

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (4): 598.doi: 10.7503/cjcu20160684
• Organic Chemistry • Previous Articles Next Articles
ZHANG Yunxiao1,2, SHANG Wangji1,2, SUN Liwen1, LIU Dan1, TONG Dingyi1,*( ), CAI Tao1,*(
), CAI Tao1,*( ), LIU Shenggao1
), LIU Shenggao1
Received:2016-09-26
Online:2017-04-10
Published:2017-03-23
Contact:
TONG Dingyi,CAI Tao
E-mail:tongdingyi@nimte.ac.cn;caitao@nimte.ac.cn
Supported by:CLC Number:
TrendMD:
ZHANG Yunxiao, SHANG Wangji, SUN Liwen, LIU Dan, TONG Dingyi, CAI Tao, LIU Shenggao. Synthesis, Characterization and Tribological Properties of Imidazol Cheat Boron Ionic Liquids with Different Alkyl Length†[J]. Chem. J. Chinese Universities, 2017, 38(4): 598.
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(400 MHz, CDCl3), δ |
|---|---|---|
| C2MImBScB | 9.09(s, 1H, Imi—CH), 7.82(dd, J=7.9, 1.5 Hz, 2H, Imi—CH—CH—), 7.41—7.33(m, 2H, C6H4), 7.09(dd, J=11.7, 5.5 Hz, 2H, C6H4), 6.89—6.91(m, 4H, C6H4), 4.01(q, J=7.4 Hz, 2H, CH2), 3.70(s, 3H, CH3), 1.32(t, 3H, J=7.4 Hz, 3H, CH3) | 165.66, 159.17, 135.12, 129.58, 123.48, 121.71, 119.30, 118.30, 115.03, 44.94, 35.89, 14.92 |
| C4MImBScB | 9.19(s, 1H, Imi—CH), 7.85(dd, J=7.8, 1.5 Hz, 2H, Imi—CH—CH—), 7.43—7.36(m, 2H, C6H4), 7.10(d, J=13.5 Hz, 2H, C6H4), 6.93—6.83(m, 4H, C6H4), 4.02(t, J=7.4 Hz, 2H, CH2), 3.79(s, 3H, CH3), 1.73—1.63(m, 2H, CH2), 1.21(dq, J=14.7, 7.4 Hz, 2H, CH2), 0.84(t, J=7.4 Hz, 3H, CH3) | 165.59, 159.18, 135.10, 129.59, 123.51, 122.06, 119.22, 118.27, 115.03, 49.56, 35.99, 31.61, 19.42, 13.20 |
| C6MImBScB | 9.06(s, 1H, Imi—CH), 7.85(d, J=7.8 Hz, 2H, Imi—CH—CH—), 7.39—7.31(m, 2H, C6H4), 7.11(d, J=11.1 Hz, 2H, C6H4), 6.84(t, J=7.5 Hz, 4H, C6H4), 3.94(t, J=7.5 Hz, 2H, CH2), 3.69(s, 3H, CH3), 1.77—1.51(m, 2H, CH2), 1.16(dd, J=14.2, 7.6 Hz, 6H, CH2CH2CH2), 0.78(t, J=6.7 Hz, 3H, CH3) | 165.62, 158.18, 135.05, 129.59, 123.50, 122.05, 119.19, 118.26, 115.07, 49.82, 35.98, 30.86, 29.72, 25.66, 22.21, 13.84 |
| C8MImBScB | 9.14 , (s, 1H, Imi—CH), 7.85(dd, J=9.8, 3.3 Hz, 2H, Imi—CH—CH—), 7.42—7.35(m, 2H, C6H4), 7.11(d, J=14.6 Hz, 2H, C6H4), 6.87(t, J=7.5 Hz, 4H, C6H4), 3.99(t, J=7.5 Hz, 2H, CH2), 3.75(s, 3H, CH3), 1.69(dd, J=13.8, 7.0 Hz, 2H, CH2), 1.37—1.04(m, 10H, CH2CH2CH2CH2CH2), 0.86(q, J=7.0 Hz, 3H, CH3) | 165.65, 159.16, 135.07, 129.58, 123.50, 122.05, 119.17, 118.26, 115.05, 48.81, 35.94, 31.56, 29.77, 28.55, 28.73, 26.00, 22.48, 14.02 |
Table 1 1H NMR and 13C NMR data for CnMImBScB(n=2,4,6,8)
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(400 MHz, CDCl3), δ |
|---|---|---|
| C2MImBScB | 9.09(s, 1H, Imi—CH), 7.82(dd, J=7.9, 1.5 Hz, 2H, Imi—CH—CH—), 7.41—7.33(m, 2H, C6H4), 7.09(dd, J=11.7, 5.5 Hz, 2H, C6H4), 6.89—6.91(m, 4H, C6H4), 4.01(q, J=7.4 Hz, 2H, CH2), 3.70(s, 3H, CH3), 1.32(t, 3H, J=7.4 Hz, 3H, CH3) | 165.66, 159.17, 135.12, 129.58, 123.48, 121.71, 119.30, 118.30, 115.03, 44.94, 35.89, 14.92 |
| C4MImBScB | 9.19(s, 1H, Imi—CH), 7.85(dd, J=7.8, 1.5 Hz, 2H, Imi—CH—CH—), 7.43—7.36(m, 2H, C6H4), 7.10(d, J=13.5 Hz, 2H, C6H4), 6.93—6.83(m, 4H, C6H4), 4.02(t, J=7.4 Hz, 2H, CH2), 3.79(s, 3H, CH3), 1.73—1.63(m, 2H, CH2), 1.21(dq, J=14.7, 7.4 Hz, 2H, CH2), 0.84(t, J=7.4 Hz, 3H, CH3) | 165.59, 159.18, 135.10, 129.59, 123.51, 122.06, 119.22, 118.27, 115.03, 49.56, 35.99, 31.61, 19.42, 13.20 |
| C6MImBScB | 9.06(s, 1H, Imi—CH), 7.85(d, J=7.8 Hz, 2H, Imi—CH—CH—), 7.39—7.31(m, 2H, C6H4), 7.11(d, J=11.1 Hz, 2H, C6H4), 6.84(t, J=7.5 Hz, 4H, C6H4), 3.94(t, J=7.5 Hz, 2H, CH2), 3.69(s, 3H, CH3), 1.77—1.51(m, 2H, CH2), 1.16(dd, J=14.2, 7.6 Hz, 6H, CH2CH2CH2), 0.78(t, J=6.7 Hz, 3H, CH3) | 165.62, 158.18, 135.05, 129.59, 123.50, 122.05, 119.19, 118.26, 115.07, 49.82, 35.98, 30.86, 29.72, 25.66, 22.21, 13.84 |
| C8MImBScB | 9.14 , (s, 1H, Imi—CH), 7.85(dd, J=9.8, 3.3 Hz, 2H, Imi—CH—CH—), 7.42—7.35(m, 2H, C6H4), 7.11(d, J=14.6 Hz, 2H, C6H4), 6.87(t, J=7.5 Hz, 4H, C6H4), 3.99(t, J=7.5 Hz, 2H, CH2), 3.75(s, 3H, CH3), 1.69(dd, J=13.8, 7.0 Hz, 2H, CH2), 1.37—1.04(m, 10H, CH2CH2CH2CH2CH2), 0.86(q, J=7.0 Hz, 3H, CH3) | 165.65, 159.16, 135.07, 129.58, 123.50, 122.05, 119.17, 118.26, 115.05, 48.81, 35.94, 31.56, 29.77, 28.55, 28.73, 26.00, 22.48, 14.02 |
| Compd. | Measured mass fraction(%) | Nominal mass fraction(%) | Error(%) |
|---|---|---|---|
| C2MImBScB | 2.708 | 2.77 | 2.23 |
| C4MImBScB | 2.550 | 2.61 | 2.30 |
| C6MImBScB | 2.620 | 2.67 | 1.90 |
| C8MImBScB | 2.270 | 2.34 | 2.99 |
Table 2 Boron concentration of CnMImBScB(n=2,4,6,8)
| Compd. | Measured mass fraction(%) | Nominal mass fraction(%) | Error(%) |
|---|---|---|---|
| C2MImBScB | 2.708 | 2.77 | 2.23 |
| C4MImBScB | 2.550 | 2.61 | 2.30 |
| C6MImBScB | 2.620 | 2.67 | 1.90 |
| C8MImBScB | 2.270 | 2.34 | 2.99 |
| Temperature/℃ | Kinematic viscosity/(mm2·s-1) | ||||
|---|---|---|---|---|---|
| Base oil | Lubricant blend | ||||
| PEG200 | C2MImBscB | C4MImBscB | C6MImBscB | C8MImBscB | |
| 40 | 22.99 | 23.97 | 23.80 | 23.12 | 23.62 |
| 100 | 4.20 | 4.07 | 4.16 | 4.13 | 4.26 |
Table 3 Kinematic viscosity of blends of ionic liquids(mass fraction 2%) with PEG200
| Temperature/℃ | Kinematic viscosity/(mm2·s-1) | ||||
|---|---|---|---|---|---|
| Base oil | Lubricant blend | ||||
| PEG200 | C2MImBscB | C4MImBscB | C6MImBscB | C8MImBscB | |
| 40 | 22.99 | 23.97 | 23.80 | 23.12 | 23.62 |
| 100 | 4.20 | 4.07 | 4.16 | 4.13 | 4.26 |
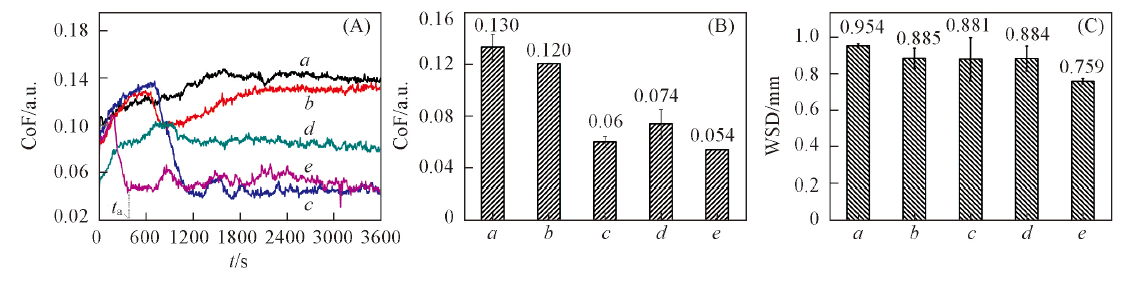
Fig.5 Evolution of coefficient of friction(CoF) with contact time(A), average coefficient of friction(B) and average wear scar diameter(WSD) of PEG200 and CnMImBScB(n=2,4,6,8, mass fraction 2%) individually blended in PEG200(C)a. PEG200; b. PEG200+G2MImBScB; c. PEG200+C4MImBScB; d. PEG200+C6MImBScB; e. PEG200+C8MImBScB.
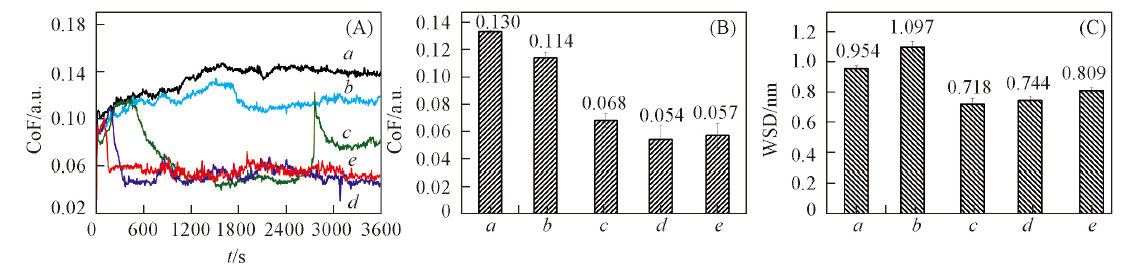
Fig.6 Evolution of friction coefficient(CoF) with contact time(A), average friction coefficient(B) and wear scar diameter(C) of PEG200, C8MImBScB blended in PEG200 with mass fraction of 0(a), 0.5%(b), 1%(c), 2%(d), 4%(e), respectively
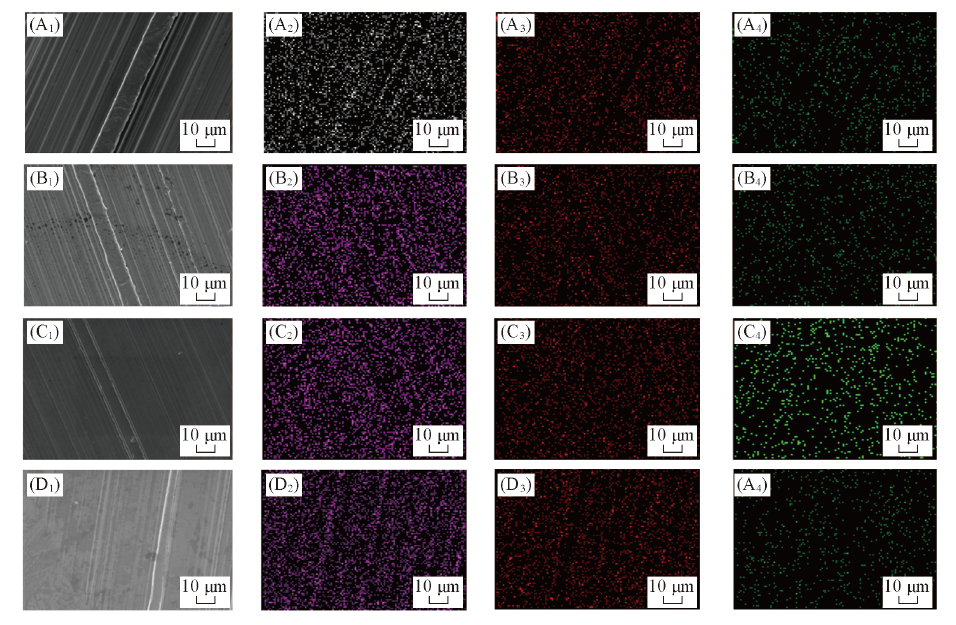
Fig.7 Elemental distribution of worn area lubricated with mass fraction of 2% C2MImBScB(A1—A4), C4MImBScB(B1—B4), C6MImBScB(C1—C4) and C8MImBScB(D1—D4) ionic liquids blends with PEG200(A1—D1) SEM images; (A2—D2) element C; (A3—D3) element N; (A4—D4) element B.
| Oil sample | Element | Mass fraction(%) | Atomic(%) | Oil sample | Element | Mass fraction(%) | Atomic(%) |
|---|---|---|---|---|---|---|---|
| PEG200 | C | 6.66 | 22.82 | PEG200+2%C8MImBScB | O | 2.28 | 4.76 |
| O | 4.41 | 11.35 | Si | 0.26 | 0.30 | ||
| Si | 0.28 | 0.41 | Cr | 1.28 | 0.82 | ||
| Cr | 1.56 | 1.23 | Fe | 79.64 | 47.63 | ||
| Fe | 87.09 | 64.18 | B | 3.94 | 12.17 | ||
| PEG200+2%C8MImBScB | C | 12.27 | 34.11 | N | 0.34 | 0.21 |
Table 4 EDX element analysis of tribofilms formed by PEG200 and PEG200 with 2% C8MImBScB
| Oil sample | Element | Mass fraction(%) | Atomic(%) | Oil sample | Element | Mass fraction(%) | Atomic(%) |
|---|---|---|---|---|---|---|---|
| PEG200 | C | 6.66 | 22.82 | PEG200+2%C8MImBScB | O | 2.28 | 4.76 |
| O | 4.41 | 11.35 | Si | 0.26 | 0.30 | ||
| Si | 0.28 | 0.41 | Cr | 1.28 | 0.82 | ||
| Cr | 1.56 | 1.23 | Fe | 79.64 | 47.63 | ||
| Fe | 87.09 | 64.18 | B | 3.94 | 12.17 | ||
| PEG200+2%C8MImBScB | C | 12.27 | 34.11 | N | 0.34 | 0.21 |
| [1] | Minami I., Molecules, 2009, 6(14), 2286—2305 |
| [2] | Duan H. F., Zhang S. B., Lin Y. J., Qiu Z. M., Wang Z. M., Chem. J. Chinese Universities, 2003, 24(11), 2024—2026 |
| (段海峰, 张所波, 林英杰, 邱志明, 王宗睦. 高等学校化学学报, 2003,24(11), 2024—2026) | |
| [3] | Zhao Y., Cui H., Zheng C., Chen X., Li C., Chem. Res. Chinese Universities, 2016, 32(1), 112—117 |
| [4] | Maria P., Virgil C., Chem. Res. Chinese Universities, 2014, 30(1), 119—124 |
| [5] | Lei P., Luo H., Shi M. K., Zhang X., Chem. J. Chinese Universities, 2016, 37(9), 1722—1727 |
| (雷蓓, 罗辉, 石孟可, 张熙. 高等学校化学学报, 2016,37(9), 1722—1727) | |
| [6] | Ye C.F., Liu W. M., Chen Y. X., Yu L. G.,Chem. Commun., 2001, (21), 2244—2245 |
| [7] | Gong Q. Y., Yu L. G., Ye C. F., Wear,2002, 253(5/6), 558—562 |
| [8] | Zhou F., Liang Y., Liu W., Chem. Soc. Rev., 2009, 38(9), 2590—2599 |
| [9] | Mahrova M., Pagano F., Pejakovic V., Valea A., Kalin M., Igartua A., Tojo E., Tribol. Int., 2015, 82, 245—254 |
| [10] | Anderson J. L., Ding R. F., Ellern A., Armstrong D. W., J. Am. Chem. Soc., 2005, 127(2), 593—604 |
| [11] | Pu J. B., Wan S. H., Zhao W. J., Mo Y. F., Zhang X. Q., Wang L. P., Xue Q. J., J. Phys. Chem. C, 2011, 115(27), 13275—13284 |
| [12] | Jimenez A. E., Bermudez M. D., Lglesias P., Carrion F. J., Nicolas G., Wear,2006, 260(7/8), 766—782 |
| [13] | Sharma V., Doerr N., Erdemir A., Aswath P. B., RSC Adv., 2016, 5(58), 53148—53161 |
| [14] | Shah F. U., Glavatskih S., Antzutkin O. N., Tribol. Lett., 2013, 51(3), 281—301 |
| [15] | Dilasari B., Jung Y., Sohn J., Kim S., Kwon K., Int. J. Electrochem. Sci., 2016, 11(2), 1482—1495 |
| [16] | Fukumoto K., Yoshizawa M., Ohno H., J. Am. Chem. Soc., 2005, 127(8), 2398—2399 |
| [17] | Gusain R., Dhingra S., Khatri O. P., Ind. & Eng. Chem. Res., 2016, 55(4), 856—865 |
| [18] | Gusain R., Khatri O. P., RSC Adv., 2016, 6(5), 3462—3469 |
| [19] | Gusain R., KhatriO. P. , RSC Adv., 2015, 5(32), 25287—25294 |
| [20] | Gusain R., Gupta P., Saran S., Khatri O. P., ACS Appl. Mater. Interfaces, 2014, 6(17), 15318—15328 |
| [21] | Khatri P. K., Joshi C., Thakre G. D., Jain S. L., New J. Chem., 2016, 40(6), 5294—5299 |
| [22] | Shah F. U., Glavatskih S., MacFarlane D. R., Somers A., Forsyth M., Antzutkin O. N., Phys. Chem. Chem. Phys., 2011, 13(28), 12865—12873 |
| [23] | Pejakovic V., Tomastik C., Dorr N., Kalin M., Tribol. Int., 2016, 97, 234—243 |
| [24] | Bonhote P., Papageorgiou N., Kalyanasundaram K., Gratzel M., Inorg. Chem., 1996, 37(1), 1168—1178 |
| [25] | Zhang Y. W., Zeng X. Q., Wu H., Li Z. P., Ren T. H., Zhao Y. D., Tribol. Lett., 2014, 53(3), 533—542 |
| [1] | CUI Wei, ZHAO Deyin, BAI Wenxuan, ZHANG Xiaodong, YU Jiang. CO2 Absorption in Composite of Aprotic Solvent and Iron-based Ionic Liquid [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220120. |
| [2] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [3] | JI Shuangqi, JIN Zhao, GUAN Wenna, PAN Xiangyu, GUAN Tong. Preparation and Chromatographic Performance of Mixed-mode Silica Stationary Phase Modified by Double Cationic Ionic Liquid and Octadecyl Group [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220008. |
| [4] | JIN Keyan, BAI Pu, LI Xiaolong, ZHANG Jianan, YAN Wenfu. New Mg-Al Type Sorbent for Efficient Removal of Boron from Waste Water Containing High-concentration of Boron from Pressurized Water Reactor Nuclear Power Plants [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210516. |
| [5] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [6] | LIU Miao, LIU Ruibo, LIU Badi, QIAN Ying. Synthesis, Two-photon Fluorescence Imaging and Photodynamic Therapy of Lysosome-targeted Indole-BODIPY Photosensitizer [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220326. |
| [7] | CHEN Chongan, YANG Guoyu. Two B5On(n=11, 12) Cluster-based Borates with Deep UV Cutoff Edge [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210711. |
| [8] | ZHAO Huijun, WU Tong, SUN Yue, DUAN Lian, MA Yanyu. A Coumarin-based Ratiometric Fluorescent Probe for BF3 Detection in Solution and Air [J]. Chem. J. Chinese Universities, 2021, 42(8): 2422. |
| [9] | LIU Changhui, LIANG Guojun, LI Yanlu, CHENG Xiufeng, ZHAO Xian. Density Functional Theory Study of NH3 Adsorption on Boron Nanotubes [J]. Chem. J. Chinese Universities, 2021, 42(7): 2263. |
| [10] | WAN Ren, SONG Fan, PENG Changjun, LIU Honglai. Group Contribution Method for Infinite Dilution Molar Conductivity of Unconventional Ions in Water [J]. Chem. J. Chinese Universities, 2021, 42(12): 3672. |
| [11] | WANG Yishu, LI Xue, YAN Li, XU Hongyun, ZHU Yuxin, SONG Yanhua, CUI Yanjuan. Photocatalytic Reduction Performance of Z-scheme Two-dimensional BCN/Sn3O4 Composite Materials [J]. Chem. J. Chinese Universities, 2021, 42(12): 3722. |
| [12] | WANG Man, WANG Xin, ZHOU Jing, GAO Guohua. Efficient Synthesis of Dimethyl Carbonate via Transesterification of Methanol and Ethylene Carbonate Catalyzed by Poly(ionic liquid)s [J]. Chem. J. Chinese Universities, 2021, 42(12): 3701. |
| [13] | CUI Jinping, CHEN Wenxian, YU Feifan, CAO Shiyu, LYU Weiyang, YAO Yuyuan. Adsorption Reduction of Hexavalent Chromium and co-Catalytic Degradation of Organic Pollutants by Carbon Doped Hexagonal Boron Nitride Supported MoS2 [J]. Chem. J. Chinese Universities, 2021, 42(10): 3125. |
| [14] | ZHOU Molin, JIANG Xin, YI Ting, YANG Xiangguang, ZHANG Yibo. Improvement of Interface Stability Between Sulfide Solid Electrolyte Li10GeP2S12 and Lithium Metal [J]. Chem. J. Chinese Universities, 2020, 41(8): 1810. |
| [15] | HUANG Mingyao, ZHU Shoufei. Recent Advances of Catalytic Asymmetric C—B Bond Forming Reactions† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1426. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||