

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (7): 1223.doi: 10.7503/cjcu20160926
• Physical Chemistry • Previous Articles Next Articles
GAO Hongcheng1,2,*( ), WANG Zhenlu2, NIU Guiling1, WEN Shoudong1, WU Xiaonan1, ZHANG Xiaofei1, SU Pengchen1, YAN Xin1, QI Qiang1, GAO Binbin1
), WANG Zhenlu2, NIU Guiling1, WEN Shoudong1, WU Xiaonan1, ZHANG Xiaofei1, SU Pengchen1, YAN Xin1, QI Qiang1, GAO Binbin1
Received:2016-12-22
Online:2017-07-10
Published:2017-06-02
Contact:
GAO Hongcheng
E-mail:gaohcdpc2014@163.com
Supported by:CLC Number:
TrendMD:
GAO Hongcheng, WANG Zhenlu, NIU Guiling, WEN Shoudong, WU Xiaonan, ZHANG Xiaofei, SU Pengchen, YAN Xin, QI Qiang, GAO Binbin. Preparation and Catalytic Olefin Epoxidation Properties of Carbon Nanotube/Polyoxometalates/Polypyrrole Composite Catalyst†[J]. Chem. J. Chinese Universities, 2017, 38(7): 1223.
| Catalyst | Time/h | Conversion(%) |
|---|---|---|
| PMo | 4 | 89.8 |
| PMo/PPy | 1 | 40.9 |
| 2 | 67.2 | |
| 4 | 71.2 | |
| PMo/CNTs | 1 | 42.2 |
| 2 | 56.1 | |
| 4 | 69.8 | |
| PPy-PMo@CNTs | 1 | 36.5 |
| 2 | 45.6 | |
| 4 | 65.9 |
Table 1 Cyclooctene epoxidation results over different kinds of catalysts in MeCN*
| Catalyst | Time/h | Conversion(%) |
|---|---|---|
| PMo | 4 | 89.8 |
| PMo/PPy | 1 | 40.9 |
| 2 | 67.2 | |
| 4 | 71.2 | |
| PMo/CNTs | 1 | 42.2 |
| 2 | 56.1 | |
| 4 | 69.8 | |
| PPy-PMo@CNTs | 1 | 36.5 |
| 2 | 45.6 | |
| 4 | 65.9 |
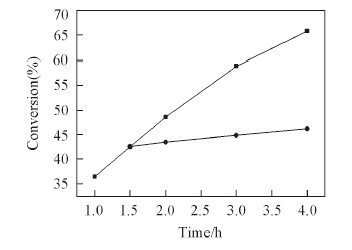
Fig.5 Kinetic profiles of epoxidation of cyclooctene with H2O2 over catalyst PPy-PMo@CNTsLeaching experiment of catalysts: solid circle indicates conversion of cyclooctene with the catalysts removed after 1.5 h at reaction temperature. Reaction condition: 1.0 mmol cyclooctene; 1.0 mmol H2O2; 10 mg catalyst; 2.0 mL MeCN; temperature: 60 ℃. All selectivities for the epoxide are nearly 100%.
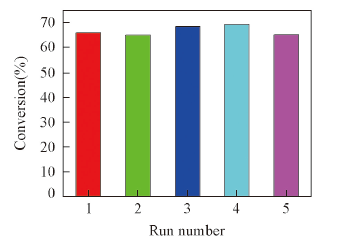
Fig.6 Recycled experiments of PPy-PMo@CNTs for the conversion of cyclooctene after 4 h reactionAll selectivities for the epoxide are greater than 99%. Reaction condition: 10 mg catalyst; 1.0 mmol cyclooctene; 2.0 mL MeCN; 1.0 mmol H2O2; temperature 60 ℃.
| [1] | Jia M.J., Thiel W. R.,Chem. Commun., 2002, 2392—2393 |
| [2] | Gao H. C., Yan Y., Xu X. H., Yu J. H., Niu H. L., Gao W. X., Zhang W. X., Jia M. J., Chin. J. Catal., 2015, 36, 1811—1817 |
| [3] | Uchida S., Hikichi S., Akatsuka T., Tanaka T., Kawamoto R., Lesbani A., Nakagawa Y., Uehara K., Mizuno N., Chem. Mater., 2007, 19, 4694—4701 |
| [4] | Zhu Y. G., Wang Q., Cornwall R. G., Shi Y., Chem. Rev., 2014, 114(16) , 8199—8256 |
| [5] | Singh G.S., D’hooghe M., Kimpe N. D.,Chem. Rev., 2007, 107,2080—2135 |
| [6] | Müller A., Peters F., Chem. Rev., 1998, 98, 239—272 |
| [7] | He J. H., Yu J. H., Pan Q. H., Chen P., Xu R. R., Chem. J. Chinese Universities,2005, 26 (5),797—800 |
| (何江华, 于吉红, 潘勤鹤, 陈鹏, 徐如人.高等学校化学学报, 2005, 26(5), 797—800) | |
| [8] | Rao C. N. R., Natarajan S., Vaidhyanathan R., Angew. Chem. Int. Ed., 2004, 43, 1466—1496 |
| [9] | Coronado E., Gómez-García C. J., Chem. Rev., 1998, 98, 273—296 |
| [10] | Wang X. L., Wang E. B., Xu X. X., Li Y. G., Chem. J. Chinese Universities,2008, 29(10),1937—1940 |
| (王晓兰, 王恩波, 徐欣欣, 李阳光.高等学校化学学报, 2008, 29(10), 1937—1940) | |
| [11] | Carlucci L., Ciani G., Proserpio D. M., Coord. Chem. Rev., 2003, 246, 247—289 |
| [12] | Jiao Y. Q., Zang H. Y., Wang X. L., Zhou E. L., Song B. Q., Wang C. G., Shao K. Z., Su Z. M., Chem. Commun., 2015, 51, 11313—11316 |
| [13] | Xu S. S., Chen W. L., Wang Y. H., Li Y. G., Liu Z. J., Shan C. H., Su Z. M., Wang E. B., Dalton Trans., 2015, 44, 18553—18562 |
| [14] | Xu D., Chen W. L., Li J. S., Sang X. J., Lu Y., Su Z. M., Wang E. B., J. Mater. Chem.A,2015, 3, 10174—10178 |
| [15] | Liao J. Z., Dui X. J., Zhang H. L., Wu X. Y., Lu C. Z., Cryst. Eng. Comm., 2014, 16, 10530—10533 |
| [16] | Shan C. H., Zhang H., Chen W. L., Su Z. M., Wang E. B., J. Mater. Chem. A,2016, 4, 3297—3303 |
| [17] | Ci C. G., Carbó J. J., Neumann R., Graaf C. D., Poblet J. M., ACS Catal., 2016, 6(10), 6422—6428 |
| [18] | Zhang C., Bu W. B., Ni D. L., Zuo C. J., Cheng C., Li Q., Zhang L. L., Wang Z., Shi J. L., J. Am. Chem. Soc., 2016, 138(26), 8156—8164 |
| [19] | Song X. J., Zhu W. C., Li K. G., Wang J., Niu H. L., Gao H. C., Gao W. X., Zhang W. X., Yu H., Jia M. J., Catalysis Today,2015, 259, 59—65 |
| [20] | Zhu Y. N., Peng T. Z., Li J. P., Chem. J. Chinese Universities,2004, 25(9), 1637—1641 |
| (朱玉奴, 彭图治, 李建平.高等学校化学学报, 2004, 25(9), 1637—1641) | |
| [21] | Iijima S., Ichihashi T., Nature,1993, 363, 603—605 |
| [22] | Yan Y. B., Miao J. W., Yang Z. H., Xiao F. X., Yang H. B., Liu B., Yang Y. H., Chem. Soc. Rev., 2015, 44, 3295—3346 |
| [23] | Iijima S., Nature, 1991, 354, 56—58 |
| [24] | Hu L. B., Hecht D. S., Gruner G., Chem. Rev., 2010, 110, 5790—5844 |
| [25] | Bhattacharya S., Samanta S. K., Chem. Rev., 2016, 116(19), 11967—12028 |
| [26] | Suo L. L., Li S. J., Li Y. T., Zhang L., Zhang X., Chem. J. Chinese Universities,2016, 37(11), 2043—2049 |
| (索路路,李生娟,李应涛,张莉,张熙.高等学校化学学报, 2016, 37(11), 2013—2049) | |
| [27] | Zeng Z. H., Li S. C., Liu Y., Xu J. J., Zhou Y. L., Chem. J. Chinese Universities,2017, 38(1), 20—27 |
| (曾泽华, 李诗纯, 刘渝, 徐金江, 周元林.高等学校化学学报, 2017, 38(1), 20—27) | |
| [28] | Rapi S., Gardini G. P., Synthetic Metals,1988, 24, 217—221 |
| [29] | Zhang Z., Chen G. M., Wang H. F., Zhai W. T., J. Mater. Chem.C,2015, 3, 1649—1654 |
| [30] | Guo H. J., Yin G. C., J. Phys. Chem.C,2011, 115, 17516—17522 |
| [31] | Predoeva A., Damyanova S., Gaigneaux E. M., Petrov L., Appl. Catal. A: Gen., 2007, 319, 14—24 |
| [1] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [2] | ZHAO Runyao, JI Guipeng, LIU Zhimin. Efficient Electrocatalytic CO2 Reduction over Pyrrole Nitrogen-coordinated Single-atom Copper Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220272. |
| [3] | LI Jiafu, ZHANG Kai, WANG Ning, SUN Qiming. Research Progress of Zeolite-encaged Single-atom Metal Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220032. |
| [4] | GAO Jing, HE Wentao, WANG Xinxin, XIANG Yushu, LONG Lijuan, QIN Shuhao. Preparation of DOPO Derivative Modified Carbon Nanotubes and Their Effect on Flame Retardancy of Polylactic Acid [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210670. |
| [5] | LIU Jie, LI Jinsheng, BAI Jingsen, JIN Zhao, GE Junjie, LIU Changpeng, XING Wei. Constructing a Water-blocking Interlayer Containing Sulfonated Carbon Tubes to Reduce Concentration Polarization in Direct Methanol Fuel Cells [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220420. |
| [6] | ZHU Haotian, JIN Meixiu, TANG Wensi, SU Fang, LI Yangguang. Properties of Transition Metal-biimidazole-Dawson-type Tungstophosphate Hybrid Compounds as Supports for Enzyme Immobilization [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220328. |
| [7] | DING Qin, ZHANG Zixuan, XU Peicheng, LI Xiaoyu, DUAN Limei, WANG Yin, LIU Jinghai. Effects of Cu, Ni and Co Hetroatoms on Constructions and Electrocatalytic Properties of Fe-based Carbon Nanotubes [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220421. |
| [8] | ZHANG Taiwen, GUO Jun, ZHANG Dan, YUAN Changmei, QIU Shuangyan. Synthesis, Characterization and Catalytic Oxidation Iodine Ion Performance of trz-Cl-Cu-PMo12 [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220215. |
| [9] | HOU Congcong, WANG Huiying, LI Tingting, ZHANG Zhiming, CHANG Chunrui, AN Libao. Preparation and Electrochemical Properties of N-CNTs/NiCo-LDH Composite [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220351. |
| [10] | XU Xiaojian, LI Bo, LIN Mengxiao, ZHAN Shuo. Vacuum Freeze Drying to Prepare Porous Carbon Based Composite Membranes for Efficient Solar Steam Generation [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220361. |
| [11] | WANG Jie, HUO Haiyan, WANG Yang, ZHANG Zhong, LIU Shuxia. General Strategy for In situ Synthesis of NENU-n Series Polyoxometalate-based MOFs on Copper Foil [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210557. |
| [12] | WEI Zheyu, WU Zhikang, RU Shi, NI Lubin, WEI Yongge. Research Progress of Polyoxometalates-Cyclodextrin Supramolecular System [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210665. |
| [13] | WANG Jin, SHI Wenjie, JIN Linyu, MA Pengtao, WANG Jingping, NIU Jingyang. Synthesis, Structure, and Allochroic Property of Two Hetro-arsenomolybdates Hybrid Polyoxometalates [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210600. |
| [14] | CHEN Huina, LI Xinxiong, ZHENG Shoutian. Research Advance of Polyoxoniobate-based 3-Dimensional Framework Materials [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210625. |
| [15] | LIANG Yu, LIU Huan, GONG Lige, WANG Chunxiao, WANG Chunmei, YU Kai, ZHOU Baibin. Synthesis and Supercapacitor Properties of Biimidazole-modified {SiW12O40} Hybrid [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210556. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||