033(85.8) |
| c(CDDA)/(mmol·L-1) | Ecorr/mV | Jcorr/(μA·cm-2) | βa/(mV·dec-1) | βc/(mV·dec-1) | ηp(%) |
|---|---|---|---|---|---|
| 0 | -538.02 | 29.89 | 69.28 | -504.45 | —— |
| 0.05 | -495.82 | 4.90 | 222.47 | -462.29 | 83.6 |
| 0.1 | -490.06 | 2.36 | 253.04 | -484.93 | 92.1 |
| 0.3 | -492.89 | 2.01 | 252.97 | -481.25 | 93.3 |
| 0.6 | -478.53 | 1.94 | 258.49 | -478.06 | 93.5 |
| 1 | -462.73 | 1.88 | 251.29 | -479.89 | 93.7 |
Table 4 Electrochemical parameters and inhibition efficiencies for Q235 steel derived from cathodic and anodic polarization measurements
| c(CDDA)/(mmol·L-1) | Ecorr/mV | Jcorr/(μA·cm-2) | βa/(mV·dec-1) | βc/(mV·dec-1) | ηp(%) |
|---|---|---|---|---|---|
| 0 | -538.02 | 29.89 | 69.28 | -504.45 | —— |
| 0.05 | -495.82 | 4.90 | 222.47 | -462.29 | 83.6 |
| 0.1 | -490.06 | 2.36 | 253.04 | -484.93 | 92.1 |
| 0.3 | -492.89 | 2.01 | 252.97 | -481.25 | 93.3 |
| 0.6 | -478.53 | 1.94 | 258.49 | -478.06 | 93.5 |
| 1 | -462.73 | 1.88 | 251.29 | -479.89 | 93.7 |
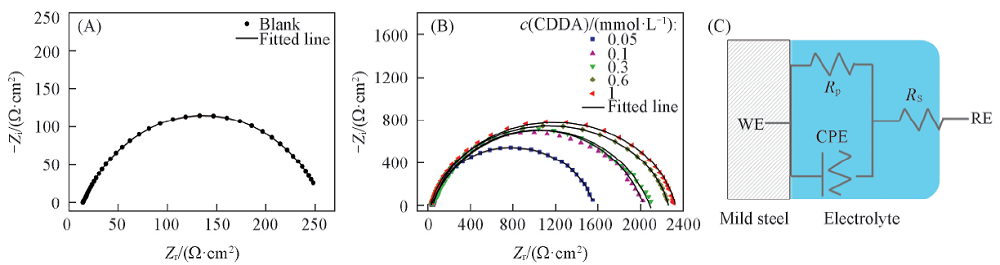
Fig.4 Impedance spectra for Q235 steel in the condensate water at 35 ℃ without(A) and with(B) various concentrations of CDDA in Nyquist form along with the equivalent electric circuit(C)WE: working electrode; RE: reference electrode.
| c(CDDA)/(mmol·L-1) | Rp/(Ω·cm2) | Cdl/(μF·cm-2) | n | ηE(%) | |
|---|---|---|---|---|---|
| 0 | 255.21 | 223.53 | 0.79 | 243.93 | —— |
| 0.05 | 1589.18 | 83.55 | 0.82 | 1598.41 | 84.7 |
| 0.1 | 1999.30 | 72.18 | 0.77 | 2003.76 | 87.8 |
| 0.3 | 2060.62 | 50.49 | 0.80 | 2079.39 | 88.3 |
| 0.6 | 2265.85 | 30.42 | 0.81 | 2289.16 | 89.3 |
| 1 | 2330.47 | 27.40 | 0.83 | 2358.32 | 89.7 |
Table 5 Impedance parameters for Q235 steel in the condensate water with various concentrations of CDDA
| c(CDDA)/(mmol·L-1) | Rp/(Ω·cm2) | Cdl/(μF·cm-2) | n | ηE(%) | |
|---|---|---|---|---|---|
| 0 | 255.21 | 223.53 | 0.79 | 243.93 | —— |
| 0.05 | 1589.18 | 83.55 | 0.82 | 1598.41 | 84.7 |
| 0.1 | 1999.30 | 72.18 | 0.77 | 2003.76 | 87.8 |
| 0.3 | 2060.62 | 50.49 | 0.80 | 2079.39 | 88.3 |
| 0.6 | 2265.85 | 30.42 | 0.81 | 2289.16 | 89.3 |
| 1 | 2330.47 | 27.40 | 0.83 | 2358.32 | 89.7 |
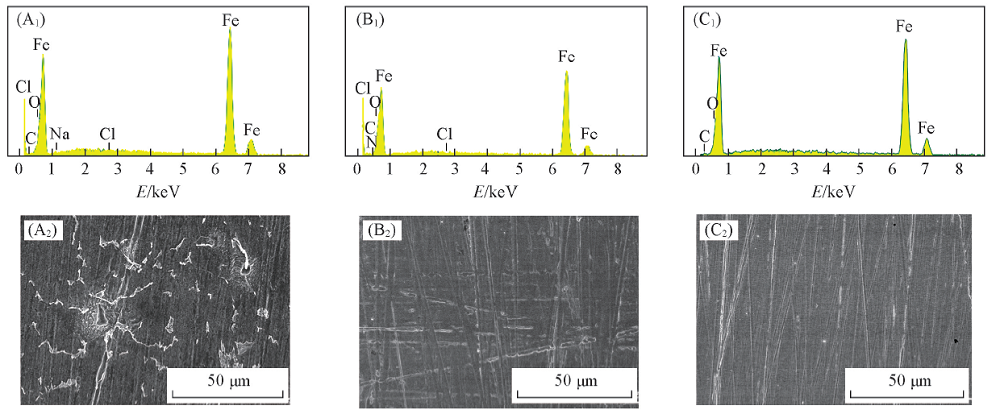
Fig.5 EDX spectra(A1—C1) and surface morphologies(A2—C2) of Q235 steel before and after immersion in condensate water for 72 h (A1, A2) Without CDDA; (B1, B2) with 1 mmol/L CDDA; (C1, C2) freshly polished.
Fig.6 Two-dimension AFM images(A1—C1) and sectional analyses(A2—C2) of Q235 steel surface before and after immersion in condensate water at 35 ℃ for 72 h(A1, A2) Without inhibitor; (B1, B2) with 1 mmol/L CDDA; (C1, C2) freshly polished.
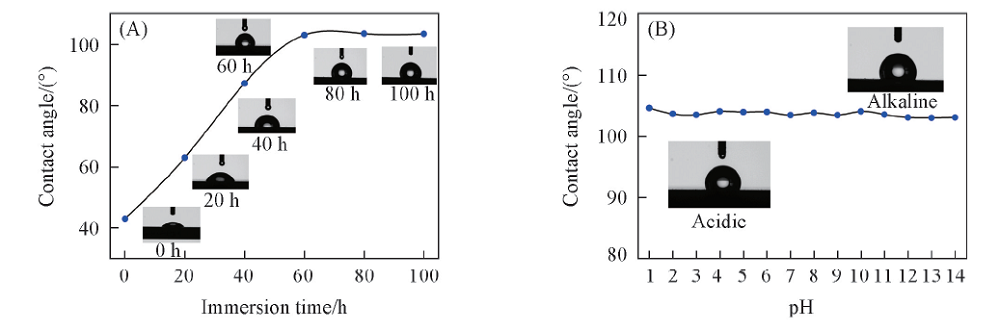
Fig.7 Variation of contact angles for Q235 steel after immersion in condensate water containing 1 mmol/L CDDA with different time(A) and under different pH values after 72 h immersion(B)
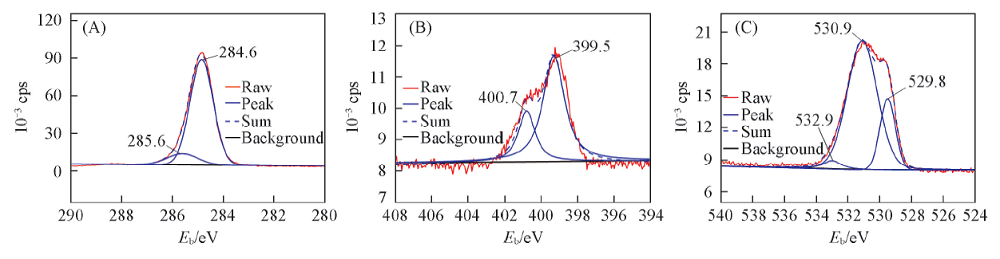
Fig.8 XPS deconvoluted spectra of major elements on Q235 steel after immersion in condensate water with 1 mmol/L CDDA at 35 ℃ for 72 h(A) C1s; (B) N1s; (C) O1s.
| Atom | Atom | ||||
|---|---|---|---|---|---|
| C1 | 0.019 | 0.004 | C11 | 0.022 | 0.005 |
| C2 | 0.010 | 0 | C12 | 0.023 | 0.008 |
| C3 | 0.019 | 0.001 | C13 | 0.030 | 0.011 |
| C4 | 0.024 | 0.002 | C14 | 0.031 | 0.017 |
| C5 | 0.028 | 0 | C15 | 0.032 | 0.025 |
| C6 | 0.027 | 0.002 | C16 | 0.059 | 0.038 |
| C7 | 0.027 | 0.001 | C17 | 0.091 | 0.058 |
| C8 | 0.026 | 0.002 | C18 | 0.121 | 0.223 |
| C9 | 0.013 | 0.003 | N19 | 0.015 | 0.367 |
| C10 | 0.022 | 0.004 |
Table 6 Condensed Fukui indices of ODA*
| Atom | Atom | ||||
|---|---|---|---|---|---|
| C1 | 0.019 | 0.004 | C11 | 0.022 | 0.005 |
| C2 | 0.010 | 0 | C12 | 0.023 | 0.008 |
| C3 | 0.019 | 0.001 | C13 | 0.030 | 0.011 |
| C4 | 0.024 | 0.002 | C14 | 0.031 | 0.017 |
| C5 | 0.028 | 0 | C15 | 0.032 | 0.025 |
| C6 | 0.027 | 0.002 | C16 | 0.059 | 0.038 |
| C7 | 0.027 | 0.001 | C17 | 0.091 | 0.058 |
| C8 | 0.026 | 0.002 | C18 | 0.121 | 0.223 |
| C9 | 0.013 | 0.003 | N19 | 0.015 | 0.367 |
| C10 | 0.022 | 0.004 |
| [1] | Fan B. M., Wei B. Y., Hao H., Feng Y. H., Yang B., Mater. Sci. Forum,2018, 913, 424—438 |
| [2] | Fan B. M., Wei G., Zhang Z., Qiao N., Corros. Sci., 2014, 83, 75—85 |
| [3] | Zhu Y., Free M. L., Woollam R., Durnie W., Prog. Mater. Sci., 2017, 90, 159—223 |
| [4] | Cao S. A., Hu J. Y., Xie J. L., Liang Q. Q., Yin L., Anti-Corros. Methods Mater., 2013, 60(1), 14—19 |
| [5] | Kolesnichenko I. V., Anslyn E. V., Chem. Soc. Rev., 2017, 46(9), 2385—2390 |
| [6] | Fang R. C., Zhang H. C., Yang L. L., Wang H. T., Tian Y., Zhang X., Jiang L., J. Am. Chem. Soc., 2016, 138(50), 16372—16379 |
| [7] | Yu H., Li C., Yuan B., Li L., Wang C., Corros. Sci., 2017, 120, 231—238 |
| [8] | Yang L. H., Wan Y. X., Qin Z. L., Xu Q. J., Min Y. L., Corros. Sci., 2018, 130, 85—94 |
| [9] | Fan B. M., Wei G., Zhang Z., Qiao N., Anti-Corros. Methods Mater.,2014, 61(2), 104—111 |
| [10] | Chasoglou D., Hryha E., Norell M., Nyborg L., Appl. Surf. Sci., 2013, 268, 496—506 |
| [11] | Solomon M. M., Gerengi H., Umoren S. A., Essien N. B., Essien U. B., Kaya E., Carbohyd. Polym., 2018, 181, 43—55 |
| [12] | Obot I. B., Macdonald D. D., Gasem Z. M., Corros.Sci., 2015, 99, 1—30 |
| [13] | Feng X. G., Lu X. Y., Zuo Y., Zhuang N., Chen D., Corros.Sci., 2016, 103, 223—229 |
| [14] | Wang T. Y., Zou C. J., Li D. X., Chen Z. L., Liu Y., Li X. K., Li M., Acta Phys. Chim. Sin., 2015, 31(12), 2294—2302 |
| (王太杨, 邹长军, 李代禧, 陈正隆, 刘圆, 李小可, 李明. 物理化学学报, 2015, 31(12), 2294—2302) | |
| [15] | Kannan P., Rao T. S., Rajendran N., J. Colloid Interf. Sci.,2018, 512, 618—628 |
| [16] | Liu L. F., Liu J. X., Zhang J., You L., Yu L. J., Qiao G. M., Chem. J. Chinese Universities,2010, 31(3), 537—541 |
| (刘林法, 刘金祥, 张军, 尤龙, 于立军, 乔贵民. 高等学校化学学报, 2010, 31(3), 537—541) | |
| [17] | Kan W. H., Chen L. R., Jiang Q. H., Wang Z., Surf. Tech.,2015, 44(4), 127—131 |
| (阚伟海, 陈莉荣, 姜庆宏, 王哲. 表面技术, 2015, 44(4), 127—131) | |
| [18] | He Z.G., Technology of Cyclodextrin Inclusion, People’s Medical Publishing House, Beijing, 2007, 24 |
| (何仲贵. 环糊精包合物技术, 北京: 人民卫生出版社, 2007, 24) | |
| [19] | Al Omari M. M., Zughul M. B., Davies J. E. D., Badwan A. A., J. Solution Chem., 2009, 38(6), 669—683 |
| [20] | Tian H. W., Li W. H., Hou B. R., Wang D. P., Corros. Sci., 2017, 117, 43—58 |
| [21] | Messali M., Larouj M., Lgaz H., Rezki N., Al-Blewi F. F., Aouad M. R., Chaouiki A., Salghi R., Chung I. M., J. Mol. Struct., 2018, 1168, 39—48 |
| [22] | Sigircik G., Yildirim D., Tuken T., Corros. Sci., 2017, 120, 184—193 |
| [23] | Shaban S. M., RSC Adv., 2016, 6(46), 39784—39800 |
| [24] | Zhang C., Zhao J. M., Acta Phys. Chim. Sin., 2014, 30(4), 677—685 |
| (张晨, 赵景茂. 物理化学学报, 2014, 30(4), 677—685) | |
| [25] | Tian H. W., Li W. H., Liu A., Gao X., Han P., Ding R., Yang C. Z., Wang D. P., Corros. Sci., 2018, 131, 1—16 |
| [26] | Hsu C. H., Mansfeld F., Corrosion,2001, 57(9), 747—748 |
| [27] | Mendonca G. L. F., Costa S. N., Freire V. N., Casciano P. N. S., Correia A. N., de Lima-Neto P., Corros. Sci., 2017, 115, 41—55 |
| [28] | Dong S. G., Gao Y. B., Guan Z. C., Wang H. P., Wang X., Du R. G., Song G. L., Chem. J. Chinese Universities,2018, 39(6), 1260—1266 |
| (董士刚, 高颖波, 官自超, 王海鹏, 王霞, 杜荣归, 宋光铃. 高等学校化学学报. 2018, 39(6), 1260—1266) | |
| [29] | Zhang D. Q., Tang Y. M., Qi S. J., Dong D. W., Cang H., Lu G., Corros. Sci, 2016, 102, 517—522 |
| [30] | Hassan M. M., Barker H., Collie S., Prog. Org. Coat., 2015, 78, 249—255 |
| [31] | Hu K., Zhuang J., Ding J. T., Ma Z., Wang F., Zeng X. G., Corros. Sci., 2017, 125, 68—76 |
| [32] | Zarrouk A., Hammouti B., Lakhlifi T., Traisnel M., Vezin H., Bentiss F., Corros. Sci., 2015, 90, 572—584 |
| [33] | Benali O., Larabi L., Traisnel M., Gengembre L., Harek Y., Appl. Surf. Sci., 2007, 253(14), 6130—6139 |
| [34] | Gao X., Zhao C. C., Lu H. F.,Gao F, Ma H.Y., Electrochim. Acta,2014, 150, 188—196 |
| [35] | Xu C. H., Wang J. C., Wan L., Lin J. J., Wang X. B., J. Mater. Chem., 2011, 21(28), 10463—10471 |
| [36] | Jamil D. M., Al-Okbi A. K., Al-Baghdadi S. B., Al-Amiery A. A., Kadhim A., Gaaz T. S., Kadhum A. A. H., Mohamad A. B., Chem. Cent. J., 2018, 12(1), 7—15 |
| [37] | Avazbaeva Z., Sung W., Lee J., Phan M. D., Shin K., Vaknin D., Kim D., Langmuir,2015, 31(51), 13753—13758 |
| [1] | GAO Zhiwei, LI Junwei, SHI Sai, FU Qiang, JIA Junru, AN Hailong. Analysis of Gating Characteristics of TRPM8 Channel Based on Molecular Dynamics [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220080. |
| [2] | ZENG Xianyang, ZHAO Xi, HUANG Xuri. Mechanism of Inhibition of Glucose and Proton Cotransport Protein GlcPSe by Cytochalasin B [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210822. |
| [3] | CHEN Hanxiang, BIAN Shaoju, HU Bin, LI Wu. Molecular Simulation of the Osmotic Pressures for LiCl-NaCl-KCl-H2O Solution System [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210727. |
| [4] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [5] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [6] | ZHANG Lingyu, ZHANG Jilong, QU Zexing. Dynamics Study of Intramolecular Vibrational Energy Redistribution in RDX Molecule [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220393. |
| [7] | LI Congcong, LIU Minghao, HAN Jiarui, ZHU Jingxuan, HAN Weiwei, LI Wannan. Theoretical Study of the Catalytic Activity of VmoLac Non-specific Substrates Based on Molecular Dynamics Simulations [J]. Chem. J. Chinese Universities, 2021, 42(8): 2518. |
| [8] | LEI Xiaotong, JIN Yiqing, MENG Xuanyu. Prediction of the Binding Site of PIP2 in the TREK-1 Channel Based on Molecular Modeling [J]. Chem. J. Chinese Universities, 2021, 42(8): 2550. |
| [9] | LIU Shasha, ZHANG Heng, YUAN Shiling, LIU Chengbu. Molecular Dynamics Simulation of Pulsed Electric Field O/W Emulsion Demulsification [J]. Chem. J. Chinese Universities, 2021, 42(7): 2170. |
| [10] | ZENG Yonghui, YAN Tianying. Vibrational Density of States Analysis of Proton Hydration Structure [J]. Chem. J. Chinese Universities, 2021, 42(6): 1855. |
| [11] | QI Renrui, LI Minghao, CHANG Hao, FU Xueqi, GAO Bo, HAN Weiwei, HAN Lu, LI Wannan. Theoretical Study on the Unbinding Pathway of Xanthine Oxidase Inhibitors Based on Steered Molecular Dynamics Simulation [J]. Chem. J. Chinese Universities, 2021, 42(3): 758. |
| [12] | LIU Aiqing, XU Wensheng, XU Xiaolei, CHEN Jizhong, AN Lijia. Molecular Dynamics Simulation of Polymer/rod Nanocomposite [J]. Chem. J. Chinese Universities, 2021, 42(3): 875. |
| [13] | HE Jinlu, LONG Run, FANG Weihai. A-site Cation Effects on Hot Carrier Relaxation in Perovskites by Nonadiabatic Molecular Dynamics Simulations † [J]. Chem. J. Chinese Universities, 2020, 41(3): 439. |
| [14] | ZHU Yuquan, ZHAO Xiaojie, ZHONG Yuan, CHEN Ziru, YAN Hong, DUAN Xue. Theoretical Study on the Construction and Characteristics of the Host-guest Intercalated Structure of Layered Double Hydroxides [J]. Chem. J. Chinese Universities, 2020, 41(11): 2287. |
| [15] | QU Siying, XU Qin. Different Roles of Some Key Residues in the S4 Pocket of Coagulation Factor Xa for Rivaroxaban Binding † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1918. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||