

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (4): 591.doi: 10.7503/cjcu20160798
• Organic Chemistry • Previous Articles Next Articles
YAN Chang1, ZOU Yu1, FU Junjie2, HUANG Zhangjian1, ZHANG Dayong1,*( ), ZHANG Yihua1,*(
), ZHANG Yihua1,*( )
)
Received:2016-11-16
Online:2017-04-10
Published:2017-03-13
Contact:
ZHANG Dayong,ZHANG Yihua
E-mail:cpuzdy@163.com;zyhtgd@163.com
Supported by:CLC Number:
TrendMD:
YAN Chang, ZOU Yu, FU Junjie, HUANG Zhangjian, ZHANG Dayong, ZHANG Yihua. Design, Synthesis and Biological Evaluation of Novel O2-(2,4-Dinitrophenyl)diazeniumdiolates as Anti-tumor Agents†[J]. Chem. J. Chinese Universities, 2017, 38(4): 591.
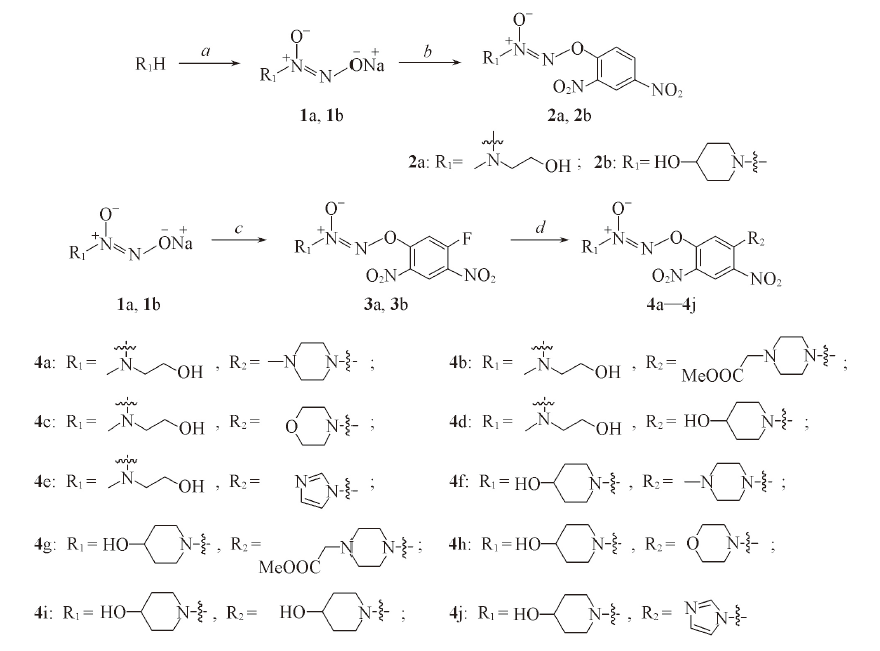
Scheme 3 Synthetic route of compounds 4a—4ja. NO, NaOMe/MeOH/Et2O, nanometer-sized TiO2, 25 ℃, 48 h; b. 1-fluoro-2,4-dinitrobenzene, Me2CO, NaHCO3(mass fraction 5%), N2, 0—25 ℃; c. 1,5-difluoro-2,4-dinitrobenzene, Me2CO, NaHCO3(mass fraction 5%), N2, 0—25 ℃; d. R2, K2CO3, Me2CO, 0 ℃—r. t.
| Compd. | Appearance | Yield(%) | Chemical formula | m. p./℃ | HRMS(calcd. )[M+Na]+ |
|---|---|---|---|---|---|
| 2a | Yellow solid | 88 | C9H11N5O7 | 149—153 | 324.0564(324.0556) |
| 2b | Yellow solid | 83 | C11H13N5O7 | 148—150 | 350.0721(350.0713) |
| 4a | Yellow solid | 68 | C14H21N7O7 | 166—171 | 422.1411(422.1400) |
| 4b | Yellow solid | 62 | C16H23N7O9 | 155—158 | 480.1466(480.1455) |
| 4c | Yellow solid | 65 | C13H18N6O8 | 153—156 | 409.1093(409.1084) |
| 4d | Yellow solid | 68 | C14H20N6O8 | 143—147 | 423.1249(423.1240) |
| 4e | Yellow solid | 62 | C12H13N7O7 | 145—147 | 390.0784(390.0774) |
| 4f | Yellow solid | 70 | C16H23N7O7 | 170—173 | 448.1566(448.1557) |
| 4g | Yellow solid | 69 | C18H25N7O9 | 117—119 | 506.1622(506.1611) |
| 4h | Yellow solid | 72 | C15H20N6O8 | 146—150 | 435.1246(435.1240) |
| 4i | Yellow solid | 68 | C16H22N6O8 | 158—162 | 449.1407(449.1397) |
| 4j | Yellow solid | 71 | C14H15N7O7 | 173—176 | 416.0942(416.0931) |
Table 1 Appearance, yields, melting points and HRMS data of target compounds 2 and 4
| Compd. | Appearance | Yield(%) | Chemical formula | m. p./℃ | HRMS(calcd. )[M+Na]+ |
|---|---|---|---|---|---|
| 2a | Yellow solid | 88 | C9H11N5O7 | 149—153 | 324.0564(324.0556) |
| 2b | Yellow solid | 83 | C11H13N5O7 | 148—150 | 350.0721(350.0713) |
| 4a | Yellow solid | 68 | C14H21N7O7 | 166—171 | 422.1411(422.1400) |
| 4b | Yellow solid | 62 | C16H23N7O9 | 155—158 | 480.1466(480.1455) |
| 4c | Yellow solid | 65 | C13H18N6O8 | 153—156 | 409.1093(409.1084) |
| 4d | Yellow solid | 68 | C14H20N6O8 | 143—147 | 423.1249(423.1240) |
| 4e | Yellow solid | 62 | C12H13N7O7 | 145—147 | 390.0784(390.0774) |
| 4f | Yellow solid | 70 | C16H23N7O7 | 170—173 | 448.1566(448.1557) |
| 4g | Yellow solid | 69 | C18H25N7O9 | 117—119 | 506.1622(506.1611) |
| 4h | Yellow solid | 72 | C15H20N6O8 | 146—150 | 435.1246(435.1240) |
| 4i | Yellow solid | 68 | C16H22N6O8 | 158—162 | 449.1407(449.1397) |
| 4j | Yellow solid | 71 | C14H15N7O7 | 173—176 | 416.0942(416.0931) |
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
|---|---|---|
| 2a | 8.88(s, 1H, ArH), 8.46(d,J=9.24 Hz, 1H, ArH), 7.69(d, J=8.85 Hz, 1H, ArH), 3.82—3.92(m, 4H, NCH2CH2OH), 3.41(s, 3H, CH3) | 129.8, 129.2, 122.3, 121.8, 120.5, 117.5, 57.5, 54.8, 39.6 |
| 2b | 8.89(d,J=2.55 Hz, 1H, ArH), 8.46(dd, J1=9.3 Hz, J2=2.64 Hz, 1H, ArH), 7.68(d, J=9.27 Hz, 1H, ArH), 4.03(s, 1H, NCH2CH2CHOH), 3.63—3.88(m, 4H, 2×NCH2CH2CHOH), 1.84—2.05(m, 4H, 2×NCH2CH2CHOH) | 152.9, 142.0, 136.8, 129.8, 121.8, 118.0, 63.6, 47.4, 31.8 |
| 4a | 8.72(s, 1H, ArH), 6.98(s, 1H, ArH), 3.81—3.88(m, 4H, NCH2CH2OH), 3.33(s, 3H, CH3), 3.24—3.27(m, 4H, 2×ArNCH2CH2N), 2.58—2.63(m, 4H, 2×ArNCH2CH2N), 2.37(s, 3H, CH3) | 153.6, 149.6, 132.2, 127.3, 127.1, 105.4, 57.6, 54.8, 53.9, 50.1, 45.5, 39.6 |
| 4b | 8.72(s, 1H, ArH), 6.98(s, 1H, ArH), 3.91(t, J=5.1 Hz, 2H, NCH2CH2OH), 3.76—3.82(m, 6H, 2×ArNCH2CH2N, NCH2CH2OH), 3.34(s, 3H, CH3), 3.31—3.35(m, 7H, 2×ArNCH2CH2N, NCH3), 2.78(s, 2H, CH2) | 170.3, 153.6, 149.6, 132.3, 127.7, 127.2, 105.5, 57.7, 57.6, 54.8, 51.2, 51.1, 50.2, 39.6 |
| 4c | 8.75(s, 1H, ArH), 6.98(s, 1H, ArH), 3.85—3.89(m, 6H, 2×NCH2CH2O, NCH2CH2OH), 3.76(t, J=4.71 Hz, 2H, NCH2CH2OH), 3.34(s, 3H, CH3), 3.24(t, J=4.17 Hz, 4H, 2×NCH2CH2O) | 153.6, 149.6, 132.3, 127.4, 127.2, 105.4, 85.5, 57.6, 54.8, 50.5, 39.6 |
| 4d | 8.71(s, 1H, ArH), 6.32(s, 1H, ArH), 4.00(s, 2H, NCH2CH2OH), 3.87—3.92(m, 3H, NCH2CH2OH, NCH2CH2CHOH), 3.49(s, 3H, CH3), 3.34—3.42(m, 4H, 2×NCH2CH2CHOH), 1.23—1.28(m, 4H, 2×NCH2CH2CHOH) | 153.6, 149.8, 131.9, 129.5, 127.4, 107.1, 105.0, 64.9, 64.4, 57.6, 54.8, 48.0, 39.6, 33.5 |
| 4e | 8.95(s, 1H, ArH), 8.03(s, 1H, Imidazole), 7.86(s, 1H, ArH), 7.52(s, 1H, Imidazole), 7.14(s, 1H, Imidazole), 4.90(s, 1H, OH), 3.75(t, J=5.2 Hz, 2H, NCH2CH2OH), 3.63(t, J=4.8 Hz, 2H, NCH2CH2OH), 3.14(s, 3H, CH3) | 152.4, 137.6, 136.5, 135.3, 128.5, 124.4, 123.1, 121.0, 116.7, 57.6, 55.5, 54.7 |
| 4f | 8.73(s, 1H, ArH), 6.96(s, 1H, ArH), 3.51—3.55(m, 4H, 2×ArNCH2CH2N), 3.23—3.29(m, 5H, 2×ArNCH2CH2N, NCH2CH2CHOH), 2.58—2.63(m, 4H, 2×NCH2CH2CHOH), 2.37(s, 3H, CH3), 2.03—2.08(m, 4H, 2×NCH2CH2CHOH) | 153.0, 149.5, 132.6, 127.4, 127.2, 106.4, 63.7, 53.9, 50.1, 47.4, 45.5, 31.8 |
| 4g | 8.72(s, 1H, ArH), 6.96(s, 1H, ArH), 3.72(s, 3H, CH3), 3.29—3.33(m, 5H, 2×ArNCH2CH2N, NCH2CH2CHOH), 3.22(s, 2H, NCH2COOMe), 2.63—2.66(m, 8H, 2×NCH2CH2CHOH, 2×ArNCH2CH2N), 2.02—2.05(m, 4H, 2×NCH2CH2CHOH) | 170.3, 153.0, 149.5, 132.6, 127.5, 127.2, 106.4, 63.7, 58.3, 57.7, 51.9, 51.2, 50.3, 47.4, 31.8 |
| 4h | 8.74(s, 1H, ArH), 6.96(s, 1H, ArH), 3.88(t, J=4.5 Hz, 4H, 2×NCH2CH2O), 3.81—3.86(m, 1H, NCH2CH2CHOH), 3.55—3.58(m, 4H, 2×NCH2CH2CHOH), 3.24(t, J=4.38 Hz, 4H, 2×NCH2CH2O), 1.84—2.06(m, 4H, 2×NCH2CH2CHOH) | 150.8, 150.7, 129.9, 127.5, 107.4, 106.3, 66.5, 66.4, 65.1, 51.5, 51.3, 48.4, 47.7, 33.7, 32.1 |
| 4i | 8.61(s, 1H, ArH), 7.12(s, 1H, ArH), 4.85(s, 2H, 2×OH), 3.75—3.79(m, 2H, 2×NCH2CH2CHOH), 3.42—3.45(m, 4H, 2×ArNCH2CH2CHOH), 3.12—3.15(m, 4H, 2×NCH2CH2CHOH), 1.85—1.88(m, 8H, 4×NCH2CH2CHOH) | 153.0, 149.6, 132.3, 127.3, 126.9, 106.0, 64.4, 63.7, 48.0, 47.4, 33.4, 31.8 |
| 4j | 8.95(s, 1H, ArH), 8.05(s, 1H, Imidazole), 7.96(s, 1H, ArH), 7.53(s, 1H, Imidazole), 7.14(s, 1H, Imidazole), 4.85(s, 1H, OH), 3.71(s, 1H, NCH2CH2CHOH), 3.44—3.51(m, 4H, 2×NCH2CH2CHOH), 1.58—1.85(m, 4H, NCH2CH2CHOH) | 141.7, 137.6, 134.1, 130.2, 129.3, 128.0, 126.9, 123.9, 120.3, 64.3, 47.8, 33.6 |
Table 2 1H NMR and 13C NMR data of target compounds 2 and 4
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
|---|---|---|
| 2a | 8.88(s, 1H, ArH), 8.46(d,J=9.24 Hz, 1H, ArH), 7.69(d, J=8.85 Hz, 1H, ArH), 3.82—3.92(m, 4H, NCH2CH2OH), 3.41(s, 3H, CH3) | 129.8, 129.2, 122.3, 121.8, 120.5, 117.5, 57.5, 54.8, 39.6 |
| 2b | 8.89(d,J=2.55 Hz, 1H, ArH), 8.46(dd, J1=9.3 Hz, J2=2.64 Hz, 1H, ArH), 7.68(d, J=9.27 Hz, 1H, ArH), 4.03(s, 1H, NCH2CH2CHOH), 3.63—3.88(m, 4H, 2×NCH2CH2CHOH), 1.84—2.05(m, 4H, 2×NCH2CH2CHOH) | 152.9, 142.0, 136.8, 129.8, 121.8, 118.0, 63.6, 47.4, 31.8 |
| 4a | 8.72(s, 1H, ArH), 6.98(s, 1H, ArH), 3.81—3.88(m, 4H, NCH2CH2OH), 3.33(s, 3H, CH3), 3.24—3.27(m, 4H, 2×ArNCH2CH2N), 2.58—2.63(m, 4H, 2×ArNCH2CH2N), 2.37(s, 3H, CH3) | 153.6, 149.6, 132.2, 127.3, 127.1, 105.4, 57.6, 54.8, 53.9, 50.1, 45.5, 39.6 |
| 4b | 8.72(s, 1H, ArH), 6.98(s, 1H, ArH), 3.91(t, J=5.1 Hz, 2H, NCH2CH2OH), 3.76—3.82(m, 6H, 2×ArNCH2CH2N, NCH2CH2OH), 3.34(s, 3H, CH3), 3.31—3.35(m, 7H, 2×ArNCH2CH2N, NCH3), 2.78(s, 2H, CH2) | 170.3, 153.6, 149.6, 132.3, 127.7, 127.2, 105.5, 57.7, 57.6, 54.8, 51.2, 51.1, 50.2, 39.6 |
| 4c | 8.75(s, 1H, ArH), 6.98(s, 1H, ArH), 3.85—3.89(m, 6H, 2×NCH2CH2O, NCH2CH2OH), 3.76(t, J=4.71 Hz, 2H, NCH2CH2OH), 3.34(s, 3H, CH3), 3.24(t, J=4.17 Hz, 4H, 2×NCH2CH2O) | 153.6, 149.6, 132.3, 127.4, 127.2, 105.4, 85.5, 57.6, 54.8, 50.5, 39.6 |
| 4d | 8.71(s, 1H, ArH), 6.32(s, 1H, ArH), 4.00(s, 2H, NCH2CH2OH), 3.87—3.92(m, 3H, NCH2CH2OH, NCH2CH2CHOH), 3.49(s, 3H, CH3), 3.34—3.42(m, 4H, 2×NCH2CH2CHOH), 1.23—1.28(m, 4H, 2×NCH2CH2CHOH) | 153.6, 149.8, 131.9, 129.5, 127.4, 107.1, 105.0, 64.9, 64.4, 57.6, 54.8, 48.0, 39.6, 33.5 |
| 4e | 8.95(s, 1H, ArH), 8.03(s, 1H, Imidazole), 7.86(s, 1H, ArH), 7.52(s, 1H, Imidazole), 7.14(s, 1H, Imidazole), 4.90(s, 1H, OH), 3.75(t, J=5.2 Hz, 2H, NCH2CH2OH), 3.63(t, J=4.8 Hz, 2H, NCH2CH2OH), 3.14(s, 3H, CH3) | 152.4, 137.6, 136.5, 135.3, 128.5, 124.4, 123.1, 121.0, 116.7, 57.6, 55.5, 54.7 |
| 4f | 8.73(s, 1H, ArH), 6.96(s, 1H, ArH), 3.51—3.55(m, 4H, 2×ArNCH2CH2N), 3.23—3.29(m, 5H, 2×ArNCH2CH2N, NCH2CH2CHOH), 2.58—2.63(m, 4H, 2×NCH2CH2CHOH), 2.37(s, 3H, CH3), 2.03—2.08(m, 4H, 2×NCH2CH2CHOH) | 153.0, 149.5, 132.6, 127.4, 127.2, 106.4, 63.7, 53.9, 50.1, 47.4, 45.5, 31.8 |
| 4g | 8.72(s, 1H, ArH), 6.96(s, 1H, ArH), 3.72(s, 3H, CH3), 3.29—3.33(m, 5H, 2×ArNCH2CH2N, NCH2CH2CHOH), 3.22(s, 2H, NCH2COOMe), 2.63—2.66(m, 8H, 2×NCH2CH2CHOH, 2×ArNCH2CH2N), 2.02—2.05(m, 4H, 2×NCH2CH2CHOH) | 170.3, 153.0, 149.5, 132.6, 127.5, 127.2, 106.4, 63.7, 58.3, 57.7, 51.9, 51.2, 50.3, 47.4, 31.8 |
| 4h | 8.74(s, 1H, ArH), 6.96(s, 1H, ArH), 3.88(t, J=4.5 Hz, 4H, 2×NCH2CH2O), 3.81—3.86(m, 1H, NCH2CH2CHOH), 3.55—3.58(m, 4H, 2×NCH2CH2CHOH), 3.24(t, J=4.38 Hz, 4H, 2×NCH2CH2O), 1.84—2.06(m, 4H, 2×NCH2CH2CHOH) | 150.8, 150.7, 129.9, 127.5, 107.4, 106.3, 66.5, 66.4, 65.1, 51.5, 51.3, 48.4, 47.7, 33.7, 32.1 |
| 4i | 8.61(s, 1H, ArH), 7.12(s, 1H, ArH), 4.85(s, 2H, 2×OH), 3.75—3.79(m, 2H, 2×NCH2CH2CHOH), 3.42—3.45(m, 4H, 2×ArNCH2CH2CHOH), 3.12—3.15(m, 4H, 2×NCH2CH2CHOH), 1.85—1.88(m, 8H, 4×NCH2CH2CHOH) | 153.0, 149.6, 132.3, 127.3, 126.9, 106.0, 64.4, 63.7, 48.0, 47.4, 33.4, 31.8 |
| 4j | 8.95(s, 1H, ArH), 8.05(s, 1H, Imidazole), 7.96(s, 1H, ArH), 7.53(s, 1H, Imidazole), 7.14(s, 1H, Imidazole), 4.85(s, 1H, OH), 3.71(s, 1H, NCH2CH2CHOH), 3.44—3.51(m, 4H, 2×NCH2CH2CHOH), 1.58—1.85(m, 4H, NCH2CH2CHOH) | 141.7, 137.6, 134.1, 130.2, 129.3, 128.0, 126.9, 123.9, 120.3, 64.3, 47.8, 33.6 |
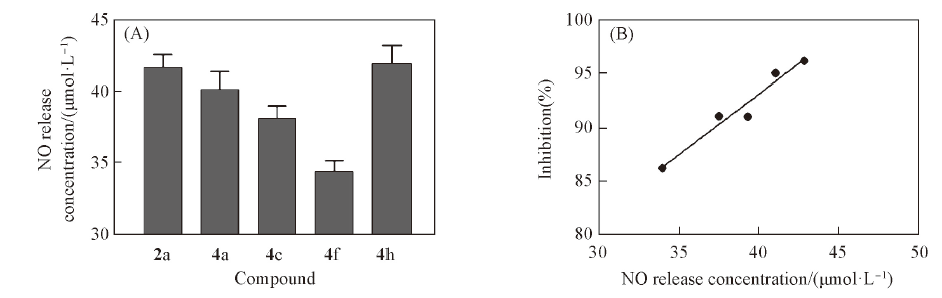
Fig.2 Quantitative measurement of intracellular NO production(A), the positive correlation between NO release in HCT-116 cells and anti-proliferative activity of target compounds against HCT-116 cells(B)
| [1] | Strange R. C., Jones P. W., Fryer A. A., Toxicol. Lett., 2000, 112/113, 357—363 |
| [2] | Hayes J. D., Flanagan J. U., Jowsey I. R., Annu. Rev. Pharmacol. Toxicol., 2005, 45, 51—88 |
| [3] | Obrien M. L., Tew K. D., Eur. J. Cancer, 1996, 32(6), 967—978 |
| [4] | Habig W. H., Pabst M. J., Jakoby W. B., J. Biol. Chem., 1974, 249(22), 7130—7139 |
| [5] | Ji X., Pal A., Kalathur R., Hu X., Gu Y., Saavedra J. E., Buzard G. S., Srinivasan A., Keefer L. K., Singh S. V., Drug Des. Devel. Ther., 2008, 2, 123—130 |
| [6] | Kim Y., Maciag A. E., Cao Z., Deschamps J. R., Saavedra J. E., Keefer L. K., Holland R. J., Bioorg. Med. Chem., 2015, 23, 4980—4988 |
| [7] | Huerta S., Chilka S., Bonavida B., Int. J. Oncol., 2008, 33(5), 909—927 |
| [8] | Burke A. J., Sullivan F. J., Giles F. J., Glynn S. A., Carcinogenesis., 2013, 34(3), 503—512 |
| [9] | Mocellin S., Bronte V., Nitti D., Med. Res. Rev., 2007, 27(3), 317—352 |
| [10] | He L. Q., Gu H. X., Yin D. K., Zhang Y. H., Wang X. S., Chem. J. Chinese Universities, 2010, 31(8), 1541—1547 |
| (何黎琴, 顾宏霞, 尹登科, 张奕华, 王效山. 高等学校化学学报, 2010,31(8), 1541—1547) | |
| [11] | Saavedra J. E., Dunams T. M., Flippen J. L., Keefer L. K., J. Org. Chem., 1992, 57(23), 6134—6138 |
| [12] | Saavedra J. E., Srinivasan A., Buzard G. S., Davies K. M., Waterhouse D. J., J. Med. Chem., 2006, 49(3), 1157—1164 |
| [13] | Findlay V. J., Townsend D. M., Saavedra J. E., Buzard G. S., Citro M. L., Keefer L. K., Ji X., Tew K. D., Mol. Pharmacol., 2004, 65(5), 1070—1079 |
| [14] | Chakrapani H., Wilde T. C., Citro M. L., Bioorg. Med. Chem., 2008, 16, 2657—2664 |
| [15] | Sies H., Free. Radic. Biol. Med., 1999, 27(9/10), 916—921 |
| [16] | Fu J. J., Liu L., Huang Z. J., Lai Y. S., Ji H., Peng S. X., Tian J. D., Zhang Y. H., J. Med. Chem., 2013, 56(11), 4641—4655 |
| [17] | Huang Z.J., Zhang Y. H., Fang L., Zhang Z. G., Lai Y. S., Ding Y., Cao F. Q., Zhang J., Peng S. X.,Chem. Commun., 2009, (13), 1763—1765 |
| [18] | Saavedra J. E., Srinivasan A., Bonifant C. L., Chu J., Shanklin A. P., Rice W. G., Turpin J. A., Davies K. M., Keefer L. K., J. Org. Chem., 2001, 66(9), 3090—3098 |
| [19] | Fu J. J., Zou Y., Huang Z. J., Yan C., Zhou Q. M., Zhang H. B., Lai Y. S., Peng S. X., Zhang Y. H., RSC Adv., 2015, 5(25), 19445—19454 |
| [20] | Gross G., Tardio J., Kuhlmann O., Int. J. Pharm., 2012, 437(1/2), 103—109 |
| [1] | CHU Yuyi, LAN Chang, LUO Ergui, LIU Changpeng, GE Junjie, XING Wei. Single-atom Cerium Sites Designed for Durable Oxygen Reduction Reaction Catalyst with Weak Fenton Effect [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220294. |
| [2] | ZHENG Anni, JIN Lei, YANG Jiaqiang, WANG Zhaoyun, LI Weiqing, YANG Fangzu, ZHAN Dongping, TIAN Zhongqun. Effects of 5,5-Dimethylhydantoin on Electroless Copper Plating [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220191. |
| [3] | WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, JIANG Wei, HUANG Weiqiu, CHEN Ruoyu. Activation of Biochar from Cattail and the VOCs Adsorption Application [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210824. |
| [4] | LI Weihui, LI Haobo, ZENG Cheng, LIANG Haoyue, CHEN Jiajun, LI Junyong, LI Huiqiao. Hot-pressed PVDF-based Difunctional Protective Layer for Lithium Metal Anodes [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210629. |
| [5] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [6] | YUE Shengli, WU Guangbao, LI Xing, LI Kang, HUANG Gaosheng, TANG Yi, ZHOU Huiqiong. Research Progress of Quasi-two-dimensional Perovskite Solar Cells [J]. Chem. J. Chinese Universities, 2021, 42(6): 1648. |
| [7] | WANG Hongning, HUANG Li, SONG Fujiao, ZHU Ting, HUANG Weiqiu, ZHONG Jing, CHEN Ruoyu. Synthesis and VOCs Adsorption Properties of Hollow Carbon Nanospheres [J]. Chem. J. Chinese Universities, 2021, 42(6): 1704. |
| [8] | WANG Kunhua, YAO Jisong, YANG Junnan, SONG Yonghui, LIU Yuying, YAO Hongbin. Synthesis and Device Optimization of Highly Efficient Metal Halide Perovskite Light-emitting Diodes [J]. Chem. J. Chinese Universities, 2021, 42(5): 1464. |
| [9] | LIU Yao, DENG Zhengtao. Fast Synthesis of Highly Luminescent Two-dimensional Tin-halide Perovskites by Anti-solvent Method [J]. Chem. J. Chinese Universities, 2021, 42(12): 3774. |
| [10] | ZHANG Jun, WANG Bin, PAN Li, MA Zhe, LI Yuesheng. Synthesis and Properties of Imidazolium-based Polyethylene Ionomer [J]. Chem. J. Chinese Universities, 2020, 41(9): 2070. |
| [11] | WANG Tingting, LEI Yuhan, LIN Yujuan, HUANG Jialing, LIU Cuie, ZHENG Fengying, LI Shunxing. Preparation of Liposome-terminated CsPbX3(X=Cl,Br,I) Nanocrystals and Applications in Light-emitting Diode Devices [J]. Chem. J. Chinese Universities, 2020, 41(8): 1896. |
| [12] | WU Chunxiao, AI Xin, CHEN Yingxin, CUI Zhiyuan, LI Feng. Effects of Introducing Halogen Atoms to Biphenylmethyl Radical on Photostability, Photophysical and Electroluminescent Properties † [J]. Chem. J. Chinese Universities, 2020, 41(5): 972. |
| [13] | ZHANG Xuan,ZHANG Tianci,JIANG Ping,GE Jijiang,ZHANG Guicai. Enhancement of CO2 Foam Stability with Modified Silica Nanoparticles in High Salinity Brine † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1076. |
| [14] | REN Wen, ZHANG Guoli, YAN Han, HU Xinghua, LI Kun, WANG Jingfeng, LI Ruiqi. Preparation of Superhydrophobic Polyaniline/Polytetrafluoroethylenethylene Composite Membrane and Its Separation Ability for Oil-Water Emulsion † [J]. Chem. J. Chinese Universities, 2020, 41(4): 846. |
| [15] | JIANG Yefang, DONG Ru, CAI Xuediao, FENG Jiangshan, LIU Zhike, LIU Shengzhong. Effect of Amphiphilic Quaternary Ammonium Salt Additive on Performance and Stability of Perovskite Solar Cells [J]. Chem. J. Chinese Universities, 2019, 40(8): 1697. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||