

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (10): 1809.doi: 10.7503/cjcu20160375
• Physical Chemistry • Previous Articles Next Articles
SHEN Wen1, SHAO Xueguang1,2, CAI Wensheng1,*( )
)
Received:2016-05-25
Online:2016-10-10
Published:2016-09-23
Contact:
CAI Wensheng
E-mail:wscai@nankai.edu.cn
Supported by:CLC Number:
TrendMD:
SHEN Wen, SHAO Xueguang, CAI Wensheng. Inclusion Mechanism of Cyclodextrins with Glutathione†[J]. Chem. J. Chinese Universities, 2016, 37(10): 1809.
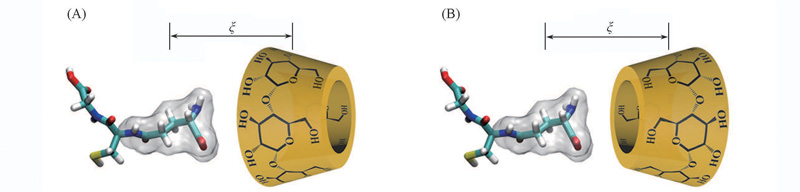
Fig.2 Initial spacial arrangements of molecular systems with two orientations of GSH towards the CDs(A) Orien(Ⅰ); (B) Orien(Ⅱ). ξ: Projection onto the Z axis of the distance between the center of mass of Glu and that of CD.
| System | Method | Number of atoms | Time/ns |
|---|---|---|---|
| GSH: α-CD[Gly(Ⅰ)]a | MD | 7243 | 10 |
| GSH: α-CD[Gly(Ⅱ)]b | MD | 7147 | 10 |
| GSH: α-CD[Glu(Ⅰ)]c | MD + ABF | 7243 | 10+100 |
| GSH: α-CD[Glu(Ⅱ)]d | MD + ABF | 7579 | 10+100 |
| GSH: α-CD[Cys(Ⅰ)]e | MD | 7099 | 10 |
| GSH: α-CD[Cys(Ⅱ)]f | MD | 7228 | 10 |
| GSH: β-CD[Gly(Ⅰ)]a | MD | 7546 | 10 |
| GSH: β-CD[Gly(Ⅱ)]b | MD | 7753 | 10 |
| GSH: β-CD[Glu(Ⅰ)]c | MD + ABF | 7270 | 10+100 |
| GSH: β-CD[Glu(Ⅱ)]d | MD + ABF | 7777 | 10+100 |
| GSH: β-CD[Cys(Ⅰ)]e | MD | 7168 | 10 |
| GSH: β-CD[Cys(Ⅱ)]f | MD | 7753 | 10 |
| GSH: γ-CD[Gly(Ⅰ)]a | MD | 7450 | 10 |
| GSH: γ-CD[Gly(Ⅱ)]b | MD | 8008 | 10 |
| GSH: γ-CD[Glu(Ⅰ)]c | MD + ABF | 7450 | 10+100 |
| GSH: γ-CD[Glu(Ⅱ)]d | MD + ABF | 8008 | 10+100 |
| GSH: γ-CD[Cys(Ⅰ)]e | MD | 7783 | 10 |
| GSH: γ-CD[Cys(Ⅱ)]f | MD | 7666 | 10 |
Table 1 Summary of simulation systems with GSH and CDs
| System | Method | Number of atoms | Time/ns |
|---|---|---|---|
| GSH: α-CD[Gly(Ⅰ)]a | MD | 7243 | 10 |
| GSH: α-CD[Gly(Ⅱ)]b | MD | 7147 | 10 |
| GSH: α-CD[Glu(Ⅰ)]c | MD + ABF | 7243 | 10+100 |
| GSH: α-CD[Glu(Ⅱ)]d | MD + ABF | 7579 | 10+100 |
| GSH: α-CD[Cys(Ⅰ)]e | MD | 7099 | 10 |
| GSH: α-CD[Cys(Ⅱ)]f | MD | 7228 | 10 |
| GSH: β-CD[Gly(Ⅰ)]a | MD | 7546 | 10 |
| GSH: β-CD[Gly(Ⅱ)]b | MD | 7753 | 10 |
| GSH: β-CD[Glu(Ⅰ)]c | MD + ABF | 7270 | 10+100 |
| GSH: β-CD[Glu(Ⅱ)]d | MD + ABF | 7777 | 10+100 |
| GSH: β-CD[Cys(Ⅰ)]e | MD | 7168 | 10 |
| GSH: β-CD[Cys(Ⅱ)]f | MD | 7753 | 10 |
| GSH: γ-CD[Gly(Ⅰ)]a | MD | 7450 | 10 |
| GSH: γ-CD[Gly(Ⅱ)]b | MD | 8008 | 10 |
| GSH: γ-CD[Glu(Ⅰ)]c | MD + ABF | 7450 | 10+100 |
| GSH: γ-CD[Glu(Ⅱ)]d | MD + ABF | 8008 | 10+100 |
| GSH: γ-CD[Cys(Ⅰ)]e | MD | 7783 | 10 |
| GSH: γ-CD[Cys(Ⅱ)]f | MD | 7666 | 10 |
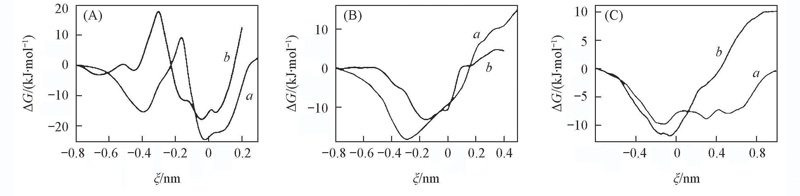
Fig.4 Free-energy profiles for the inclusion of the Glu of GSH into CDs in two orientations(A) GSH:α-CD; (B) GSH:β-CD; (C) GSH:γ-CD. a. Orien(Ⅰ); b. Orien(Ⅱ).
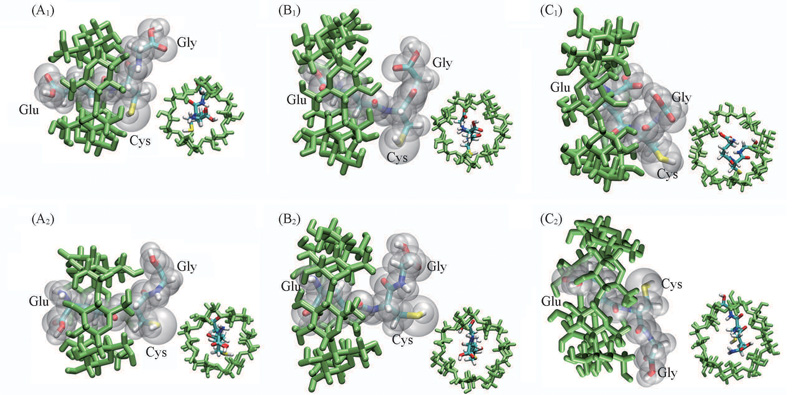
Fig.5 Structures of the inclusion complexes of GSH with CD near the global minima of the PMFs in Fig.4(A1) GSH with α-CD, Orien(Ⅰ): ξ=-0.02 nm, d=0.05 nm, θ=14°; (A2) Orien(Ⅱ): ξ=-0.04 nm, d=0.22 nm, θ=12°; (B1) GSH with β-CD, Orien(Ⅰ): ξ=-0.30 nm, d=0.31 nm, θ=28°; (B2) Orien(Ⅱ): ξ=-0.18 nm, d=0.28 nm, θ=22°; (C1) GSH with γ-CD, Orien(Ⅰ): ξ=-0.10 nm, d=0.49 nm, θ=43°; (C2) Orien(Ⅱ): ξ=-0.05 nm, d=0.44 nm, θ=37°. ξ: Reaction coordinate in Fig.4; d: distance between the center of mass of Glu and the principal axis of CD; θ: angle between the side chain of Glu and the principal axis of CD.
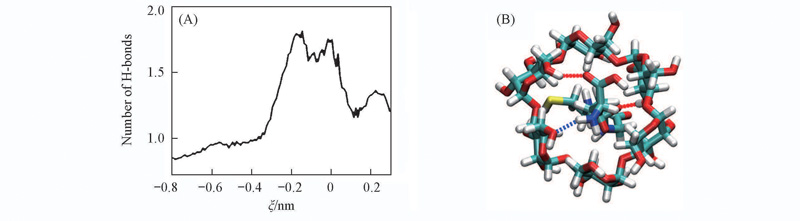
Fig.6 Variation of average number of hydrogen bonds formed between GSH and α-CD as a function of the model reaction coordinate(A) and hydrogen bonds formed between GSH and α-CD(near the global minima of the PMF)(B)
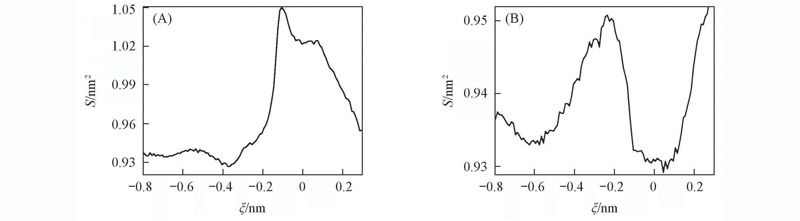
Fig.7 Fluctuation of the area of the central plane formed by the six primary side carbon atoms(A) and the six glycosidic oxygen atoms(B) of the α-CD in the course of the threading process
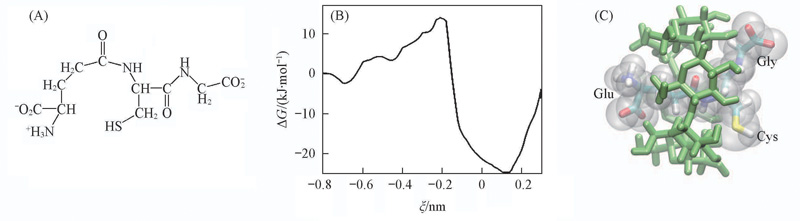
Fig.9 Structure of GSH-(A), free-energy profile for the inclusion of the Glu of GSH- into α-CD in Orien(I)(B) and structure of the inclusion complex of α-CD with GSH- near the global minima of the PMF(C)
| [1] | Zetterstrom R., Eijkman C., Hopkins F. G., Acta Paediatr., 2006, 95(11), 1331—1333 |
| [2] | Dixon D. P., Skipsey M., Grundy N. M., Plant Physiol., 2005, 138,2233—2244 |
| [3] | Garcia-Fuentes M., Trapani A., Alonso M. J., Eur. J. Pharm. Biopharm., 2006, 64(2), 146—153 |
| [4] | Song L. X., Guo Z. J., Chinese J. Inorg. Chem., 2001, 17(4), 457—470 |
| (宋乐新, 郭子建.无机化学学报, 2001,17(4), 457—470) | |
| [5] | You C.C., Liu Y., Chem. J. Chinese Universities, 2000, 21(2), 249—251 |
| (尤长城, 刘育.高等学校化学学报, 2000,21(2), 249—251) | |
| [6] | You C.C., Zhao Y. L., Liu Y., Chem. J. Chinese Universities, 2001, 22(2), 218—222 |
| (尤长城, 赵彦利, 刘育.高等学校化学学报, 2001,22(2), 218—222) | |
| [7] | Song L. X.,Wang H. M., Yang Y., Acta Chim. Sinica, 2007, 65(16), 1593—1599 |
| (宋乐新, 王海名, 杨燕.化学学报, 2007,65(16), 1593—1599) | |
| [8] | He J., Feng X. Z., Shao X. G., Cai W. S., Chem. J. Chinese Universities, 2015, 36(1), 110—115 |
| (何佳, 冯喜增, 邵学广, 蔡文生.高等学校化学学报, 2015,36(1), 110—115) | |
| [9] | Cai W. S., Sun T. T., Shao X. G., Chipot C., J. Phys. Chem. B, 2009, 113(22), 7836—7843 |
| [10] | Harata K., Bull. Chem. Soc. Jpn., 1975, 48(9), 2409—2413 |
| [11] | Lindner K., Saenger W., Carbohydr Res., 1982, 99(2), 103—115 |
| [12] | Harata K., Bull. Chem. Soc. Jpn., 1987, 60(8), 2763—2767 |
| [13] | Phillips J. C., Braun R., Wang W., Gumbart J., Tajkhorshid E., Villa E., Chipot C., Skeel R. D., Kale L., Schulten K., J. Comput. Chem., 2005, 26(16), 1781—1802 |
| [14] | MacKerell A. D., Bashford D., Bellott M., Dunbrack R. L., Evanseck J. D., Field M. J., Fischer S., Gao J., Guo H., Ha S., Joseph-McCarthy D., Kuchnir L., Kuczera K., Lau F. T. K., Mattos C., Michnick S., Ngo T., Nguyen D. T., Prodhom B., Reiher W. E., Roux B., Schlenkrich M., Smith J. C., Stote R., Straub J., Watanabe M., Wiorkiewicz Kuczera J., Yin D., Karplus M., J. Phys. Chem. B, 1998, 102(18), 3586—3616 |
| [15] | MacKerell A. D., Feig M., Brooks C. L., J. Comput. Chem., 2004, 25(11), 1400—1415 |
| [16] | Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., Klein M. L., J. Chem. Phys., 1983, 79(2), 926—935 |
| [17] | Ryckaert J. P., Ciccotti G., Berendsen H. J. C., J. Comput. Phys., 1977, 23(3), 327—341 |
| [18] | Andersen H. C., J. Comput. Phys., 1983, 52(1), 24—34 |
| [19] | Miyamoto S., Kollman P. A., J. Comput. Chem., 1992, 13(8), 952—962 |
| [20] | Feller S. E., Zhang Y. H., Pastor R. W., Brooks B. R., J. Chem. Phys., 1995, 103(11), 4613—4621 |
| [21] | Humphrey W., Dalke A., Schulten K., J. Mol. Graphics, 1996, 14(1), 33—38 |
| [22] | Wang S. S., Liu P., Cai W. S., Shao X. G., Chem. J. Chinese Universities, 2015, 36(11), 2211—2219 |
| (汪双双, 刘鹏, 蔡文生, 邵学广.高等学校化学学报, 2015,36(11), 2211—2219) | |
| [23] | Darden T., York D., Pedersen L., J. Chem. Phys., 1993, 98(12), 10089—10092 |
| [24] | Darve E., Pohorille A., J. Chem. Phys., 2001, 115(20), 9169—9183 |
| [25] | Hénin J., Chipot C., J. Chem. Phys., 2004, 121(7), 2904—2914 |
| [26] | Rodríguez-Gómez D., Darve E., Pohorille A., J. Chem. Phys., 2004, 120(8), 3563—3578 |
| [27] | Chipot C., Hénin J., J. Chem. Phys., 2005, 123(24), 244906 |
| [28] | Hénin J., Fiorin G., Chipot C., Klein M. L., J. Chem. Theory Comput., 2010, 6(1), 35—47 |
| [29] | Lampela O., Juffer A. H., Rauk A., J. Phys. Chem. A, 2003, 107(43), 9208—9220 |
| [1] | GAO Zhiwei, LI Junwei, SHI Sai, FU Qiang, JIA Junru, AN Hailong. Analysis of Gating Characteristics of TRPM8 Channel Based on Molecular Dynamics [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220080. |
| [2] | CUI Shaoli, ZHANG Weijia, SHAO Xueguang, CAI Wensheng. Revealing the Effect of Threonine on the Binding Ability of Antifreeze Proteins with Ice Crystals by Free-energy Calculations [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210838. |
| [3] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [4] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [5] | TAN Lejian, ZHONG Xuanshu, WANG Jin, LIU Zongjian, ZHANG Aiying, YE Lin, FENG Zengguo. Low Critical Dissolution Temperature Behavior of β⁃Cyclodextrin and Its Application in the Preparation of β⁃Cyclodextrin Sheet Crystal with Ordered Nano⁃channel [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220405. |
| [6] | WEI Zheyu, WU Zhikang, RU Shi, NI Lubin, WEI Yongge. Research Progress of Polyoxometalates-Cyclodextrin Supramolecular System [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210665. |
| [7] | LEI Xiaotong, JIN Yiqing, MENG Xuanyu. Prediction of the Binding Site of PIP2 in the TREK-1 Channel Based on Molecular Modeling [J]. Chem. J. Chinese Universities, 2021, 42(8): 2550. |
| [8] | LI Congcong, LIU Minghao, HAN Jiarui, ZHU Jingxuan, HAN Weiwei, LI Wannan. Theoretical Study of the Catalytic Activity of VmoLac Non-specific Substrates Based on Molecular Dynamics Simulations [J]. Chem. J. Chinese Universities, 2021, 42(8): 2518. |
| [9] | ZENG Yonghui, YAN Tianying. Vibrational Density of States Analysis of Proton Hydration Structure [J]. Chem. J. Chinese Universities, 2021, 42(6): 1855. |
| [10] | LIU Aiqing, XU Wensheng, XU Xiaolei, CHEN Jizhong, AN Lijia. Molecular Dynamics Simulation of Polymer/rod Nanocomposite [J]. Chem. J. Chinese Universities, 2021, 42(3): 875. |
| [11] | QI Renrui, LI Minghao, CHANG Hao, FU Xueqi, GAO Bo, HAN Weiwei, HAN Lu, LI Wannan. Theoretical Study on the Unbinding Pathway of Xanthine Oxidase Inhibitors Based on Steered Molecular Dynamics Simulation [J]. Chem. J. Chinese Universities, 2021, 42(3): 758. |
| [12] | ZHANG Juan, HU Xinyue, WANG Hongbo, LIAN Ying, LE Jinyu, YANG Zihao. Crystal-like Hydrogels Consisting of Parallel Hexahedrons Obtained from the Self-assembly ofβ⁃Cyclodextrin/perfluorononanoic Acid Inclusion Complexes [J]. Chem. J. Chinese Universities, 2021, 42(10): 3187. |
| [13] | MIAO Mengyao, GUO Yichang, SHAO Xueguang, CAI Wensheng. Mechanism of Ion Transport Across Membranes Assisted by Molecular Shuttles [J]. Chem. J. Chinese Universities, 2021, 42(10): 3116. |
| [14] | FU Kefei, LIAN Huiting, WEI Xiaofeng, SUN Xiangying, LIU Bin. Construction of Cyclodextrin-based Impedance Sensor for Recognition of L-Cysteine † [J]. Chem. J. Chinese Universities, 2020, 41(4): 706. |
| [15] | LING Yunyun,LI Li,LIANG Xiurong,XIA Yunsheng. Glutathione Sensing: from Colorimetry to Single Particle Spectroscopy Based on Gold NP@MnO2 Nanosheets Supraparticles [J]. Chem. J. Chinese Universities, 2020, 41(4): 623. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||