

Chem. J. Chinese Universities ›› 2022, Vol. 43 ›› Issue (3): 20210727.doi: 10.7503/cjcu20210727
• Physical Chemistry • Previous Articles Next Articles
CHEN Hanxiang1,2,3, BIAN Shaoju1,2, HU Bin1,2( ), LI Wu1,2
), LI Wu1,2
Received:2021-10-19
Online:2022-03-10
Published:2022-01-07
Contact:
HU Bin
E-mail:hubin@isl.ac.cn
Supported by:CLC Number:
TrendMD:
CHEN Hanxiang, BIAN Shaoju, HU Bin, LI Wu. Molecular Simulation of the Osmotic Pressures for LiCl-NaCl-KCl-H2O Solution System[J]. Chem. J. Chinese Universities, 2022, 43(3): 20210727.
| System | Number of models | Molality/(mol?kg-1) | Number of particles in models | r |
|---|---|---|---|---|
| LiCl?H2O | 8 | 0.5—4.7 | 8000 water a, 80—640 ion pairs | |
| NaCl?H2O | 8 | 0.5—4.7 | 8000 water a, 80—640 ion pairs | |
| KCl?H2O | 8 | 0.5—4.7 | 8000 water a, 80—640 ion pairs | |
| LiCl?NaCl?H2O | 28 | 1.1—4.7 | 8000 water a, 160—640 ion pairs | 1∶4, 2∶3, 3∶2, 4∶1 b |
| LiCl?KCl?H2O | 28 | 1.1—4.7 | 8000 water a, 160—640 ion pairs | 1∶4, 2∶3, 3∶2, 4∶1 b |
| NaCl?KCl?H2O | 28 | 1.1—4.7 | 8000 water a, 160—640 ion pairs | 1∶4, 2∶3, 3∶2, 4∶1 b |
| LiCl?NaCl?KCl?H2O | 24 | 0.8—4.7 | 8000 water a, 160—640 ion pairs | 2∶1∶1, 1∶2∶1, 1∶1∶2 c |
Table 1 Number of models, molalities and number of particles in models of all solution systems for simulation
| System | Number of models | Molality/(mol?kg-1) | Number of particles in models | r |
|---|---|---|---|---|
| LiCl?H2O | 8 | 0.5—4.7 | 8000 water a, 80—640 ion pairs | |
| NaCl?H2O | 8 | 0.5—4.7 | 8000 water a, 80—640 ion pairs | |
| KCl?H2O | 8 | 0.5—4.7 | 8000 water a, 80—640 ion pairs | |
| LiCl?NaCl?H2O | 28 | 1.1—4.7 | 8000 water a, 160—640 ion pairs | 1∶4, 2∶3, 3∶2, 4∶1 b |
| LiCl?KCl?H2O | 28 | 1.1—4.7 | 8000 water a, 160—640 ion pairs | 1∶4, 2∶3, 3∶2, 4∶1 b |
| NaCl?KCl?H2O | 28 | 1.1—4.7 | 8000 water a, 160—640 ion pairs | 1∶4, 2∶3, 3∶2, 4∶1 b |
| LiCl?NaCl?KCl?H2O | 24 | 0.8—4.7 | 8000 water a, 160—640 ion pairs | 2∶1∶1, 1∶2∶1, 1∶1∶2 c |
| Solution | Molality/(mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[ |
|---|---|---|---|---|---|
| LiCl | 0.56 | -0.174 | 2.80 | 3.12 | 2.75 |
| LiCl | 1.13 | -0.248 | 6.31 | 5.30 | 5.79 |
| LiCl | 1.70 | -0.364 | 9.27 | 8.71 | 9.23 |
| LiCl | 2.28 | -0.570 | 10.11 | 14.77 | 13.11 |
| LiCl | 2.87 | -0.752 | 16.12 | 20.09 | 17.47 |
| LiCl | 3.48 | -0.720 | 12.14 | 19.16 | 22.34 |
| LiCl | 4.08 | -0.992 | 21.11 | 27.05 | 27.82 |
| LiCl | 4.70 | -1.240 | 26.17 | 34.20 | 33.89 |
| NaCl | 0.56 | -0.133 | 1.84 | 2.96 | 2.62 |
| NaCl | 1.12 | -0.262 | 7.00 | 5.07 | 5.30 |
| NaCl | 1.69 | -0.406 | 10.07 | 7.62 | 8.13 |
| NaCl | 2.27 | -0.611 | 13.97 | 11.58 | 11.14 |
| NaCl | 2.86 | -0.807 | 15.43 | 15.75 | 14.33 |
| NaCl | 3.45 | -0.819 | 18.76 | 16.04 | 17.75 |
| NaCl | 4.05 | -1.087 | 24.00 | 22.37 | 21.42 |
| NaCl | 4.67 | -1.175 | 25.76 | 24.62 | 25.32 |
| KCl | 0.56 | -0.113 | 2.79 | 2.53 | 2.54 |
| KCl | 1.13 | -0.331 | 7.15 | 5.18 | 5.05 |
| KCl | 1.71 | -0.466 | 10.11 | 7.46 | 7.58 |
| KCl | 2.30 | -0.600 | 13.42 | 10.18 | 10.16 |
| KCl | 2.90 | -0.699 | 14.47 | 12.50 | 12.81 |
| KCl | 3.51 | -0.912 | 19.66 | 18.38 | 15.55 |
| KCl | 4.15 | -0.902 | 20.39 | 18.07 | 18.36 |
| KCl | 4.78 | -0.934 | 23.97 | 19.05 | 21.29 |
Table 2 Main numeric results of binary solution system simulations
| Solution | Molality/(mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[ |
|---|---|---|---|---|---|
| LiCl | 0.56 | -0.174 | 2.80 | 3.12 | 2.75 |
| LiCl | 1.13 | -0.248 | 6.31 | 5.30 | 5.79 |
| LiCl | 1.70 | -0.364 | 9.27 | 8.71 | 9.23 |
| LiCl | 2.28 | -0.570 | 10.11 | 14.77 | 13.11 |
| LiCl | 2.87 | -0.752 | 16.12 | 20.09 | 17.47 |
| LiCl | 3.48 | -0.720 | 12.14 | 19.16 | 22.34 |
| LiCl | 4.08 | -0.992 | 21.11 | 27.05 | 27.82 |
| LiCl | 4.70 | -1.240 | 26.17 | 34.20 | 33.89 |
| NaCl | 0.56 | -0.133 | 1.84 | 2.96 | 2.62 |
| NaCl | 1.12 | -0.262 | 7.00 | 5.07 | 5.30 |
| NaCl | 1.69 | -0.406 | 10.07 | 7.62 | 8.13 |
| NaCl | 2.27 | -0.611 | 13.97 | 11.58 | 11.14 |
| NaCl | 2.86 | -0.807 | 15.43 | 15.75 | 14.33 |
| NaCl | 3.45 | -0.819 | 18.76 | 16.04 | 17.75 |
| NaCl | 4.05 | -1.087 | 24.00 | 22.37 | 21.42 |
| NaCl | 4.67 | -1.175 | 25.76 | 24.62 | 25.32 |
| KCl | 0.56 | -0.113 | 2.79 | 2.53 | 2.54 |
| KCl | 1.13 | -0.331 | 7.15 | 5.18 | 5.05 |
| KCl | 1.71 | -0.466 | 10.11 | 7.46 | 7.58 |
| KCl | 2.30 | -0.600 | 13.42 | 10.18 | 10.16 |
| KCl | 2.90 | -0.699 | 14.47 | 12.50 | 12.81 |
| KCl | 3.51 | -0.912 | 19.66 | 18.38 | 15.55 |
| KCl | 4.15 | -0.902 | 20.39 | 18.07 | 18.36 |
| KCl | 4.78 | -0.934 | 23.97 | 19.05 | 21.29 |
| Solution | c2 | c1 | c0 |
|---|---|---|---|
| LiCl | -7.884 | -124.7 | -2.066 |
| NaCl | 85.21 | -60.30 | 0.9623 |
| KCl | 231.1 | -26.40 | 1.648 |
Table 3 Final fitted parameters of MEF method
| Solution | c2 | c1 | c0 |
|---|---|---|---|
| LiCl | -7.884 | -124.7 | -2.066 |
| NaCl | 85.21 | -60.30 | 0.9623 |
| KCl | 231.1 | -26.40 | 1.648 |
| Solution | RMSE MD?FS(%) | RMSE MD?MEF(%) |
|---|---|---|
| LiCl?KCl?H2O | 7.9 | 2.6 |
| LiCl?NaCl?H2O | 8.6 | 2.7 |
| NaCl?KCl?H2O | 12.4 | 2.6 |
| LiCl?NaCl?KCl?H2O | 6.8 | 2.0 |
Table 4 Error analysis of computed osmotic pressures of ternary and quaternary systems
| Solution | RMSE MD?FS(%) | RMSE MD?MEF(%) |
|---|---|---|
| LiCl?KCl?H2O | 7.9 | 2.6 |
| LiCl?NaCl?H2O | 8.6 | 2.7 |
| NaCl?KCl?H2O | 12.4 | 2.6 |
| LiCl?NaCl?KCl?H2O | 6.8 | 2.0 |
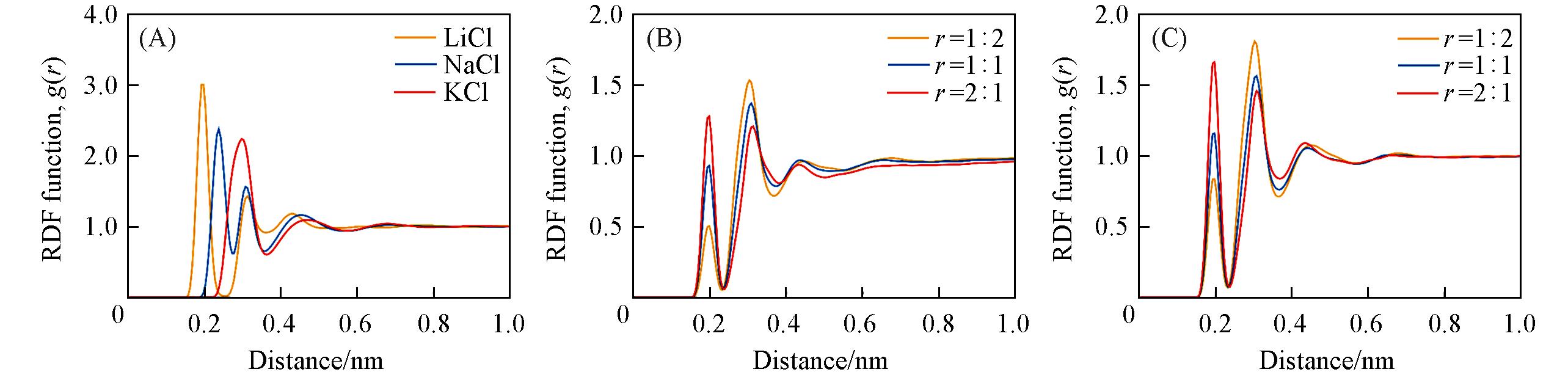
Fig.8 Radial distribution functions of water?ion pairs of binary systems and ternary system LiCl?KCl?H2O(A) 4.4 mol/kg MCl?H2O water?ion RDF; (B) 6.0 mol/kg LiCl?KCl?H2O water?ion RDF; (C) 3.0 mol/kg LiCl?KCl?H2O water?ion RDF.
| Solution | r | Molality/ (mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[44―46]/MPa |
|---|---|---|---|---|---|---|
| LiCl?KCl | 1∶4 | 1.13 | -0.270 | 7.73 | 4.63 | 5.17 |
| LiCl?KCl | 1∶4 | 1.71 | -0.378 | 10.78 | 6.57 | 7.82 |
| LiCl?KCl | 1∶4 | 2.29 | -0.521 | 12.15 | 9.48 | 10.59 |
| LiCl?KCl | 1∶4 | 2.89 | -0.806 | 20.80 | 16.59 | 13.46 |
| LiCl?KCl | 1∶4 | 3.51 | -0.919 | 25.48 | 19.88 | 16.42 |
| LiCl?KCl | 1∶4 | 4.13 | -1.040 | 30.94 | 23.68 | 19.55 |
| LiCl?KCl | 1∶4 | 4.76 | -1.113 | 30.67 | 26.13 | 22.82 |
| LiCl?KCl | 2∶3 | 1.13 | -0.296 | 9.94 | 5.50 | 5.30 |
| LiCl?KCl | 2∶3 | 1.71 | -0.506 | 16.14 | 10.10 | 8.11 |
| LiCl?KCl | 2∶3 | 2.29 | -0.545 | 17.59 | 11.01 | 11.09 |
| LiCl?KCl | 2∶3 | 2.89 | -0.792 | 22.55 | 17.44 | 14.23 |
| LiCl?KCl | 2∶3 | 3.50 | -0.809 | 22.10 | 17.94 | 17.55 |
| LiCl?KCl | 2∶3 | 4.12 | -0.844 | 24.73 | 18.95 | 21.10 |
| LiCl?KCl | 2∶3 | 4.75 | -1.157 | 35.18 | 28.70 | 24.82 |
| LiCl?KCl | 3∶2 | 1.13 | -0.327 | 7.51 | 6.63 | 5.44 |
| LiCl?KCl | 3∶2 | 1.70 | -0.371 | 11.43 | 7.67 | 8.44 |
| LiCl?KCl | 3∶2 | 2.29 | -0.572 | 13.64 | 12.73 | 11.67 |
| LiCl?KCl | 3∶2 | 2.88 | -0.655 | 20.31 | 14.95 | 15.17 |
| LiCl?KCl | 3∶2 | 3.49 | -0.742 | 20.81 | 17.31 | 18.92 |
| LiCl?KCl | 3∶2 | 4.10 | -0.875 | 22.01 | 21.11 | 22.99 |
| LiCl?KCl | 3∶2 | 4.73 | -1.185 | 32.23 | 30.63 | 27.35 |
| LiCl?KCl | 4∶1 | 1.13 | -0.276 | 6.95 | 5.78 | 5.61 |
| LiCl?KCl | 4∶1 | 1.70 | -0.326 | 8.24 | 7.11 | 8.81 |
| LiCl?KCl | 4∶1 | 2.29 | -0.492 | 11.90 | 11.58 | 12.34 |
| LiCl?KCl | 4∶1 | 2.88 | -0.719 | 19.43 | 17.92 | 16.24 |
| LiCl?KCl | 4∶1 | 3.48 | -0.868 | 28.16 | 22.20 | 20.52 |
| LiCl?KCl | 4∶1 | 4.09 | -1.034 | 26.05 | 27.08 | 25.23 |
| LiCl?KCl | 4∶1 | 4.71 | -1.226 | 30.80 | 32.89 | 30.38 |
| LiCl?NaCl | 1∶4 | 1.12 | -0.274 | 7.43 | 5.43 | 5.42 |
| LiCl?NaCl | 1∶4 | 1.69 | -0.513 | 14.15 | 10.34 | 8.38 |
| LiCl?NaCl | 1∶4 | 2.27 | -0.583 | 16.73 | 11.84 | 11.57 |
| LiCl?NaCl | 1∶4 | 2.86 | -0.689 | 19.53 | 14.22 | 15.01 |
| LiCl?NaCl | 1∶4 | 3.45 | -0.950 | 28.48 | 20.40 | 18.75 |
| LiCl?NaCl | 1∶4 | 4.06 | -1.027 | 31.16 | 22.34 | 22.77 |
| LiCl?NaCl | 1∶4 | 4.66 | -1.338 | 39.75 | 30.57 | 27.13 |
| LiCl?NaCl | 2∶3 | 1.12 | -0.261 | 11.24 | 5.32 | 5.52 |
| Solution | r | Molality/ (mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[44―46]/MPa |
| LiCl?NaCl | 2∶3 | 1.69 | -0.502 | 14.38 | 10.76 | 8.61 |
| LiCl?NaCl | 2∶3 | 2.27 | -0.604 | 18.93 | 13.18 | 12.00 |
| LiCl?NaCl | 2∶3 | 2.86 | -0.686 | 20.43 | 15.15 | 15.66 |
| LiCl?NaCl | 2∶3 | 3.46 | -0.812 | 28.41 | 18.25 | 19.67 |
| LiCl?NaCl | 2∶3 | 4.06 | -1.022 | 29.51 | 23.64 | 24.07 |
| LiCl?NaCl | 2∶3 | 4.67 | -1.200 | 30.19 | 28.38 | 28.85 |
| LiCl?NaCl | 3∶2 | 1.12 | -0.296 | 11.00 | 6.29 | 5.62 |
| LiCl?NaCl | 3∶2 | 1.70 | -0.494 | 15.00 | 11.24 | 8.82 |
| LiCl?NaCl | 3∶2 | 2.28 | -0.476 | 12.19 | 10.78 | 12.37 |
| LiCl?NaCl | 3∶2 | 2.86 | -0.678 | 21.28 | 15.95 | 16.30 |
| LiCl?NaCl | 3∶2 | 3.46 | -0.869 | 26.12 | 20.97 | 20.61 |
| LiCl?NaCl | 3∶2 | 4.07 | -1.119 | 31.95 | 27.70 | 25.36 |
| LiCl?NaCl | 3∶2 | 4.68 | -1.215 | 35.19 | 30.36 | 30.56 |
| LiCl?NaCl | 4∶1 | 1.13 | -0.224 | 7.82 | 4.55 | 5.71 |
| LiCl?NaCl | 4∶1 | 1.70 | -0.409 | 10.65 | 9.57 | 9.03 |
| LiCl?NaCl | 4∶1 | 2.28 | -0.440 | 12.07 | 10.41 | 12.75 |
| LiCl?NaCl | 4∶1 | 2.87 | -0.935 | 25.25 | 24.07 | 16.90 |
| LiCl?NaCl | 4∶1 | 3.47 | -0.860 | 25.68 | 21.98 | 21.49 |
| LiCl?NaCl | 4∶1 | 4.07 | -1.018 | 24.98 | 26.40 | 26.60 |
| LiCl?NaCl | 4∶1 | 4.69 | -1.084 | 31.94 | 28.22 | 32.23 |
| NaCl?KCl | 1∶4 | 1.12 | -0.282 | 9.00 | 4.66 | 5.10 |
| NaCl?KCl | 1∶4 | 1.69 | -0.505 | 17.10 | 8.46 | 7.70 |
| NaCl?KCl | 1∶4 | 2.28 | -0.478 | 14.66 | 7.94 | 10.36 |
| NaCl?KCl | 1∶4 | 2.86 | -0.662 | 21.18 | 11.81 | 13.12 |
| NaCl?KCl | 1∶4 | 3.46 | -0.777 | 24.48 | 14.64 | 15.96 |
| NaCl?KCl | 1∶4 | 4.06 | -1.145 | 34.74 | 25.73 | 18.95 |
| NaCl?KCl | 1∶4 | 4.68 | -1.249 | 37.30 | 29.42 | 22.01 |
| NaCl?KCl | 2∶3 | 1.13 | -0.263 | 10.25 | 4.57 | 5.13 |
| NaCl?KCl | 2∶3 | 1.70 | -0.542 | 16.49 | 9.44 | 7.76 |
| NaCl?KCl | 2∶3 | 2.28 | -0.568 | 16.18 | 9.99 | 10.48 |
| NaCl?KCl | 2∶3 | 2.87 | -0.750 | 23.77 | 14.08 | 13.30 |
| NaCl?KCl | 2∶3 | 3.47 | -0.829 | 26.98 | 16.06 | 16.23 |
| NaCl?KCl | 2∶3 | 4.08 | -0.923 | 29.60 | 18.61 | 19.30 |
| NaCl?KCl | 2∶3 | 4.71 | -1.160 | 34.48 | 25.73 | 22.48 |
| NaCl?KCl | 3∶2 | 1.13 | -0.284 | 7.54 | 5.07 | 5.18 |
| NaCl?KCl | 3∶2 | 1.70 | -0.481 | 14.36 | 8.50 | 7.85 |
| NaCl?KCl | 3∶2 | 2.29 | -0.568 | 16.58 | 10.23 | 10.63 |
| NaCl?KCl | 3∶2 | 2.88 | -0.799 | 23.79 | 15.39 | 13.54 |
| NaCl?KCl | 3∶2 | 3.49 | -0.866 | 25.25 | 17.06 | 16.58 |
| NaCl?KCl | 3∶2 | 4.08 | -0.981 | 29.55 | 20.09 | 19.76 |
| NaCl?KCl | 3∶2 | 4.73 | -1.154 | 34.85 | 25.06 | 23.15 |
| NaCl?KCl | 4∶1 | 1.13 | -0.308 | 7.74 | 5.65 | 5.22 |
| NaCl?KCl | 4∶1 | 1.70 | -0.392 | 13.18 | 7.11 | 7.95 |
| NaCl?KCl | 4∶1 | 2.29 | -0.572 | 15.97 | 10.55 | 10.82 |
| NaCl?KCl | 4∶1 | 2.89 | -0.684 | 17.87 | 12.90 | 13.82 |
| NaCl?KCl | 4∶1 | 3.49 | -0.806 | 21.23 | 15.66 | 17.03 |
| NaCl?KCl | 4∶1 | 4.12 | -0.897 | 28.55 | 17.84 | 20.39 |
| NaCl?KCl | 4∶1 | 4.76 | -1.001 | 30.74 | 20.45 | 23.97 |
| LiCl?NaCl?KCl | 1∶1∶2 | 0.90 | -0.282 | 9.00 | 5.17 | 4.39 |
| LiCl?NaCl?KCl | 1∶1∶2 | 1.36 | -0.332 | 8.97 | 6.12 | 6.75 |
| Solution | r | Molality/ (mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[44―46]/MPa |
| LiCl?NaCl?KCl | 1∶1∶2 | 1.82 | -0.455 | 11.78 | 8.61 | 9.21 |
| LiCl?NaCl?KCl | 1∶1∶2 | 2.29 | -0.561 | 15.12 | 10.94 | 11.78 |
| LiCl?NaCl?KCl | 1∶1∶2 | 2.76 | -0.709 | 20.41 | 14.50 | 14.42 |
| LiCl?NaCl?KCl | 1∶1∶2 | 3.24 | -0.813 | 25.55 | 17.18 | 17.14 |
| LiCl?NaCl?KCl | 1∶1∶2 | 3.73 | -0.909 | 26.39 | 19.84 | 19.96 |
| LiCl?NaCl?KCl | 1∶1∶2 | 4.22 | -0.894 | 30.80 | 19.42 | 22.87 |
| LiCl?NaCl?KCl | 1∶1∶2 | 4.73 | -1.202 | 32.89 | 28.77 | 25.85 |
| LiCl?NaCl?KCl | 1∶2∶1 | 0.90 | -0.260 | 9.95 | 4.97 | 4.43 |
| LiCl?NaCl?KCl | 1∶2∶1 | 1.35 | -0.307 | 8.22 | 5.89 | 6.83 |
| LiCl?NaCl?KCl | 1∶2∶1 | 1.81 | -0.389 | 12.59 | 7.53 | 9.36 |
| LiCl?NaCl?KCl | 1∶2∶1 | 2.28 | -0.643 | 17.73 | 13.13 | 12.00 |
| LiCl?NaCl?KCl | 1∶2∶1 | 2.75 | -0.693 | 23.24 | 14.32 | 14.75 |
| LiCl?NaCl?KCl | 1∶2∶1 | 3.23 | -0.859 | 26.71 | 18.47 | 17.58 |
| LiCl?NaCl?KCl | 1∶2∶1 | 3.71 | -0.957 | 26.29 | 21.07 | 20.55 |
| LiCl?NaCl?KCl | 1∶2∶1 | 4.21 | -0.952 | 28.65 | 20.92 | 23.59 |
| LiCl?NaCl?KCl | 1∶2∶1 | 4.70 | -1.191 | 31.36 | 27.68 | 26.77 |
| LiCl?NaCl?KCl | 2∶1∶1 | 0.90 | -0.246 | 7.44 | 4.82 | 4.51 |
| LiCl?NaCl?KCl | 2∶1∶1 | 1.35 | -0.330 | 10.92 | 6.71 | 7.01 |
| LiCl?NaCl?KCl | 2∶1∶1 | 1.82 | -0.360 | 11.20 | 7.40 | 9.66 |
| LiCl?NaCl?KCl | 2∶1∶1 | 2.28 | -0.654 | 18.29 | 14.59 | 12.47 |
| LiCl?NaCl?KCl | 2∶1∶1 | 2.76 | -0.708 | 22.02 | 15.98 | 15.42 |
| LiCl?NaCl?KCl | 2∶1∶1 | 3.23 | -0.977 | 29.43 | 23.35 | 18.53 |
| LiCl?NaCl?KCl | 2∶1∶1 | 3.72 | -0.851 | 24.05 | 19.82 | 21.78 |
| LiCl?NaCl?KCl | 2∶1∶1 | 4.21 | -0.883 | 23.87 | 20.70 | 25.18 |
| LiCl?NaCl?KCl | 2∶1∶1 | 4.72 | -1.090 | 28.52 | 26.60 | 28.74 |
Table 5 Main numeric results of ternary solution simulations
| Solution | r | Molality/ (mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[44―46]/MPa |
|---|---|---|---|---|---|---|
| LiCl?KCl | 1∶4 | 1.13 | -0.270 | 7.73 | 4.63 | 5.17 |
| LiCl?KCl | 1∶4 | 1.71 | -0.378 | 10.78 | 6.57 | 7.82 |
| LiCl?KCl | 1∶4 | 2.29 | -0.521 | 12.15 | 9.48 | 10.59 |
| LiCl?KCl | 1∶4 | 2.89 | -0.806 | 20.80 | 16.59 | 13.46 |
| LiCl?KCl | 1∶4 | 3.51 | -0.919 | 25.48 | 19.88 | 16.42 |
| LiCl?KCl | 1∶4 | 4.13 | -1.040 | 30.94 | 23.68 | 19.55 |
| LiCl?KCl | 1∶4 | 4.76 | -1.113 | 30.67 | 26.13 | 22.82 |
| LiCl?KCl | 2∶3 | 1.13 | -0.296 | 9.94 | 5.50 | 5.30 |
| LiCl?KCl | 2∶3 | 1.71 | -0.506 | 16.14 | 10.10 | 8.11 |
| LiCl?KCl | 2∶3 | 2.29 | -0.545 | 17.59 | 11.01 | 11.09 |
| LiCl?KCl | 2∶3 | 2.89 | -0.792 | 22.55 | 17.44 | 14.23 |
| LiCl?KCl | 2∶3 | 3.50 | -0.809 | 22.10 | 17.94 | 17.55 |
| LiCl?KCl | 2∶3 | 4.12 | -0.844 | 24.73 | 18.95 | 21.10 |
| LiCl?KCl | 2∶3 | 4.75 | -1.157 | 35.18 | 28.70 | 24.82 |
| LiCl?KCl | 3∶2 | 1.13 | -0.327 | 7.51 | 6.63 | 5.44 |
| LiCl?KCl | 3∶2 | 1.70 | -0.371 | 11.43 | 7.67 | 8.44 |
| LiCl?KCl | 3∶2 | 2.29 | -0.572 | 13.64 | 12.73 | 11.67 |
| LiCl?KCl | 3∶2 | 2.88 | -0.655 | 20.31 | 14.95 | 15.17 |
| LiCl?KCl | 3∶2 | 3.49 | -0.742 | 20.81 | 17.31 | 18.92 |
| LiCl?KCl | 3∶2 | 4.10 | -0.875 | 22.01 | 21.11 | 22.99 |
| LiCl?KCl | 3∶2 | 4.73 | -1.185 | 32.23 | 30.63 | 27.35 |
| LiCl?KCl | 4∶1 | 1.13 | -0.276 | 6.95 | 5.78 | 5.61 |
| LiCl?KCl | 4∶1 | 1.70 | -0.326 | 8.24 | 7.11 | 8.81 |
| LiCl?KCl | 4∶1 | 2.29 | -0.492 | 11.90 | 11.58 | 12.34 |
| LiCl?KCl | 4∶1 | 2.88 | -0.719 | 19.43 | 17.92 | 16.24 |
| LiCl?KCl | 4∶1 | 3.48 | -0.868 | 28.16 | 22.20 | 20.52 |
| LiCl?KCl | 4∶1 | 4.09 | -1.034 | 26.05 | 27.08 | 25.23 |
| LiCl?KCl | 4∶1 | 4.71 | -1.226 | 30.80 | 32.89 | 30.38 |
| LiCl?NaCl | 1∶4 | 1.12 | -0.274 | 7.43 | 5.43 | 5.42 |
| LiCl?NaCl | 1∶4 | 1.69 | -0.513 | 14.15 | 10.34 | 8.38 |
| LiCl?NaCl | 1∶4 | 2.27 | -0.583 | 16.73 | 11.84 | 11.57 |
| LiCl?NaCl | 1∶4 | 2.86 | -0.689 | 19.53 | 14.22 | 15.01 |
| LiCl?NaCl | 1∶4 | 3.45 | -0.950 | 28.48 | 20.40 | 18.75 |
| LiCl?NaCl | 1∶4 | 4.06 | -1.027 | 31.16 | 22.34 | 22.77 |
| LiCl?NaCl | 1∶4 | 4.66 | -1.338 | 39.75 | 30.57 | 27.13 |
| LiCl?NaCl | 2∶3 | 1.12 | -0.261 | 11.24 | 5.32 | 5.52 |
| Solution | r | Molality/ (mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[44―46]/MPa |
| LiCl?NaCl | 2∶3 | 1.69 | -0.502 | 14.38 | 10.76 | 8.61 |
| LiCl?NaCl | 2∶3 | 2.27 | -0.604 | 18.93 | 13.18 | 12.00 |
| LiCl?NaCl | 2∶3 | 2.86 | -0.686 | 20.43 | 15.15 | 15.66 |
| LiCl?NaCl | 2∶3 | 3.46 | -0.812 | 28.41 | 18.25 | 19.67 |
| LiCl?NaCl | 2∶3 | 4.06 | -1.022 | 29.51 | 23.64 | 24.07 |
| LiCl?NaCl | 2∶3 | 4.67 | -1.200 | 30.19 | 28.38 | 28.85 |
| LiCl?NaCl | 3∶2 | 1.12 | -0.296 | 11.00 | 6.29 | 5.62 |
| LiCl?NaCl | 3∶2 | 1.70 | -0.494 | 15.00 | 11.24 | 8.82 |
| LiCl?NaCl | 3∶2 | 2.28 | -0.476 | 12.19 | 10.78 | 12.37 |
| LiCl?NaCl | 3∶2 | 2.86 | -0.678 | 21.28 | 15.95 | 16.30 |
| LiCl?NaCl | 3∶2 | 3.46 | -0.869 | 26.12 | 20.97 | 20.61 |
| LiCl?NaCl | 3∶2 | 4.07 | -1.119 | 31.95 | 27.70 | 25.36 |
| LiCl?NaCl | 3∶2 | 4.68 | -1.215 | 35.19 | 30.36 | 30.56 |
| LiCl?NaCl | 4∶1 | 1.13 | -0.224 | 7.82 | 4.55 | 5.71 |
| LiCl?NaCl | 4∶1 | 1.70 | -0.409 | 10.65 | 9.57 | 9.03 |
| LiCl?NaCl | 4∶1 | 2.28 | -0.440 | 12.07 | 10.41 | 12.75 |
| LiCl?NaCl | 4∶1 | 2.87 | -0.935 | 25.25 | 24.07 | 16.90 |
| LiCl?NaCl | 4∶1 | 3.47 | -0.860 | 25.68 | 21.98 | 21.49 |
| LiCl?NaCl | 4∶1 | 4.07 | -1.018 | 24.98 | 26.40 | 26.60 |
| LiCl?NaCl | 4∶1 | 4.69 | -1.084 | 31.94 | 28.22 | 32.23 |
| NaCl?KCl | 1∶4 | 1.12 | -0.282 | 9.00 | 4.66 | 5.10 |
| NaCl?KCl | 1∶4 | 1.69 | -0.505 | 17.10 | 8.46 | 7.70 |
| NaCl?KCl | 1∶4 | 2.28 | -0.478 | 14.66 | 7.94 | 10.36 |
| NaCl?KCl | 1∶4 | 2.86 | -0.662 | 21.18 | 11.81 | 13.12 |
| NaCl?KCl | 1∶4 | 3.46 | -0.777 | 24.48 | 14.64 | 15.96 |
| NaCl?KCl | 1∶4 | 4.06 | -1.145 | 34.74 | 25.73 | 18.95 |
| NaCl?KCl | 1∶4 | 4.68 | -1.249 | 37.30 | 29.42 | 22.01 |
| NaCl?KCl | 2∶3 | 1.13 | -0.263 | 10.25 | 4.57 | 5.13 |
| NaCl?KCl | 2∶3 | 1.70 | -0.542 | 16.49 | 9.44 | 7.76 |
| NaCl?KCl | 2∶3 | 2.28 | -0.568 | 16.18 | 9.99 | 10.48 |
| NaCl?KCl | 2∶3 | 2.87 | -0.750 | 23.77 | 14.08 | 13.30 |
| NaCl?KCl | 2∶3 | 3.47 | -0.829 | 26.98 | 16.06 | 16.23 |
| NaCl?KCl | 2∶3 | 4.08 | -0.923 | 29.60 | 18.61 | 19.30 |
| NaCl?KCl | 2∶3 | 4.71 | -1.160 | 34.48 | 25.73 | 22.48 |
| NaCl?KCl | 3∶2 | 1.13 | -0.284 | 7.54 | 5.07 | 5.18 |
| NaCl?KCl | 3∶2 | 1.70 | -0.481 | 14.36 | 8.50 | 7.85 |
| NaCl?KCl | 3∶2 | 2.29 | -0.568 | 16.58 | 10.23 | 10.63 |
| NaCl?KCl | 3∶2 | 2.88 | -0.799 | 23.79 | 15.39 | 13.54 |
| NaCl?KCl | 3∶2 | 3.49 | -0.866 | 25.25 | 17.06 | 16.58 |
| NaCl?KCl | 3∶2 | 4.08 | -0.981 | 29.55 | 20.09 | 19.76 |
| NaCl?KCl | 3∶2 | 4.73 | -1.154 | 34.85 | 25.06 | 23.15 |
| NaCl?KCl | 4∶1 | 1.13 | -0.308 | 7.74 | 5.65 | 5.22 |
| NaCl?KCl | 4∶1 | 1.70 | -0.392 | 13.18 | 7.11 | 7.95 |
| NaCl?KCl | 4∶1 | 2.29 | -0.572 | 15.97 | 10.55 | 10.82 |
| NaCl?KCl | 4∶1 | 2.89 | -0.684 | 17.87 | 12.90 | 13.82 |
| NaCl?KCl | 4∶1 | 3.49 | -0.806 | 21.23 | 15.66 | 17.03 |
| NaCl?KCl | 4∶1 | 4.12 | -0.897 | 28.55 | 17.84 | 20.39 |
| NaCl?KCl | 4∶1 | 4.76 | -1.001 | 30.74 | 20.45 | 23.97 |
| LiCl?NaCl?KCl | 1∶1∶2 | 0.90 | -0.282 | 9.00 | 5.17 | 4.39 |
| LiCl?NaCl?KCl | 1∶1∶2 | 1.36 | -0.332 | 8.97 | 6.12 | 6.75 |
| Solution | r | Molality/ (mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[44―46]/MPa |
| LiCl?NaCl?KCl | 1∶1∶2 | 1.82 | -0.455 | 11.78 | 8.61 | 9.21 |
| LiCl?NaCl?KCl | 1∶1∶2 | 2.29 | -0.561 | 15.12 | 10.94 | 11.78 |
| LiCl?NaCl?KCl | 1∶1∶2 | 2.76 | -0.709 | 20.41 | 14.50 | 14.42 |
| LiCl?NaCl?KCl | 1∶1∶2 | 3.24 | -0.813 | 25.55 | 17.18 | 17.14 |
| LiCl?NaCl?KCl | 1∶1∶2 | 3.73 | -0.909 | 26.39 | 19.84 | 19.96 |
| LiCl?NaCl?KCl | 1∶1∶2 | 4.22 | -0.894 | 30.80 | 19.42 | 22.87 |
| LiCl?NaCl?KCl | 1∶1∶2 | 4.73 | -1.202 | 32.89 | 28.77 | 25.85 |
| LiCl?NaCl?KCl | 1∶2∶1 | 0.90 | -0.260 | 9.95 | 4.97 | 4.43 |
| LiCl?NaCl?KCl | 1∶2∶1 | 1.35 | -0.307 | 8.22 | 5.89 | 6.83 |
| LiCl?NaCl?KCl | 1∶2∶1 | 1.81 | -0.389 | 12.59 | 7.53 | 9.36 |
| LiCl?NaCl?KCl | 1∶2∶1 | 2.28 | -0.643 | 17.73 | 13.13 | 12.00 |
| LiCl?NaCl?KCl | 1∶2∶1 | 2.75 | -0.693 | 23.24 | 14.32 | 14.75 |
| LiCl?NaCl?KCl | 1∶2∶1 | 3.23 | -0.859 | 26.71 | 18.47 | 17.58 |
| LiCl?NaCl?KCl | 1∶2∶1 | 3.71 | -0.957 | 26.29 | 21.07 | 20.55 |
| LiCl?NaCl?KCl | 1∶2∶1 | 4.21 | -0.952 | 28.65 | 20.92 | 23.59 |
| LiCl?NaCl?KCl | 1∶2∶1 | 4.70 | -1.191 | 31.36 | 27.68 | 26.77 |
| LiCl?NaCl?KCl | 2∶1∶1 | 0.90 | -0.246 | 7.44 | 4.82 | 4.51 |
| LiCl?NaCl?KCl | 2∶1∶1 | 1.35 | -0.330 | 10.92 | 6.71 | 7.01 |
| LiCl?NaCl?KCl | 2∶1∶1 | 1.82 | -0.360 | 11.20 | 7.40 | 9.66 |
| LiCl?NaCl?KCl | 2∶1∶1 | 2.28 | -0.654 | 18.29 | 14.59 | 12.47 |
| LiCl?NaCl?KCl | 2∶1∶1 | 2.76 | -0.708 | 22.02 | 15.98 | 15.42 |
| LiCl?NaCl?KCl | 2∶1∶1 | 3.23 | -0.977 | 29.43 | 23.35 | 18.53 |
| LiCl?NaCl?KCl | 2∶1∶1 | 3.72 | -0.851 | 24.05 | 19.82 | 21.78 |
| LiCl?NaCl?KCl | 2∶1∶1 | 4.21 | -0.883 | 23.87 | 20.70 | 25.18 |
| LiCl?NaCl?KCl | 2∶1∶1 | 4.72 | -1.090 | 28.52 | 26.60 | 28.74 |
| 1 | Zheng M. P., Acta Geo. Sin., 2010, 84(11), 1613—1622(郑绵平. 地质学报, 2010, 84(11), 1613—1622) |
| 2 | Nie Z., Bu L. Z., Zheng M. P., Acta Geosci. Sin., 2010, 31(1), 95—101(乜贞, 卜令忠, 郑绵平. 地球学报, 2010, 31(1), 95—101) |
| 3 | Yan H., J. Salt Lake Res., 2018, 26(4), 85—90(颜辉. 盐湖研究, 2018, 26(4), 85—90) |
| 4 | Catlow C. R. A., Diller K. M., Norgett M. J., J. Phys. C: Solid State Physics, 1977, 10(9), 1395—1412 |
| 5 | Dang L. X., Chang T. M., J. Chem. Phys., 1997, 106(19), 8149—8159 |
| 6 | Maginn E. J., J. Phys.: Condensed Matter., 2009, 21(37), 373101 |
| 7 | Yang J. M., Yao Y., Zhang A. Y., Song P. S., Chem. J. Chinese Universities, 2006, 27(4), 735—738(杨吉民, 姚燕, 张爱云, 宋彭生. 高等学校化学学报, 2006, 27(4), 735—738) |
| 8 | Zhang A. Y., Yao Y., Song P. S., Chem. J. Chinese Universities, 2004, 25(10), 1934—1936(张爱云, 姚燕, 宋彭生. 高等学校化学学报, 2004, 25(10), 1934—1936) |
| 9 | Song P. S., Yao Y., Sun B., Li W., Sci. Sin. Chim., 2010, 40(9), 1286—1296(宋彭生, 姚燕, 孙柏, 李武. 中国科学: 化学, 2010, 40(9), 1286—1296) |
| 10 | Song P. S., Yao Y., J. Salt Lake Res., 2003, 11(3), 1—8(宋彭生, 姚燕. 盐湖研究, 2003, 11(3), 1—8) |
| 11 | Song P. S., Yao Y., J. Salt Lake Res., 2003, 11(4), 1—12,19(宋彭生, 姚燕. 盐湖研究, 2003, 11(4), 1—12,19) |
| 12 | Taylor R. S., Dang L. X., Garrett B. C., J. Phys. Chem., 1996, 100(28), 11720—11725 |
| 13 | Takagi R., Sakurai M., Z. Naturforsh. A, 1998, 53(1/2), 13—16 |
| 14 | Smith P. E., Van Gunsteren W. F., Chem. Phys. Lett., 1993, 215(4), 315—318 |
| 15 | Satarifard V., Kashefolgheta S., Vila Verde A., GrafmüLler A., J. Chem. Theor. Comput., 2017, 13(5), 2112—2122 |
| 16 | Saric D., Kohns M., Vrabec J., J. Chem. Phys., 2020, 152(1), 164502 |
| 17 | Sanz E., Vega C., J. Chem. Phys., 2007, 126(1), 014507 |
| 18 | Rajabpour A., Akizi F. Y., Heyhat M. M., Gordiz K., Int. Nano Lett., 2013, 3(58), 1—6 |
| 19 | Raim V., Srebnik S., J. Membrane Sci., 2018, 563(14), 183—190 |
| 20 | Song T., Liu S. J., Xiao L. P., Chen Q. Y., Chem. J. Chinese Universities, 2012, 33(1), 114—118(宋婷, 刘士军, 肖刘萍, 陈启元. 高等学校化学学报, 2012, 33(1), 114—118) |
| 21 | Liu Q. Z., Yang D. F., Hu Y. D., Chem. J. Chinese Universities, 2009, 30(3), 568—572(刘清芝, 杨登峰, 胡仰栋. 高等学校化学学报, 2009, 30(3), 568—572) |
| 22 | Kohns M., Reiser S., Horsch M., Hasse H., J. Chem. Phys., 2016, 144(8), 084112 |
| 23 | Kohns M., Horsch M., Hasse H., J. Chem. Phys., 2017, 147(14), 144108 |
| 24 | Moucka F., Lisal M., Skvor J., Jirsak J., Nezbeda I., Smith W. R., J. Phys. Chem. B, 2011, 115(24), 7849—7861 |
| 25 | Luo Y., Roux B., J. Phys. Chem. Lett., 2009, 1(1), 183—189 |
| 26 | Luo Y., Jiang W., Yu H., Mackerell A. D., Roux B., Faraday Discuss., 2013, 160(9), 135—149 |
| 27 | Joung I. S., Thomas E., Cheatham I., J. Phys. Chem. B, 2009, 113(1), 13279—13290 |
| 28 | Joung I. S., Thomas E. Cheatham I., J. Phys. Chem. B, 2008, 112(30), 9020—9041 |
| 29 | Yagasaki T., Matsumoto M., Tanaka H., J. Chem. Thero. Comput., 2020, 16(4), 2460—2473 |
| 30 | Boda D., Henderson D., Mol. Phys., 2008, 106(20), 2367—2370 |
| 31 | Delhommelle J., Millie P., Mol. Phys., 2001, 99(8), 619—625 |
| 32 | Kulkarni M., Yang C., Pak Y., B. Korean Chem. Soc., 2018, 39(8), 931—935 |
| 33 | Aragones J. L., Sanz E., Vega C., J. Chem. Phys., 2012, 136(24), 244508 |
| 34 | Mester Z., Panagiotopoulos A. Z., J. Chem. Phys., 2015, 142(4), 044507 |
| 35 | Moučka F., Nezbeda I., Smith W. R., J. Chem. Theor. Comput., 2015, 11(4), 1756—1764 |
| 36 | Price D. J., Brooks Iii C. L., J. Chem. Phys., 2004, 121(20), 10096—10103 |
| 37 | Mark P., Nilsson L., J. Phys. Chem. A, 2001, 105(43), 9954—9960 |
| 38 | Kiss P. T., Baranyai A., J. Chem. Phys., 2011, 134(5), 054106 |
| 39 | Jorgensen W. L., Jenson C., J. Comput. Chem., 1998, 19(10), 1179—1186 |
| 40 | Harrach M. F., Drossel B., J. Chem. Phys., 2014, 140(17), 174501 |
| 41 | Guendouzi M. E., Dinane A., Mounir A., Calphad, 2001, 33(9), 1059—1072 |
| 42 | Martínez L., Andrade R., Birgin E. G., Martínez J. M., J. Comput. Chem., 2009, 30(13), 2157—2164 |
| 43 | Thompson A. P., Aktulga H. M., Berger R., Bolintineanu D. S., Brown W. M., Crozier P. S., In 'T Veld P. J., Kohlmeyer A., Moore S. G., Nguyen T. D., Shan R., Stevens M. J., Tranchida J., Trott C., Plimpton S. J., Comput. Phys. Comm., 2022, 271, 108171 |
| 44 | Dinane A., J. Chem. Thermodyn., 2007, 39(1), 96—103 |
| 45 | Guendouzi M. E., Benbiyi A., Dinane A., Azougen R., Calphad, 2004, 28(1), 97—103 |
| 46 | Guendouzi M. E., Benbiyi A., Dinane A., Azougen R., Calphad, 2003, 27(23), 213—219 |
| 47 | Dinane A., Guendouzi M. E., Mounir A., J. Chem. Thermodyn., 2002, 34(4), 423—441 |
| [1] | GAO Zhiwei, LI Junwei, SHI Sai, FU Qiang, JIA Junru, AN Hailong. Analysis of Gating Characteristics of TRPM8 Channel Based on Molecular Dynamics [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220080. |
| [2] | ZENG Xianyang, ZHAO Xi, HUANG Xuri. Mechanism of Inhibition of Glucose and Proton Cotransport Protein GlcPSe by Cytochalasin B [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210822. |
| [3] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [4] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [5] | ZHANG Lingyu, ZHANG Jilong, QU Zexing. Dynamics Study of Intramolecular Vibrational Energy Redistribution in RDX Molecule [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220393. |
| [6] | LEI Xiaotong, JIN Yiqing, MENG Xuanyu. Prediction of the Binding Site of PIP2 in the TREK-1 Channel Based on Molecular Modeling [J]. Chem. J. Chinese Universities, 2021, 42(8): 2550. |
| [7] | LI Congcong, LIU Minghao, HAN Jiarui, ZHU Jingxuan, HAN Weiwei, LI Wannan. Theoretical Study of the Catalytic Activity of VmoLac Non-specific Substrates Based on Molecular Dynamics Simulations [J]. Chem. J. Chinese Universities, 2021, 42(8): 2518. |
| [8] | LIU Shasha, ZHANG Heng, YUAN Shiling, LIU Chengbu. Molecular Dynamics Simulation of Pulsed Electric Field O/W Emulsion Demulsification [J]. Chem. J. Chinese Universities, 2021, 42(7): 2170. |
| [9] | ZENG Yonghui, YAN Tianying. Vibrational Density of States Analysis of Proton Hydration Structure [J]. Chem. J. Chinese Universities, 2021, 42(6): 1855. |
| [10] | QI Renrui, LI Minghao, CHANG Hao, FU Xueqi, GAO Bo, HAN Weiwei, HAN Lu, LI Wannan. Theoretical Study on the Unbinding Pathway of Xanthine Oxidase Inhibitors Based on Steered Molecular Dynamics Simulation [J]. Chem. J. Chinese Universities, 2021, 42(3): 758. |
| [11] | LIU Aiqing, XU Wensheng, XU Xiaolei, CHEN Jizhong, AN Lijia. Molecular Dynamics Simulation of Polymer/rod Nanocomposite [J]. Chem. J. Chinese Universities, 2021, 42(3): 875. |
| [12] | HE Jinlu, LONG Run, FANG Weihai. A-site Cation Effects on Hot Carrier Relaxation in Perovskites by Nonadiabatic Molecular Dynamics Simulations † [J]. Chem. J. Chinese Universities, 2020, 41(3): 439. |
| [13] | ZHU Yuquan, ZHAO Xiaojie, ZHONG Yuan, CHEN Ziru, YAN Hong, DUAN Xue. Theoretical Study on the Construction and Characteristics of the Host-guest Intercalated Structure of Layered Double Hydroxides [J]. Chem. J. Chinese Universities, 2020, 41(11): 2287. |
| [14] | QU Siying, XU Qin. Different Roles of Some Key Residues in the S4 Pocket of Coagulation Factor Xa for Rivaroxaban Binding † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1918. |
| [15] | MA Yucong, FAN Baomin, WANG Manman, YANG Biao, HAO Hua, SUN Hui, ZHANG Huijuan. Two-step Preparation of Trazodone and Its Corrosion Inhibition Mechanism for Carbon Steel [J]. Chem. J. Chinese Universities, 2019, 40(8): 1706. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||