

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (1): 1.doi: 10.7503/cjcu20180133
• Inorganic Chemistry • Previous Articles Next Articles
ZHANG Lirong1, WANG Zhipeng1, LIU Ying1, XU Chao2,*( ), CHEN Jing2, DING Songdong1,*(
), CHEN Jing2, DING Songdong1,*( )
)
Received:2018-02-12
Online:2019-01-10
Published:2018-12-12
Contact:
XU Chao,DING Songdong
E-mail:xuchao@tsinghua.edu.cn;dsd68@163.com
Supported by:CLC Number:
TrendMD:
ZHANG Lirong,WANG Zhipeng,LIU Ying,XU Chao,CHEN Jing,DING Songdong. Extraction of Trivalent Americium and Europiumfrom Nitric Acid Solution with Di(2-ethylhexyl)dithiophosphinic Acid†[J]. Chem. J. Chinese Universities, 2019, 40(1): 1.
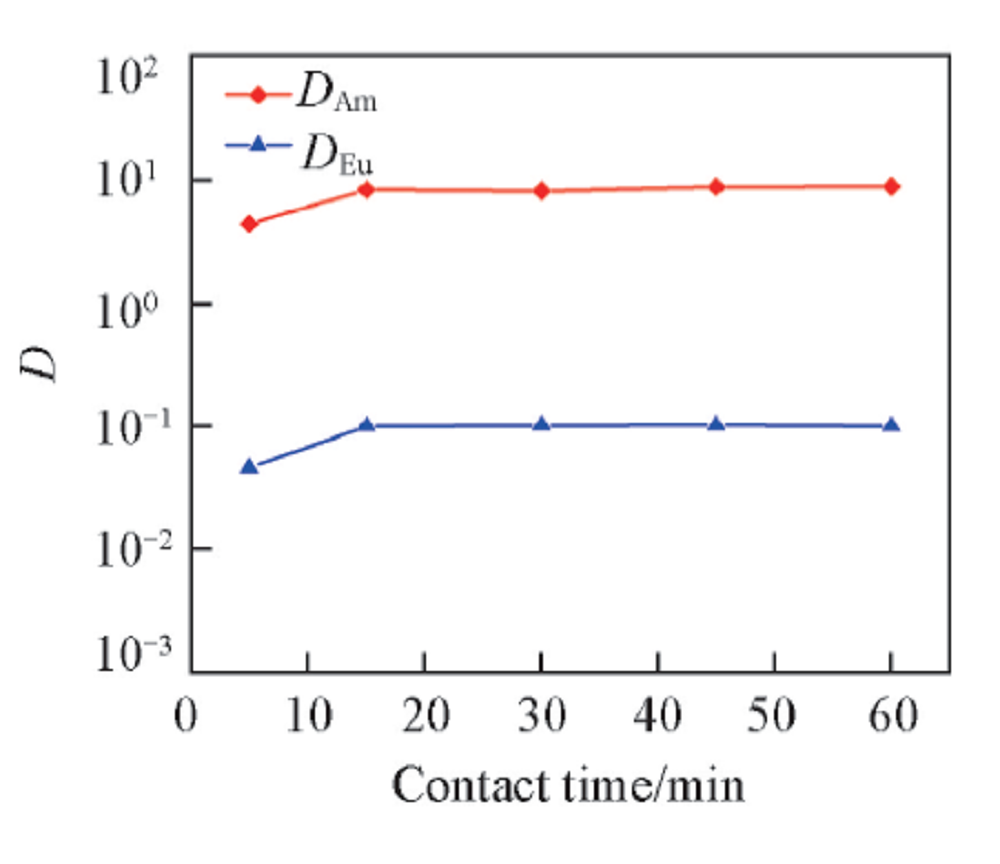
Fig.2 Influence of contact time on the distribution ratio of Am3+ and Eu3+Organic phase: 0.50 mol/L D2EHDTPA in n-dodecane; aqueous phase: trace amount of 241Am3+ or 152,154Eu3+ in 1.0 mol/L NaNO3 solution with pH of 3.35 and 4.05 for Am3+ and Eu3+, respectively.
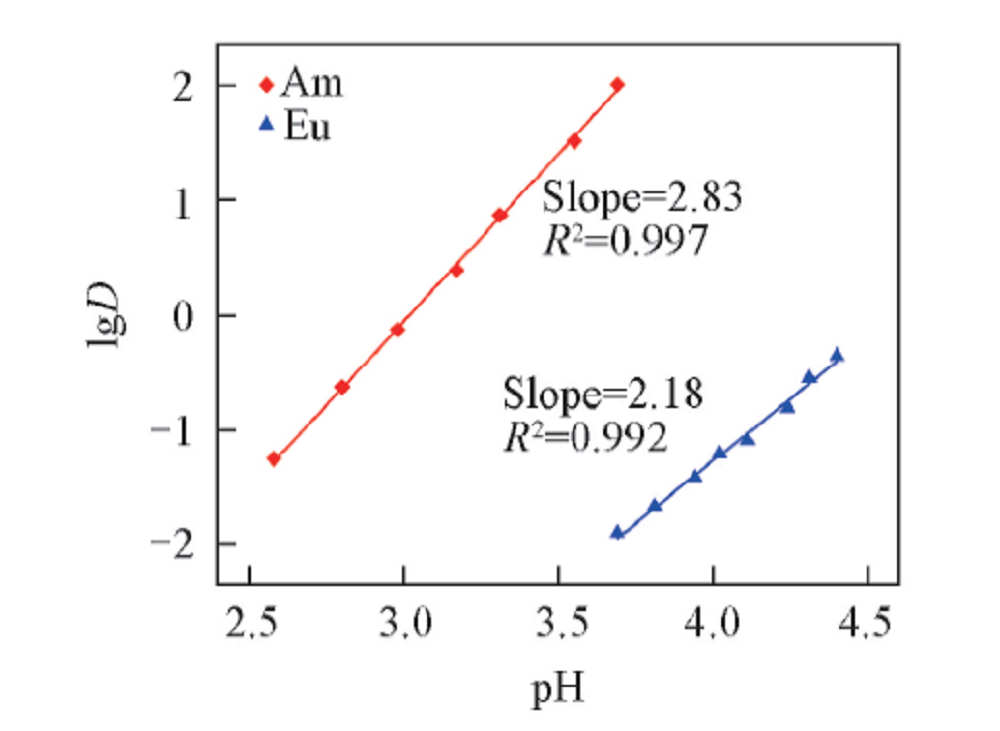
Fig.3 Influence of pH on the distribution ratio of Am3+ and Eu3+Organic phase: 0.50 mol/L D2EHDTPA in n-dodecane; aqueous phase: trace amount of 241Am3+ or 152,154Eu3+ in 1.0 mol/L NaNO3 solution with various pH.
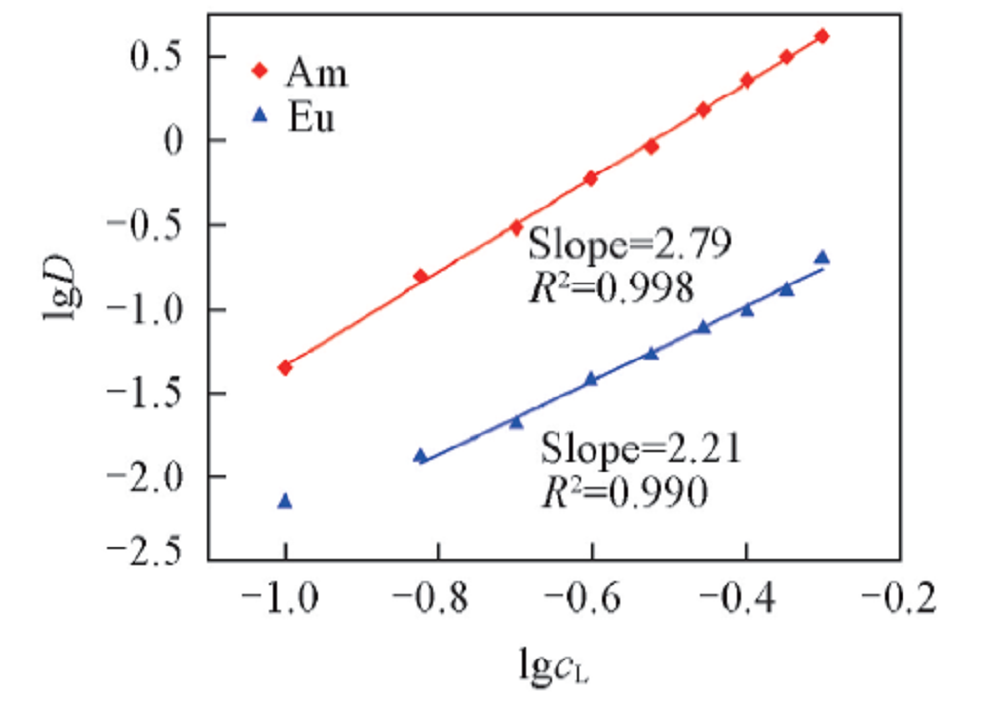
Fig.4 Influence of D2EHDTPA concentration on the extraction of Am3+ and Eu3+Organic phase: various concentration of D2EHDTPA in n-dodecane; aqueous phase: trace amount of 241Am3+ or 152,154Eu3+ in 1.0 mol/L NaNO3 solution with pH of 3.24 and 4.40 for Am3+ and Eu3+, respectively.
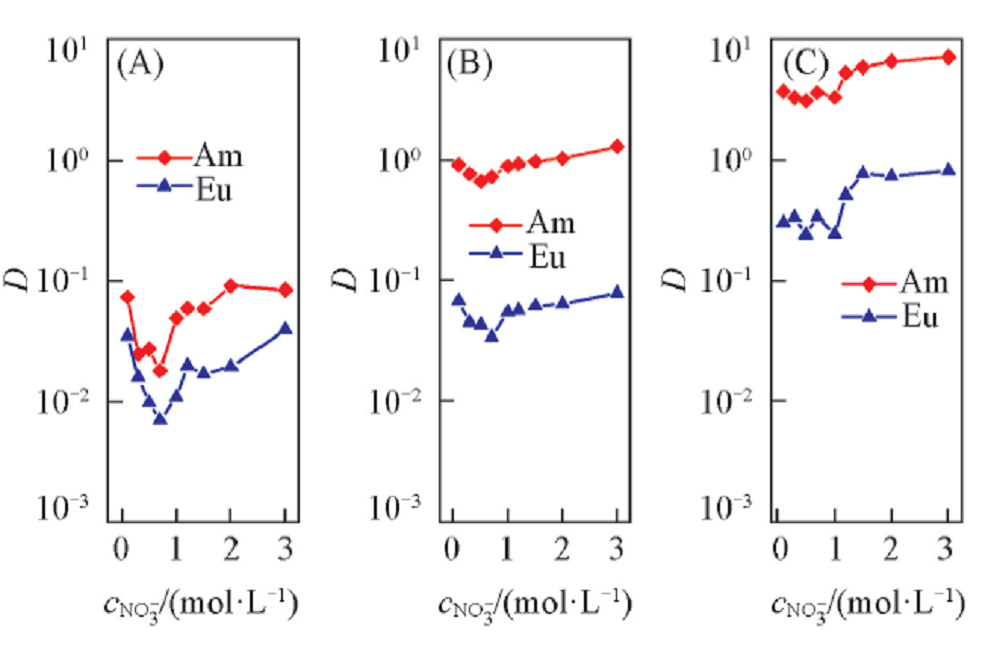
Fig.5 Influence of NO3- concentration on the extraction of Am3+ and Eu3+Organic phase: 0.10(A), 0.30(B) or 0.50(C) mol/L D2EHDTPA in n-dodecane; aqueous phase: trace amount of 241Am3+ or 152,154Eu3+ in various concentration NaNO3 solutions with pH of 3.24 and 4.40 for Am3+ and Eu3+, respectively.
| cL /(mol·L-1) | lg | lg | cL /(mol·L-1) | lg | lg |
|---|---|---|---|---|---|
| 0.10 | -7.22 | -9.00 | 0.35 | -7.21 | -9.16 |
| 0.15 | -7.17 | -9.11 | 0.40 | -7.19 | -9.19 |
| 0.20 | -7.22 | -9.19 | 0.45 | -7.20 | -9.18 |
| 0.25 | -7.21 | -9.15 | 0.50 | -7.20 | -9.09 |
| 0.30 | -7.24 | -9.15 | Average value | -7.21 | -9.14 |
Table 1 Apparent extraction equilibrium constants for Am3+ and Eu3+ by D2EHDTPA at 25 ℃
| cL /(mol·L-1) | lg | lg | cL /(mol·L-1) | lg | lg |
|---|---|---|---|---|---|
| 0.10 | -7.22 | -9.00 | 0.35 | -7.21 | -9.16 |
| 0.15 | -7.17 | -9.11 | 0.40 | -7.19 | -9.19 |
| 0.20 | -7.22 | -9.19 | 0.45 | -7.20 | -9.18 |
| 0.25 | -7.21 | -9.15 | 0.50 | -7.20 | -9.09 |
| 0.30 | -7.24 | -9.15 | Average value | -7.21 | -9.14 |
| Saponification degree(%) | 0 | 1 | 3 | 5 | 10 | 15 | 20 |
|---|---|---|---|---|---|---|---|
| SFAm/Eu | 5.55 | 5.05 | 1.34×104 | 2.51×104 | 1.34×104 | 4.86×103 | 5.07×103 |
Table 2 Influence of the saponification degree of D2EHDTPA on the separation factor*
| Saponification degree(%) | 0 | 1 | 3 | 5 | 10 | 15 | 20 |
|---|---|---|---|---|---|---|---|
| SFAm/Eu | 5.55 | 5.05 | 1.34×104 | 2.51×104 | 1.34×104 | 4.86×103 | 5.07×103 |
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
|---|---|---|---|---|---|
| 0.022 | 0.022 | 0.022 | 0.023 | 0.022 |
Table 3 Eu3+ concentration in organic phase after extraction by D2EHDTPAa
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
|---|---|---|---|---|---|
| 0.022 | 0.022 | 0.022 | 0.023 | 0.022 |
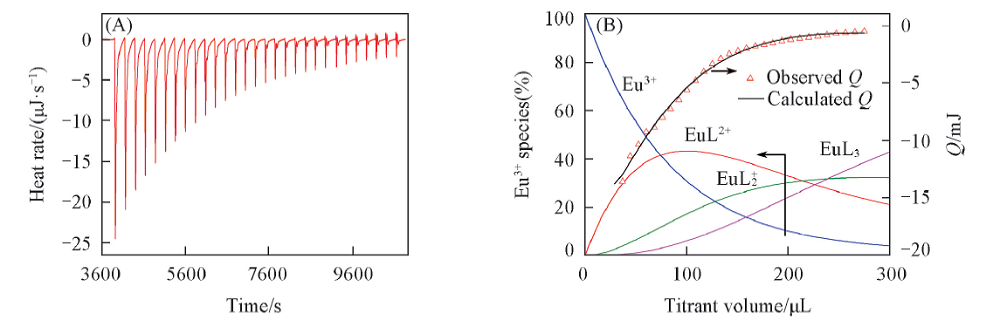
Fig.9 Microcalorimentric titration of Eu(ClO4)3 with D2EHDTPA in EtOH-H2O(volume ratio 99:1)(A) and the observed and fitted cumulative heat and the speciation of Eu3+ along the titration(B)Initial conditions: [Eu3+]=1.0×10-3 mol/L, volume=1.40 mL; Titration conditions: [D2EHDTPA]=1.0×10-2 mol/L, 30 additions of 0.01 mL each.
| Reaction | lgβ | ΔH/(kJ·mol-1) | ΔS/(J·mol-1·K-1) | ΔG/(kJ·mol-1) |
|---|---|---|---|---|
| Eu3++L-=EuL2+ | 3.56 | 12.7 | 72.3 | -8.85 |
| Eu3++2L-=Eu | 6.57 | 16.6 | 113 | -17.2 |
| Eu3++3L-=EuL3 | 9.52 | 18.5 | 162 | -29.8 |
Table 4 Thermodynamic parameters for the complexation of Eu3+ with D2EHDTPA at 25 ℃
| Reaction | lgβ | ΔH/(kJ·mol-1) | ΔS/(J·mol-1·K-1) | ΔG/(kJ·mol-1) |
|---|---|---|---|---|
| Eu3++L-=EuL2+ | 3.56 | 12.7 | 72.3 | -8.85 |
| Eu3++2L-=Eu | 6.57 | 16.6 | 113 | -17.2 |
| Eu3++3L-=EuL3 | 9.52 | 18.5 | 162 | -29.8 |
| [1] | Bhattacharyya A., Ghanty T. K., Mohapatra P. K., Manchanda V. K., Inorg.Chem.,2011, 50, 3913—3921 |
| [2] | Xue W. J., Zhang A. Y., Chai Z. F., China Science Paper.,2012, 7(9), 657—665 |
| (薛文静, 张安运, 柴之芳. 中国科技论文, 2012, 7(9), 657—665) | |
| [3] | Mathur J. N., Murali M. S., Nash K. L., Solvent Extr. IonExch.,2001, 19(3), 357—390 |
| [4] | Nash K. L., Solvent Extr. Ion Exch., 1993, 11(4), 729—768 |
| [5] | Mincher B. J., Modolo G., Mezyk S. P., Solvent Extr. Ion Exch.,2010, 28(4), 415—436 |
| [6] | Chen J., Wang F., He X. H., Pan D. F., Prog.Chem.,2011, 23(7), 1338—1344 |
| (陈靖, 王芳, 何喜红, 盘登芳. 化学进展, 2011, 23(7), 1338—1344) | |
| [7] | Dam H. H., Reinhoudt D. N., Verboom W., Chem. Soc.Rev.,2007, 36, 367—377 |
| [8] | Jensen M. P., Bond A. H., J. Am. Chem.Soc.,2002, 124, 9870—9877 |
| [9] | Kolarik Z., Müllich U., Gassner F., Solvent Extr. Ion Exch.,1999, 17(1), 23—32 |
| [10] | Trumm S., Geist A., Panak P. J., Fanghänel T., Solvent Extr. Ion Exch., 2011, 29(4), 213—229 |
| [11] | Foreman M. R. S., Hudson M. J., Geist A., Madic C., Weigl M., Solvent Extr. Ion Exch., 2005, 23, 645—662 |
| [12] | Retegan T., Ekberg C., Dubois I., Fermvik A., Wass T. J., Skarnemark G., Solvent Extr. Ion Exch., 2007, 25(4), 417—431 |
| [13] | Galletta M., Scaravaggi S., Macerata E., Famulari A., Mele A., Panzeri W., Sansone F., Casnati A., Mariani M., Dalton Trans.,2013, 42, 16930—16938 |
| [14] | Edwards A. C., Wagner C., Geist A., Burton N. A., Sharrad C. A., Adams R. W., Pritchard R. G., Panak P. J., Whitehead R. C., Harwood L. M., Dalton Trans.,2016, 45, 18102—18112 |
| [15] | Bremer A., Ruff C. M., Girnt D., Müllich U., Rothe J., Roesky P. W., Panak P. J., Karpov A., Müllich T. J. J., Denecke M. A., Geist A., Inorg.Chem., 2012, 51, 5199—5207 |
| [16] | Wang J. R., Su D. P., Wang D. Q., Ding S. D., Huang C., Huang H., Hu X. Y., Wang Z. P., Li S. M., Inorg.Chem., 2015, 54, 10648—10655 |
| [17] | Su D.P., Liu Y., Li S. M., Ding S. D., Jin Y. D., Wang Z. P., Hu X. Y., Zhang L. R.,Eur. J. Inorg. Chem., 2017, 651—658 |
| [18] | Hu X. Y., Su D. P., Li S. M., Wang Z. P., Zhang L. R., Liu Y., Song L. J., Chen Z. L., Ding S. D., Chem. J. Chinese Universities.,2017, 38(8), 1324—1333 |
| (胡晓阳, 苏冬萍, 李诗萌, 王志鹏, 张利荣, 刘莹, 宋莲君, 陈志力, 丁颂东. 高等学校化学学报. , 2017, 38(8), 1324—1333) | |
| [19] | Geist A., Hill C., Modold G., Foreman M. R. St. J., Weigl M., Gompper K., Hudson M. J., Solvent Extr. Ion Exch., 2007, 24, 463—483 |
| [20] | Zhu Y. J., Chen J., Jiao R. Z., Solvent Extr. IonExch., 1996, 14(1), 61—68 |
| [21] | Wang X. H., Zhu Y. J., Jiao R. Z., J. Radioanal. Nucl. Chem.,., 2002, 251 487—492 |
| [22] | Bhattacharyya A., Mohapatra P. K., Manchanda V. K., Solvent Extr. Ion Exch.,2006, 24(1), 1—17 |
| [23] | Bhattacharyya A., Mohapatra P. K., Manchanda V. K., Solvent Extr. Ion Exch.,2007, 25(1), 27—39 |
| [24] | Ionova G., Rabbe C., Hill C., Madic C., Guillaumont R., Krupa C., Solvent Extr. Ion Exch., 2001, 19(3), 391—414 |
| [25] | Cao X. Y., Heidelberg D., Ciupka J., Dolg M., Inorg.Chem.,2010, 49(22), 10307—10315 |
| [26] | Tian G. X., Zhu Y. J., Xu J. M., Hu T. D., Xie Y. N., J. Alloys Compd.,2002, 334(1/2), 86—91 |
| [27] | Tian G. X., Zhu Y. J., Xu J. M., Zhang P., Hu T. D., Xie Y. N., Zhang J., Inorg.Chem. ,2003, 42, 735—741 |
| [28] | Tian G. X., Kimura T., Yoshida Z., Zhu Y. J., Rao L. F., Radiochim .Acta.,2004, 92(8), 495—499 |
| [29] | Sole K. C., Hiskey J. B., Ferguson T. L., Solvent Extr. IonExch.,1993, 11(5), 783—796 |
| [30] | Chen J., The Separation of Amerieium from Lanthanides by Bis(2,4,4-trimethylpentyl)dithiophosphinic Acid Extraction, Tsinghua University, Beijing, 1996 |
| (陈靖. 二(2,4,4-三甲基戊基)二硫代膦酸萃取分离镅与镧系元素, 北京: 清华大学, 1996) | |
| [31] | Chen J., Wang S. W., Xu C., Wang X. H., Feng X. G., Procedia Chemistry.,2012, 7, 172—177 |
| [32] | Sole K. C., Hiskey J. B., Hydrometallurgy.,1995, 37, 129—147 |
| [33] | Tian G. X., Zhu Y. J., Xu J. M., Solvent Extr. Ion Exch., 2001, 19(6), 993—1005 |
| [34] | Xu Q. C., Wu J. F., Chang Y. Z., Zhang L. X., Yang Y. S., Radiochim.Acta.,2008, 96, 771—779 |
| [35] | Xu C., Rao L. F., Chem. Eur.J.,2014, 20, 14807—14815 |
| [36] | Xu G.X., Wang W. Q., Wu J. G., Gao H. C., Shi N., Extraction Chemistry Principle., Shanghai Science and Technology Press, Shanghai, 1985, 87—91 |
| (徐光宪, 王文清, 吴瑾光, 高宏成, 施鼐. 萃取化学原理., 上海: 上海科学技术出版社, 1985, 87—91) | |
| [37] | Peterman D. R., Greenhalgh M. R., Tillotson R. D., Klaehn J. R., Harrup M. K., Luther T. A., Law J. D., Sep. Sci.Technol.,2010, 45, 1711—1717 |
| [38] | Jensen M. P., Chiarizia R., Urban V., Solvent Extr. Ion Exch., 2001, 19(5), 865—884 |
| [39] | Pattee D., Musikas C.,Faure A., Chachaty C., J. Less-Common Metals.,1986, 122, 295—302 |
| [40] | Horwitz E. P., Muscatello A. L., Kalina D. G., Kaplan L., Sep. Sci.Technol.,1981, 16(4), 417—437 |
| [41] | Chen J., Zhu Y. J., Jiao R. Z., Solvent Extr. Ion Exch., 1996, 31(19), 2723—2731 |
| [42] | Chen H. H., Zhao X., Luan X. H., Yu G. L., Chem. J. Chinese Universities.,2015, 36(1), 1—8 |
| (陈欢欢, 赵峡, 栾晓红, 于广利. 高等学校化学学报., 2015, 36(1), 1—8) | |
| [43] | Zhang L., Li H. F., Chen P., Sun W. B., Yan P. F., Chem. Res. Chinese Universities.,2016, 32(4), 534—538 |
| [44] | Ferraro J. R., J. Inorg. Nucl.Chem., 1959, 10, 319—324 |
| [45] | Bernardo P. D., Melchior A., Tolazzi M., Zanonato P. L., Coord. Chem.Rev., 2012, 256, 328—351 |
| [46] | Xu C., Sun T. X., Rao L. F., Inorg.Chem.,2017, 56, 2556—2565 |
| [47] | Wang L. F., He D. Q., Chen W., Yu H. Q., Water Research.,2015, 81, 325—332 |
| [48] | Shi X. L., Zhang T., Li X. W., Feng Y., Tan X., Jin Y. R., Chem. Res. Chinese Universities.,2016, 32(4), 556—560 |
| [49] | Wang L.F., Interactions Between Natural Organic MatterNOM) and Metal Ions/Nanoparticles and Their Effects in Membrane Fouling Process, University of Science and Technology of China,Hefei, 2016 |
| (王龙飞. 天然有机脂与金属离子/纳米颗粒的相互作用及其对膜污染过程的影响, 合肥: 中国科学技术大学, 2016) |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||