

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (4): 687.doi: 10.7503/cjcu20140875
• Organic Chemistry • Previous Articles Next Articles
ZHANG Jing1, MU Boshuai1, WU Meng2, BIAN Yanqing2,*( ), LI Yuan1,*(
), LI Yuan1,*( )
)
Received:2014-09-26
Online:2015-04-10
Published:2015-03-19
Contact:
BIAN Yanqing,LI Yuan
E-mail:bianyanqing151@sohu.com;liyuanhbsd@163.com
Supported by:CLC Number:
TrendMD:
ZHANG Jing, MU Boshuai, WU Meng, BIAN Yanqing, LI Yuan. Synthesis, Antifungal Activity and Structure-activity Relationship of -Fluorophenyl-2,3-dihydro-1,5-benzothiazepines Derivatives†[J]. Chem. J. Chinese Universities, 2015, 36(4): 687.
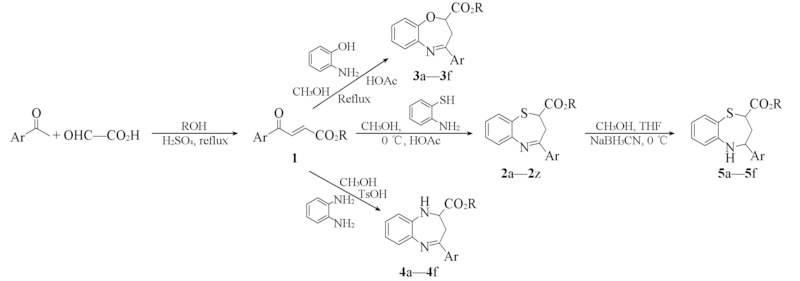
Scheme 1 Synthesis routes of 1,5-benzothiazepines 2—5 a. R=CH3, Ar=2,3-F2C6H3; b. R=CH3, Ar=2,5-F2C6H3; c. R=CH3, Ar=3,4-F2C6H3; d. R=C2H5, Ar=2,3-F2C6H3; e. R=C2H5, Ar=2,5-F2C6H3; f. R=C2H5, Ar=3,4-F2C6H3; g. R=CH3, Ar=2,6-F2C6H3; h. R=CH3, Ar=3,5-F2C6H3; i. R=C2H5, Ar=2,6-F2C6H3; j. R=C2H5, Ar=3,5-F2C6H3; k. R=CH3, Ar=2,3,4-F3C6H2; l. R=CH3, Ar=2,3,6-F3C6H2; m. R=CH3, Ar=2,4,5-F3C6H2; n. R=CH3, Ar=2,4,6-F3C6H2; o. R=CH3, Ar=3,4,5-F3C6H2; p. R=C2H5, Ar=2,3,4-F3C6H2; q. R=C2H5, Ar=2,3,6-F3C6H2; r. R=C2H5, Ar=2,4,5-F3C6H2; s. R=C2H5, Ar=2,4,6-F3C6H2; t. R=C2H5, Ar=3,4,5-F3C6H2; u. R=CH3, Ar=3-CF3-C6H4; v. R=CH3, Ar=4-CF3-C6H4; w. R=CH3, Ar=3,5-bis(CF3)-C6H3; x. R=C2H5, Ar=3-CF3-C6H4; y. R=C2H5, Ar=4-CF3-C6H4; z. R=C2H5, Ar=3,5-bis(CF3)-C6H3
| Compd. | Isolated yield(%) | m.p./℃ | HRMS(calcd., [M+H]+), m/z | IR(KBr), |
|---|---|---|---|---|
| 2a | 85.0 | 106—108 | 334.0708(334.0707) | 1736.0, 1610.6 |
| 2b | 80.0 | 111—113 | 334.0705(334.0707) | 1732.1, 1608.9 |
| 2c | 86.5 | 98—100 | 334.0708(334.0707) | 1728.3, 1620.0 |
| 2d | 87.0 | 102—104 | 348.0860(348.0864) | 1724.4, 1618.3 |
| 2e | 90.2 | 99—101 | 348.0858(348.0864) | 1705.1, 1600.9 |
| 2f | 86.8 | 103—105 | 348.0860(348.0864) | 1728.3, 1600.0 |
| 2g | 82.5 | 96—97 | 334.0708(334.0707) | 1732.1, 1618.3 |
| 2h | 89.3 | 87—89 | 334.0708(334.0707) | 1726.4, 1610.6 |
| 2i | 86.0 | 101—102 | 348.0860(348.0864) | 1728.3, 1629.9 |
| Compd. | Isolated yield(%) | m.p./℃ | HRMS(calcd., [M+H]+), m/z | IR(KBr), |
| 2j | 91.0 | 105—106 | 348.0864(348.0864) | 1726.3, 1610.9 |
| 2k | 72.5 | 96—97 | 352.0609(352.0613) | 1730.1, 1640.5 |
| 2l | 75.8 | 78—79 | 352.0608(352.0613) | 1731.2, 1641.0 |
| 2m | 69.4 | 106—107 | 352.0609(352.0613) | 1732.9, 1641.3 |
| 2n | 80.0 | 108—110 | 352.0614(352.0613) | 1732.1, 1641.5 |
| 2o | 87.3 | 86—87 | 352.0609(352.0613) | 1724.4, 1601.5 |
| 2p | 69.2 | 106—107 | 366.0767(366.0770) | 1730.1, 1640.5 |
| 2q | 68.5 | 98—99 | 366.0765(366.0770) | 1731.2, 1641.0 |
| 2r | 75.3 | 91—93 | 366.0764(366.0770) | 1732.9, 1641.3 |
| 2s | 78.7 | 101—103 | 366.0770(366.0770) | 1707.1, 1618.3 |
| 2t | 82.8 | 91—93 | 366.0765(366.0770) | 1716.7, 1601.5 |
| 2u | 85.7 | 76—77 | 366.0766(366.0770) | 1724.4, 1676.2 |
| 2v | 88.9 | 86—87 | 366.0768(366.0770) | 1716.7, 1610.6 |
| 2w | 89.2 | 80—81 | 434.0636(434.0644) | 1728.6, 1620.3 |
| 2x | 81.3 | 79—81 | 380.0923(380.0926) | 1720.6, 1622.2 |
| 2y | 85.3 | 86—87 | 380.0924(380.0926) | 1708.9, 1618.3 |
| 2z | 80.2 | 80—82 | 448.0792(448.0800) | 1728.9, 1619.3 |
Table 1 Yields, melting points, HRMS and IR data for compounds 2
| Compd. | Isolated yield(%) | m.p./℃ | HRMS(calcd., [M+H]+), m/z | IR(KBr), |
|---|---|---|---|---|
| 2a | 85.0 | 106—108 | 334.0708(334.0707) | 1736.0, 1610.6 |
| 2b | 80.0 | 111—113 | 334.0705(334.0707) | 1732.1, 1608.9 |
| 2c | 86.5 | 98—100 | 334.0708(334.0707) | 1728.3, 1620.0 |
| 2d | 87.0 | 102—104 | 348.0860(348.0864) | 1724.4, 1618.3 |
| 2e | 90.2 | 99—101 | 348.0858(348.0864) | 1705.1, 1600.9 |
| 2f | 86.8 | 103—105 | 348.0860(348.0864) | 1728.3, 1600.0 |
| 2g | 82.5 | 96—97 | 334.0708(334.0707) | 1732.1, 1618.3 |
| 2h | 89.3 | 87—89 | 334.0708(334.0707) | 1726.4, 1610.6 |
| 2i | 86.0 | 101—102 | 348.0860(348.0864) | 1728.3, 1629.9 |
| Compd. | Isolated yield(%) | m.p./℃ | HRMS(calcd., [M+H]+), m/z | IR(KBr), |
| 2j | 91.0 | 105—106 | 348.0864(348.0864) | 1726.3, 1610.9 |
| 2k | 72.5 | 96—97 | 352.0609(352.0613) | 1730.1, 1640.5 |
| 2l | 75.8 | 78—79 | 352.0608(352.0613) | 1731.2, 1641.0 |
| 2m | 69.4 | 106—107 | 352.0609(352.0613) | 1732.9, 1641.3 |
| 2n | 80.0 | 108—110 | 352.0614(352.0613) | 1732.1, 1641.5 |
| 2o | 87.3 | 86—87 | 352.0609(352.0613) | 1724.4, 1601.5 |
| 2p | 69.2 | 106—107 | 366.0767(366.0770) | 1730.1, 1640.5 |
| 2q | 68.5 | 98—99 | 366.0765(366.0770) | 1731.2, 1641.0 |
| 2r | 75.3 | 91—93 | 366.0764(366.0770) | 1732.9, 1641.3 |
| 2s | 78.7 | 101—103 | 366.0770(366.0770) | 1707.1, 1618.3 |
| 2t | 82.8 | 91—93 | 366.0765(366.0770) | 1716.7, 1601.5 |
| 2u | 85.7 | 76—77 | 366.0766(366.0770) | 1724.4, 1676.2 |
| 2v | 88.9 | 86—87 | 366.0768(366.0770) | 1716.7, 1610.6 |
| 2w | 89.2 | 80—81 | 434.0636(434.0644) | 1728.6, 1620.3 |
| 2x | 81.3 | 79—81 | 380.0923(380.0926) | 1720.6, 1622.2 |
| 2y | 85.3 | 86—87 | 380.0924(380.0926) | 1708.9, 1618.3 |
| 2z | 80.2 | 80—82 | 448.0792(448.0800) | 1728.9, 1619.3 |
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
|---|---|---|
| 2a | 7.10—7.98(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.75(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.5 Hz), 3.08(dd, 1H, CH2, J=13.0, 5.5 Hz) | 170.78, 166.73, 151.42, 151.38(dd, JC—F=246.62, 12.62 Hz), 149.39(dd, JC—F=251.62, 13.37 Hz), 135.56, 130.59, 129.02(d, JC—F=8.12 Hz), 125.95, 125.23(d, JC—F=1.62 Hz), 124.98, 124.41(d, JC—F=6.87 Hz), 121.44, 119.34(d, JC—F=16.75 Hz), 55.48, 52.67, 34.90 |
| 2b | 7.11—7.82(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.0, 6.0 Hz), 3.75(s, 3H, OCH3), 3.09—3.16(m, 2H, CH2) | 170.83, 166.50, 159.12(dd, JC—F=243.75, 3.37 Hz), 157.17(dd, JC—F=247.00, 3.87 Hz), 151.38, 135.54, 130.57, 128.10(dd, JC—F=13.62, 7.25 Hz), 125.93, 124.99, 121.52, 119.15(dd, JC—F=24.62, 9.50 Hz), 117.77(dd, JC—F=26.50, 8.37 Hz), 116.72(dd, JC—F=25.37, 3.62 Hz), 55.51, 52.64, 34.70 |
| 2c | 7.00—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.23(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz) | 170.62, 163.86, 160.36(dd, JC—F=250.25, 6.75 Hz), 151.42, 135.49, 131.12(t, JC—F=10.13 Hz), 130.48, 126.14, 125.39, 121.50, 118.15(t, JC—F=18.12 Hz), 112.11(dd, JC—F=20.25, 4.87 Hz), 55.69, 52.64, 35.89 |
| 2d | 7.12—7.82(m, 7H, PhH), 4.48(dd, 1H, SCH, J=12.0, 5.5 Hz), 4.20(q, 2H, OCH2, J=7.0 Hz), 3.17(t, 1H, CH2, J=12.5 Hz), 3.07(dd, 1H, CH2, J=13.0, 5.5 Hz), 1.29(t, 3H, CH3, J=7.5 Hz) | 170.22, 166.90, 151.45, 151.36(dd, JC—F=246.62, 12.62 Hz), 149.37(dd, JC—F=251.62, 13.37 Hz), 135.58, 130.54, 129.10(d, JC—F=8.25 Hz), 125.90, 125.23(d, JC—F=1.50 Hz), 124.93, 124.39(d, JC—F=6.87 Hz), 121.55, 119.29(d, JC—F=17.12 Hz), 61.61, 55.77, 34.87, 14.07 |
| 2e | 7.12—7.83(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.0, 6.0 Hz), 4.21(q, 2H, OCH2, J=7.0 Hz), 3.10—3.18(m, 2H, CH2), 1.29(t, 3H, CH3, J=7.0 Hz) | 170.27, 166.66, 159.12(dd, JC—F=243.75, 3.37 Hz), 157.17(dd, JC—F=247.00, 3.87 Hz), 151.41, 135.57, 130.52, 128.17(dd, JC—F=13.62, 7.25 Hz), 125.87, 124.94, 121.62, 119.15(dd, JC—F=24.62, 9.50 Hz), 117.77(dd, JC—F=26.50, 8.37 Hz), 116.72(dd, JC—F=25.37, 3.62 Hz), 61.57, 55.79, 34.70, 14.08 |
| 2f | 7.02—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 4.19(q, 2H, OCH2, J=7.0 Hz), 3.25(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz), 1.28(t, 3H, CH3, J=7.0 Hz) | 170.04, 163.99, 160.33(dd, JC—F=250.37, 6.87 Hz), 151.48, 135.50, 131.11(t, JC—F=10.13 Hz), 130.43, 126.06, 125.22, 121.62, 118.21(t, JC—F=18.25 Hz), 112.08(dd, JC—F=20.50, 5.12 Hz), 61.58, 55.98, 35.88, 14.04 |
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
| 2g | 7.00—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.23(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz) | 170.62, 163.86, 160.36(dd, JC—F=250.25, 6.75 Hz), 151.42, 135.49, 131.12(t, JC—F=10.13 Hz), 130.48, 126.14, 125.39, 121.50, 118.15(t, JC—F=18.12 Hz), 112.11(dd, JC—F=20.25, 4.87 Hz), 55.69, 52.64, 35.89 |
| 2h | 6.95—7.58(m, 7H, PhH), 4.35(dd, 1H, SCH, J=12.0, 6.0 Hz), 3.78(s, 3H, OCH3), 3.07—3.16(m, 2H, CH2) | 170.54, 166.78, 163.31(dd, JC—F=247.25, 12.62 Hz), 151.97, 140.87(t, JC—F=8.87 Hz), 135.54, 130.66, 125.92, 125.05, 120.96, 110.34(dd, JC—F=20.62, 6.12 Hz), 106.44(t, JC—F=25.75 Hz), 55.82, 52.76, 31.54 |
| 2i | 7.02—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 4.19(q, 2H, OCH2, J=7.0 Hz), 3.25(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz), 1.28(t, 3H, CH3, J=7.0 Hz) | 170.04, 163.99, 160.33(dd, JC—F=250.37, 6.87 Hz), 151.48, 135.50, 131.11(t, JC—F=10.13 Hz), 130.43, 126.06, 125.22, 121.62, 118.21(t, JC—F=18.25 Hz), 112.08(dd, JC—F=20.50, 5.12 Hz), 61.58, 55.98, 35.88, 14.04 |
| 2j | 6.95—7.59(m, 7H, PhH), 4.33(dd, 1H, SCH, J=12.0, 6.0 Hz), 4.22(q, 2H, OCH2, J=7.0 Hz), 3.06—3.17(m, 2H, CH2), 1.31(t, 3H, CH3, J=7.0 Hz) | 170.54, 166.89, 163.28(dd, JC—F=247.63, 12.25 Hz), 152.02, 140.95(t, JC—F=8.62 Hz), 135.56, 130.60, 125.84, 124.98, 121.06, 110.34(dd, JC—F=20.25, 6.62 Hz), 106.38(t, JC—F=25.37 Hz), 61.77, 56.09, 31.52, 14.08 |
| 2k | 7.09—7.90(m, 6H, PhH), 4.51(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.78(s, 3H, OCH3), 3.18(t, 1H, CH2, J=13.5 Hz), 3.07(dd, 1H, CH2, J=13.0, 5.5 Hz) | 165.95, 160.96, 146.51, 142.76, 140.79, 139.17, 130.83, 125.88, 121.27, 120.19, 119.61, 113.61, 108.03, 53.67, 50.70, 30.00 |
| 2l | 6.96—7.62(m, 6H, PhH), 4.52(dd, 1H, SCH, J=11.5, 5.5 Hz), 3.76(s, 3H, OCH3), 3.26(t, 1H, CH2, J=12.0 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.5 Hz) | 170.49, 162.93, 162.99, 162.08, 161.82, 159.81, 151.19, 135.50, 130.53, 126.22, 125.22, 121.42, 114.83, 101.02, 55.80, 52.69, 35.78 |
| 2m | 7.02—8.03(m, 6H, PhH), 4.51(dd, 1H, SCH, J=11.5, 5.5 Hz), 3.78(s, 3H, OCH3), 3.15(t, 1H, CH2, J=12.0 Hz), 3.08(dd, 1H, CH2, J=13.0, 5.5 Hz) | 170.75, 165.36, 154.56, 152.02, 151.23, 149.70, 135.57, 130.63, 125.99, 124.97, 123.25, 121.50, 108.30, 55.48, 52.67, 34.70 |
| 2n | 6.77—7.59(m, 6H, PhH), 4.47(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.21(t, 1H, CH2, J=12.5 Hz), 2.82(dd, 1H, CH2, J=13.0, 5.5 Hz) | 170.75, 165.36, 162.99, 162.08, 161.82, 159.81, 151.27, 135.50, 130.53, 126.22, 125.22, 121.42, 114.83, 101.02, 55.75, 52.64, 35.81 |
| 2o | 7.12—7.72(m, 6H, PhH), 4.34(dd, 1H, SCH, J=12.0, 6.0 Hz), 3.78(s, 3H, OCH3), 3.04—3.16(m, 2H, CH2) | 170.46, 165.75, 152.42, 151.91, 150.42, 135.56, 140.87, 130.70, 125.96, 125.04, 120.85, 111.69, 55.75, 52.81, 31.15 |
| 2p | 7.09—7.89(m, 6H, PhH), 4.47(dd, 1H, SCH, J=12.0, 5.5 Hz), 4.23(q, 2H, OCH2, J=7.0 Hz), 3.19(t, 1H, CH2, J=13.5 Hz), 3.06(dd, 1H, CH2, J=13.0, 5.5 Hz) | 165.95, 160.96, 146.51, 142.76, 140.79, 139.17, 130.83, 125.88, 121.27, 120.19, 119.61, 113.61, 108.03, 53.67, 50.70, 30.00 |
| 2q | 6.96—7.61(m, 6H, PhH), 4.52(dd, 1H, SCH, J=11.5, 5.5 Hz), 4.20(q, 2H, OCH2, J=7.0 Hz), 3.26(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.5 Hz) | 169.94, 163.04, 146.51, 142.76, 140.79, 139.17, 130.83, 125.88, 121.27, 120.19, 119.61, 113.61, 108.03, 61.66, 56.10, 35.79, 14.03 |
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
| 2r | 6.78—7.59(m, 6H, PhH), 4.45(dd, 1H, SCH, J=12.0, 5.5 Hz), 4.18(q, 2H, OCH2, J=7.0 Hz), 3.22(t, 1H, CH2, J=12.5 Hz), 2.82(dd, 1H, CH2, J=13.0, 5.5 Hz), 1.27(t, 3H, CH3, J=7.0 Hz) | 169.97, 163.24, 161.64, 160.83, 151.17, 135.55, 130.50, 126.24, 125.22, 121.61, 114.80, 101.04, 61.66, 56.05, 35.85, 14.04 |
| 2s | 6.78—7.59(m, 6H, PhH), 4.45(dd, 1H, SCH, J=12.0, 5.5 Hz), 4.18(q, 2H, OCH2, J=7.0 Hz), 3.22(t, 1H, CH2, J=12.5 Hz), 2.82(dd, 1H, CH2, J=13.0, 5.5 Hz), 1.27(t, 3H, CH3, J=7.0 Hz) | 169.97, 163.24, 161.64, 160.83, 151.17, 135.55, 130.50, 126.24, 125.22, 121.61, 114.80, 101.04, 61.66, 56.05, 35.85, 14.04 |
| 2t | 7.12—7.73(m, 6H, PhH), 4.31(dd, 1H, SCH, J=12.0, 6.0 Hz), 4.22(q, 2H, OCH2, J=7.0 Hz), 3.13(t, 1H, CH2, J=12.5 Hz), 3.05(dd, 1H, CH2, J=13.0, 5.5 Hz), 1.31(t, 3H, CH3, J=7.0 Hz) | 170.54, 166.89, 163.28(dd, JC—F=247.63, 12.25 Hz), 152.02, 140.95(t, JC—F=8.62 Hz), 135.56, 130.60, 125.84, 124.98, 121.06, 110.34(dd, JC—F=20.25, 6.62 Hz), 106.38(t, JC—F=25.37 Hz), 61.77, 56.09, 31.52, 14.08 |
| 2u | 7.14—8.34(m, 8H, PhH), 4.40(dd, 1H, SCH, J=12.0, 6.0 Hz), 3.80(s, 3H, OCH3), 3.17—3.25(m, 2H, CH2) | 170.60, 167.66, 152.25, 138.33, 135.52, 131.47, 130.64, 130.49, 129.35, 127.71, 125.76, 125.03, 124.12, 120.93, 55.83, 52.73, 31.45 |
| 2v | 7.14—8.17(m, 8H, PhH), 4.39(dd, 1H, SCH, J=12.0, 6.0 Hz), 3.80(s, 3H, OCH3), 3.17—3.25(m, 2H, CH2) | 170.08, 168.17, 152.10, 140.70, 135.57, 132.81, 130.61, 127.74, 125.84, 125.75, 125.04, 122.80, 121.10, 61.16, 56.10, 31.69 |
| 2w | 7.15—8.48(m, 7H, PhH), 4.41(dd, 1H, SCH, J=12.0, J=6.0 Hz), 3.79(s, 3H, OCH3), 3.25(t, 1H, CH2, J=12.0 Hz), 3.18(dd, 1H, CH2, J=13.5, 6.0 Hz) | 170.40, 166.25, 151.78, 139.64, 135.60, 132.29, 130.77, 127.32, 126.21, 125.14, 124.47, 124.25, 122.08, 120.09, 55.89, 52.80, 31.34 |
| 2x | 7.12—8.32(m, 8H, PhH), 4.35(dd, 1H, SCH, J=12.0, 6.0 Hz), 4.27(q, 2H, OCH2, J=7.0 Hz), 3.14—3.22(m, 2H, CH2), 1.31(t, 3H, CH3, J=7.0 Hz) | 170.60, 167.66, 152.25, 138.33, 135.52, 131.47, 130.64, 130.49, 129.35, 127.71, 125.76, 125.03, 124.12, 120.93, 55.83, 52.73, 31.45 |
| 2y | 7.14—8.17(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.0, 6.0 Hz), 4.22(q, 2H, OCH2, J=7.0 Hz), 3.14—3.23(m, 2H, CH2), 1.31(t, 3H, CH3, J=7.0 Hz) | 170.08, 168.17, 152.10, 140.70, 135.57, 132.81, 130.61, 127.74, 125.84, 125.75, 125.04, 122.80, 121.10, 61.16, 56.10, 31.69, 14.08 |
| 2z | 7.17—8.50(m, 7H, PhH), 4.39(dd, 1H, SCH, J=12.0, 6.0 Hz), 4.27(q, 2H, OCH2, J=7.0 Hz), 3.26(t, 1H, CH2, J=12.0 Hz), 3.19(dd, 1H, CH2, J=13.5, 6.0 Hz), 1.30(t, 3H, CH3, J=7.0 Hz) | 169.78, 166.36, 151.83, 139.81, 135.58, 132.32, 130.67, 127.33, 126.11, 125.09, 124.38, 124.25, 122.08, 121.12, 61.84, 56.22, 31.43, 13.99 |
Table 2 1H NMR and 13C NMR data for compounds 2
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
|---|---|---|
| 2a | 7.10—7.98(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.75(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.5 Hz), 3.08(dd, 1H, CH2, J=13.0, 5.5 Hz) | 170.78, 166.73, 151.42, 151.38(dd, JC—F=246.62, 12.62 Hz), 149.39(dd, JC—F=251.62, 13.37 Hz), 135.56, 130.59, 129.02(d, JC—F=8.12 Hz), 125.95, 125.23(d, JC—F=1.62 Hz), 124.98, 124.41(d, JC—F=6.87 Hz), 121.44, 119.34(d, JC—F=16.75 Hz), 55.48, 52.67, 34.90 |
| 2b | 7.11—7.82(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.0, 6.0 Hz), 3.75(s, 3H, OCH3), 3.09—3.16(m, 2H, CH2) | 170.83, 166.50, 159.12(dd, JC—F=243.75, 3.37 Hz), 157.17(dd, JC—F=247.00, 3.87 Hz), 151.38, 135.54, 130.57, 128.10(dd, JC—F=13.62, 7.25 Hz), 125.93, 124.99, 121.52, 119.15(dd, JC—F=24.62, 9.50 Hz), 117.77(dd, JC—F=26.50, 8.37 Hz), 116.72(dd, JC—F=25.37, 3.62 Hz), 55.51, 52.64, 34.70 |
| 2c | 7.00—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.23(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz) | 170.62, 163.86, 160.36(dd, JC—F=250.25, 6.75 Hz), 151.42, 135.49, 131.12(t, JC—F=10.13 Hz), 130.48, 126.14, 125.39, 121.50, 118.15(t, JC—F=18.12 Hz), 112.11(dd, JC—F=20.25, 4.87 Hz), 55.69, 52.64, 35.89 |
| 2d | 7.12—7.82(m, 7H, PhH), 4.48(dd, 1H, SCH, J=12.0, 5.5 Hz), 4.20(q, 2H, OCH2, J=7.0 Hz), 3.17(t, 1H, CH2, J=12.5 Hz), 3.07(dd, 1H, CH2, J=13.0, 5.5 Hz), 1.29(t, 3H, CH3, J=7.5 Hz) | 170.22, 166.90, 151.45, 151.36(dd, JC—F=246.62, 12.62 Hz), 149.37(dd, JC—F=251.62, 13.37 Hz), 135.58, 130.54, 129.10(d, JC—F=8.25 Hz), 125.90, 125.23(d, JC—F=1.50 Hz), 124.93, 124.39(d, JC—F=6.87 Hz), 121.55, 119.29(d, JC—F=17.12 Hz), 61.61, 55.77, 34.87, 14.07 |
| 2e | 7.12—7.83(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.0, 6.0 Hz), 4.21(q, 2H, OCH2, J=7.0 Hz), 3.10—3.18(m, 2H, CH2), 1.29(t, 3H, CH3, J=7.0 Hz) | 170.27, 166.66, 159.12(dd, JC—F=243.75, 3.37 Hz), 157.17(dd, JC—F=247.00, 3.87 Hz), 151.41, 135.57, 130.52, 128.17(dd, JC—F=13.62, 7.25 Hz), 125.87, 124.94, 121.62, 119.15(dd, JC—F=24.62, 9.50 Hz), 117.77(dd, JC—F=26.50, 8.37 Hz), 116.72(dd, JC—F=25.37, 3.62 Hz), 61.57, 55.79, 34.70, 14.08 |
| 2f | 7.02—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 4.19(q, 2H, OCH2, J=7.0 Hz), 3.25(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz), 1.28(t, 3H, CH3, J=7.0 Hz) | 170.04, 163.99, 160.33(dd, JC—F=250.37, 6.87 Hz), 151.48, 135.50, 131.11(t, JC—F=10.13 Hz), 130.43, 126.06, 125.22, 121.62, 118.21(t, JC—F=18.25 Hz), 112.08(dd, JC—F=20.50, 5.12 Hz), 61.58, 55.98, 35.88, 14.04 |
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
| 2g | 7.00—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.23(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz) | 170.62, 163.86, 160.36(dd, JC—F=250.25, 6.75 Hz), 151.42, 135.49, 131.12(t, JC—F=10.13 Hz), 130.48, 126.14, 125.39, 121.50, 118.15(t, JC—F=18.12 Hz), 112.11(dd, JC—F=20.25, 4.87 Hz), 55.69, 52.64, 35.89 |
| 2h | 6.95—7.58(m, 7H, PhH), 4.35(dd, 1H, SCH, J=12.0, 6.0 Hz), 3.78(s, 3H, OCH3), 3.07—3.16(m, 2H, CH2) | 170.54, 166.78, 163.31(dd, JC—F=247.25, 12.62 Hz), 151.97, 140.87(t, JC—F=8.87 Hz), 135.54, 130.66, 125.92, 125.05, 120.96, 110.34(dd, JC—F=20.62, 6.12 Hz), 106.44(t, JC—F=25.75 Hz), 55.82, 52.76, 31.54 |
| 2i | 7.02—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 4.19(q, 2H, OCH2, J=7.0 Hz), 3.25(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz), 1.28(t, 3H, CH3, J=7.0 Hz) | 170.04, 163.99, 160.33(dd, JC—F=250.37, 6.87 Hz), 151.48, 135.50, 131.11(t, JC—F=10.13 Hz), 130.43, 126.06, 125.22, 121.62, 118.21(t, JC—F=18.25 Hz), 112.08(dd, JC—F=20.50, 5.12 Hz), 61.58, 55.98, 35.88, 14.04 |
| 2j | 6.95—7.59(m, 7H, PhH), 4.33(dd, 1H, SCH, J=12.0, 6.0 Hz), 4.22(q, 2H, OCH2, J=7.0 Hz), 3.06—3.17(m, 2H, CH2), 1.31(t, 3H, CH3, J=7.0 Hz) | 170.54, 166.89, 163.28(dd, JC—F=247.63, 12.25 Hz), 152.02, 140.95(t, JC—F=8.62 Hz), 135.56, 130.60, 125.84, 124.98, 121.06, 110.34(dd, JC—F=20.25, 6.62 Hz), 106.38(t, JC—F=25.37 Hz), 61.77, 56.09, 31.52, 14.08 |
| 2k | 7.09—7.90(m, 6H, PhH), 4.51(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.78(s, 3H, OCH3), 3.18(t, 1H, CH2, J=13.5 Hz), 3.07(dd, 1H, CH2, J=13.0, 5.5 Hz) | 165.95, 160.96, 146.51, 142.76, 140.79, 139.17, 130.83, 125.88, 121.27, 120.19, 119.61, 113.61, 108.03, 53.67, 50.70, 30.00 |
| 2l | 6.96—7.62(m, 6H, PhH), 4.52(dd, 1H, SCH, J=11.5, 5.5 Hz), 3.76(s, 3H, OCH3), 3.26(t, 1H, CH2, J=12.0 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.5 Hz) | 170.49, 162.93, 162.99, 162.08, 161.82, 159.81, 151.19, 135.50, 130.53, 126.22, 125.22, 121.42, 114.83, 101.02, 55.80, 52.69, 35.78 |
| 2m | 7.02—8.03(m, 6H, PhH), 4.51(dd, 1H, SCH, J=11.5, 5.5 Hz), 3.78(s, 3H, OCH3), 3.15(t, 1H, CH2, J=12.0 Hz), 3.08(dd, 1H, CH2, J=13.0, 5.5 Hz) | 170.75, 165.36, 154.56, 152.02, 151.23, 149.70, 135.57, 130.63, 125.99, 124.97, 123.25, 121.50, 108.30, 55.48, 52.67, 34.70 |
| 2n | 6.77—7.59(m, 6H, PhH), 4.47(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.21(t, 1H, CH2, J=12.5 Hz), 2.82(dd, 1H, CH2, J=13.0, 5.5 Hz) | 170.75, 165.36, 162.99, 162.08, 161.82, 159.81, 151.27, 135.50, 130.53, 126.22, 125.22, 121.42, 114.83, 101.02, 55.75, 52.64, 35.81 |
| 2o | 7.12—7.72(m, 6H, PhH), 4.34(dd, 1H, SCH, J=12.0, 6.0 Hz), 3.78(s, 3H, OCH3), 3.04—3.16(m, 2H, CH2) | 170.46, 165.75, 152.42, 151.91, 150.42, 135.56, 140.87, 130.70, 125.96, 125.04, 120.85, 111.69, 55.75, 52.81, 31.15 |
| 2p | 7.09—7.89(m, 6H, PhH), 4.47(dd, 1H, SCH, J=12.0, 5.5 Hz), 4.23(q, 2H, OCH2, J=7.0 Hz), 3.19(t, 1H, CH2, J=13.5 Hz), 3.06(dd, 1H, CH2, J=13.0, 5.5 Hz) | 165.95, 160.96, 146.51, 142.76, 140.79, 139.17, 130.83, 125.88, 121.27, 120.19, 119.61, 113.61, 108.03, 53.67, 50.70, 30.00 |
| 2q | 6.96—7.61(m, 6H, PhH), 4.52(dd, 1H, SCH, J=11.5, 5.5 Hz), 4.20(q, 2H, OCH2, J=7.0 Hz), 3.26(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.5 Hz) | 169.94, 163.04, 146.51, 142.76, 140.79, 139.17, 130.83, 125.88, 121.27, 120.19, 119.61, 113.61, 108.03, 61.66, 56.10, 35.79, 14.03 |
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
| 2r | 6.78—7.59(m, 6H, PhH), 4.45(dd, 1H, SCH, J=12.0, 5.5 Hz), 4.18(q, 2H, OCH2, J=7.0 Hz), 3.22(t, 1H, CH2, J=12.5 Hz), 2.82(dd, 1H, CH2, J=13.0, 5.5 Hz), 1.27(t, 3H, CH3, J=7.0 Hz) | 169.97, 163.24, 161.64, 160.83, 151.17, 135.55, 130.50, 126.24, 125.22, 121.61, 114.80, 101.04, 61.66, 56.05, 35.85, 14.04 |
| 2s | 6.78—7.59(m, 6H, PhH), 4.45(dd, 1H, SCH, J=12.0, 5.5 Hz), 4.18(q, 2H, OCH2, J=7.0 Hz), 3.22(t, 1H, CH2, J=12.5 Hz), 2.82(dd, 1H, CH2, J=13.0, 5.5 Hz), 1.27(t, 3H, CH3, J=7.0 Hz) | 169.97, 163.24, 161.64, 160.83, 151.17, 135.55, 130.50, 126.24, 125.22, 121.61, 114.80, 101.04, 61.66, 56.05, 35.85, 14.04 |
| 2t | 7.12—7.73(m, 6H, PhH), 4.31(dd, 1H, SCH, J=12.0, 6.0 Hz), 4.22(q, 2H, OCH2, J=7.0 Hz), 3.13(t, 1H, CH2, J=12.5 Hz), 3.05(dd, 1H, CH2, J=13.0, 5.5 Hz), 1.31(t, 3H, CH3, J=7.0 Hz) | 170.54, 166.89, 163.28(dd, JC—F=247.63, 12.25 Hz), 152.02, 140.95(t, JC—F=8.62 Hz), 135.56, 130.60, 125.84, 124.98, 121.06, 110.34(dd, JC—F=20.25, 6.62 Hz), 106.38(t, JC—F=25.37 Hz), 61.77, 56.09, 31.52, 14.08 |
| 2u | 7.14—8.34(m, 8H, PhH), 4.40(dd, 1H, SCH, J=12.0, 6.0 Hz), 3.80(s, 3H, OCH3), 3.17—3.25(m, 2H, CH2) | 170.60, 167.66, 152.25, 138.33, 135.52, 131.47, 130.64, 130.49, 129.35, 127.71, 125.76, 125.03, 124.12, 120.93, 55.83, 52.73, 31.45 |
| 2v | 7.14—8.17(m, 8H, PhH), 4.39(dd, 1H, SCH, J=12.0, 6.0 Hz), 3.80(s, 3H, OCH3), 3.17—3.25(m, 2H, CH2) | 170.08, 168.17, 152.10, 140.70, 135.57, 132.81, 130.61, 127.74, 125.84, 125.75, 125.04, 122.80, 121.10, 61.16, 56.10, 31.69 |
| 2w | 7.15—8.48(m, 7H, PhH), 4.41(dd, 1H, SCH, J=12.0, J=6.0 Hz), 3.79(s, 3H, OCH3), 3.25(t, 1H, CH2, J=12.0 Hz), 3.18(dd, 1H, CH2, J=13.5, 6.0 Hz) | 170.40, 166.25, 151.78, 139.64, 135.60, 132.29, 130.77, 127.32, 126.21, 125.14, 124.47, 124.25, 122.08, 120.09, 55.89, 52.80, 31.34 |
| 2x | 7.12—8.32(m, 8H, PhH), 4.35(dd, 1H, SCH, J=12.0, 6.0 Hz), 4.27(q, 2H, OCH2, J=7.0 Hz), 3.14—3.22(m, 2H, CH2), 1.31(t, 3H, CH3, J=7.0 Hz) | 170.60, 167.66, 152.25, 138.33, 135.52, 131.47, 130.64, 130.49, 129.35, 127.71, 125.76, 125.03, 124.12, 120.93, 55.83, 52.73, 31.45 |
| 2y | 7.14—8.17(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.0, 6.0 Hz), 4.22(q, 2H, OCH2, J=7.0 Hz), 3.14—3.23(m, 2H, CH2), 1.31(t, 3H, CH3, J=7.0 Hz) | 170.08, 168.17, 152.10, 140.70, 135.57, 132.81, 130.61, 127.74, 125.84, 125.75, 125.04, 122.80, 121.10, 61.16, 56.10, 31.69, 14.08 |
| 2z | 7.17—8.50(m, 7H, PhH), 4.39(dd, 1H, SCH, J=12.0, 6.0 Hz), 4.27(q, 2H, OCH2, J=7.0 Hz), 3.26(t, 1H, CH2, J=12.0 Hz), 3.19(dd, 1H, CH2, J=13.5, 6.0 Hz), 1.30(t, 3H, CH3, J=7.0 Hz) | 169.78, 166.36, 151.83, 139.81, 135.58, 132.32, 130.67, 127.33, 126.11, 125.09, 124.38, 124.25, 122.08, 121.12, 61.84, 56.22, 31.43, 13.99 |
| Compd. | Isolated yield(%) | m.p./℃ | HRMS(calcd., [M+H]+), m/z | IR(KBr), |
|---|---|---|---|---|
| 3a | 45.2 | 56—57 | 318.0938(318.0936) | 1734.1, 1610.6 |
| 3b | 48.3 | 68—70 | 318.0938(318.0936) | 1747.6, 1610.6 |
| 3c | 43.7 | 76—77 | 318.0937(318.0936) | 1726.4, 1616.4 |
| 3d | 37.6 | 53—55 | 332.1093(332.1092) | 1722.5, 1622.6 |
| 3e | 35.9 | 46—47 | 332.1094(332.1092) | 1736.0, 1628.0 |
| 3f | 45.5 | 36—37 | 332.1091(332.1092) | 1734.1, 1618.3 |
| 4a | 92.1 | 86—88 | 317.1093(317.1096) | 3327.3, 1743.7, 1612.5 |
| 4b | 95.3 | 90—92 | 317.1093(317.1096) | 3356.3, 1745.6, 1618.3 |
| 4c | 89.8 | 89—91 | 317.1093(317.1096) | 3362.0, 1741.8, 1601.0 |
| 4d | 87.9 | 90—92 | 331.1249(331.1252) | 3365.9, 1726.4, 1618.3 |
| 4e | 85.9 | 85—87 | 331.1248(331.1252) | 3373.6, 1736.0, 1610.6 |
| 4f | 87.7 | 81—83 | 331.1250(331.1252) | 3375.5, 1732.1, 1612.5 |
| 5a | 87.9 | 109—111 | 336.0864(336.0864) | 3391.0, 1718.6 |
| 5b | 86.8 | 336.0859(336.0864) | 3390.9, 1720.6 | |
| 5c | 89.0 | 103—105 | 336.0863(336.0864) | 3385.2, 1720.6 |
| 5d | 85.4 | 108—110 | 350.1019(350.1020) | 3387.1, 1710.9 |
| 5e | 88.5 | 350.1016(350.1020) | 3392.9, 1720.6 | |
| 5f | 90.0 | 350.1016(350.1020) | 3388.7, 1710.6 | |
| 6a | 87.3 | 91—93 | 276.0652(276.0653) | 1608.9 |
| 6b | 83.5 | 97—99 | 276.0656(276.0653) | 1612.5 |
| 6c | 79.2 | 89—91 | 276.0651(276.0653) | 1610.7 |
Table 3 Yields, melting points, HRMS and IR data for compounds 3—6
| Compd. | Isolated yield(%) | m.p./℃ | HRMS(calcd., [M+H]+), m/z | IR(KBr), |
|---|---|---|---|---|
| 3a | 45.2 | 56—57 | 318.0938(318.0936) | 1734.1, 1610.6 |
| 3b | 48.3 | 68—70 | 318.0938(318.0936) | 1747.6, 1610.6 |
| 3c | 43.7 | 76—77 | 318.0937(318.0936) | 1726.4, 1616.4 |
| 3d | 37.6 | 53—55 | 332.1093(332.1092) | 1722.5, 1622.6 |
| 3e | 35.9 | 46—47 | 332.1094(332.1092) | 1736.0, 1628.0 |
| 3f | 45.5 | 36—37 | 332.1091(332.1092) | 1734.1, 1618.3 |
| 4a | 92.1 | 86—88 | 317.1093(317.1096) | 3327.3, 1743.7, 1612.5 |
| 4b | 95.3 | 90—92 | 317.1093(317.1096) | 3356.3, 1745.6, 1618.3 |
| 4c | 89.8 | 89—91 | 317.1093(317.1096) | 3362.0, 1741.8, 1601.0 |
| 4d | 87.9 | 90—92 | 331.1249(331.1252) | 3365.9, 1726.4, 1618.3 |
| 4e | 85.9 | 85—87 | 331.1248(331.1252) | 3373.6, 1736.0, 1610.6 |
| 4f | 87.7 | 81—83 | 331.1250(331.1252) | 3375.5, 1732.1, 1612.5 |
| 5a | 87.9 | 109—111 | 336.0864(336.0864) | 3391.0, 1718.6 |
| 5b | 86.8 | 336.0859(336.0864) | 3390.9, 1720.6 | |
| 5c | 89.0 | 103—105 | 336.0863(336.0864) | 3385.2, 1720.6 |
| 5d | 85.4 | 108—110 | 350.1019(350.1020) | 3387.1, 1710.9 |
| 5e | 88.5 | 350.1016(350.1020) | 3392.9, 1720.6 | |
| 5f | 90.0 | 350.1016(350.1020) | 3388.7, 1710.6 | |
| 6a | 87.3 | 91—93 | 276.0652(276.0653) | 1608.9 |
| 6b | 83.5 | 97—99 | 276.0656(276.0653) | 1612.5 |
| 6c | 79.2 | 89—91 | 276.0651(276.0653) | 1610.7 |
| Compd. | 1H NMR(500 MHz), δ | 13C NMR(125 MHz), δ |
|---|---|---|
| 3aa | 6.93—7.81(m, 7H, PhH), 5.81(dd, 1H, OCH, J=9.5, 2.5 Hz), 3.69(s, 3H, OCH3), 2.88(dd, 1H, CH2, J=15.5, 9.5 Hz), 2.62(d, 1H, CH2, J=16.0 Hz) | 169.67, 156.46, 150.92(dd, JC—F=246.62, 12.62 Hz), 149.55(dd, JC—F=251.62, 13.62 Hz), 144.01, 132.82, 129.97, 127.92, 125.70, 125.01, 124.65(dd, JC—F=10.87, 4.37 Hz), 122.67, 119.45(d, JC—F=17.00 Hz), 116.87, 69.83, 52.08, 34.87 |
| 3ba | 6.93—7.84(m, 7H, PhH), 5.84(dd, 1H, OCH, J=9.5, 2.5 Hz), 3.70(s, 3H, OCH3), 2.88(dd, 1H, CH2, J=15.5, 9.5 Hz), 2.62(dd, 1H, CH2, J=15.5, 2.5 Hz) | 169.79, 158.98(dd, JC—F=243.75, 3.37 Hz), 157.25(dd, JC—F=247.00, 3.87 Hz), 156.13, 144.01, 132.80, 129.95, 127.90, 124.79(dd, JC—F=13.37, 8.37 Hz), 122.67, 119.42(t, JC—F=17.00 Hz), 117.31(dd, JC—F=23.50, 11.25 Hz), 116.86, 116.39(t, JC—F=22.50 Hz), 69.73, 52.07, 34.79 |
| 3ca | 6.95—8.00(m, 7H, PhH), 5.89(dd, 1H, OCH, J=9.5, 2.5 Hz), 3.76(s, 3H, OCH3), 2.88(dd, 1H, CH2, J=15.5, 9.5 Hz), 2.62(dd, 1H, CH2, J=16.0, 3.0 Hz) | 169.82, 156.62, 150.92(dd, JC—F=246.62, 12.62 Hz), 149.55(dd, JC—F=251.62, 13.62 Hz), 143.42, 132.77, 129.60, 127.79, 123.08, 122.80, 116.95(dd, JC—F=10.87, 4.37 Hz), 116.88, 67.75, 52.27, 35.26 |
| 3da | 6.93—7.81(m, 7H, PhH), 5.81(dd, 1H, OCH, J=9.5, 2.5 Hz), 4.14(q, 2H, OCH2, J=7.0 Hz), 2.86(dd, 1H, CH2, J=16.0, 9.5 Hz), 2.61(d, 1H, CH2, J=15.5 Hz), 1.24(t, 3H, CH3, J=7.0 Hz) | 169.21, 156.62, 150.92(dd, JC—F=246.62, 12.62 Hz), 149.55(dd, JC—F=251.62, 13.62 Hz), 144.10, 132.84, 129.94, 127.92, 125.78, 125.03, 124.65(dd, JC—F=6.50, 4.37 Hz), 122.62, 119.43(d, JC—F=17.00 Hz), 116.84, 69.91, 61.09, 35.20, 14.10 |
| 3ea | 6.93—7.83(m, 7H, PhH), 5.84(dd, 1H, OCH, J=9.5, 2.5 Hz), 4.14(q, 2H, OCH2, J=7.0 Hz), 2.85(dd, 1H, CH2, J=15.5, 9.5 Hz), 2.61(dd, 1H, CH2, J=15.5, 2.5 Hz), 1.24(t, 3H, CH3, J=7.0 Hz) | 169.35, 158.98(dd, JC—F=243.75, 3.37 Hz), 157.25(dd, JC—F=247.00, 3.87 Hz), 156.23, 144.10, 132.82, 129.92, 127.90, 124.84(dd, JC—F=13.37, 8.37 Hz), 122.63, 119.42(dd, JC—F=17.00, 10.00 Hz), 117.31(dd, JC—F=23.50, 11.25 Hz), 116.84, 116.39(dd, JC—F=22.50, 5.00 Hz), 69.79, 61.09, 35.12, 14.13 |
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
| 3fa | 6.95—8.00(m, 7H, PhH), 5.89(dd, 1H, OCH, J=9.5, 2.5 Hz), 4.14(q, 2H, OCH2, J=7.0 Hz), 2.88(dd, 1H, CH2, J=15.5, 9.5 Hz), 2.62(dd, 1H, CH2, J=16.0, 3.0 Hz), 1.24(t, 3H, CH3, J=7.0 Hz) | 169.38, 156.73, 152.92(dd, JC—F=246.62, 12.62 Hz), 150.45(dd, JC—F=251.62, 13.62 Hz), 143.52, 132.81, 131.84, 129.55, 127.78, 123,12(dd, JC—F=65.00, 3.50 Hz), 122.74, 116.95(dd, JC—F=10.87, 4.37 Hz), 116.86, 67.84, 61.30, 35.56, 14.15 |
| 4aa | 6.99—7.12(m, 7H, PhH), 4.70(dd, 1H, NCH, J=11.0, 4.0 Hz), 4.43(s, 1H, NH), 3.78(s, 3H, OCH3), 3.42(dd, 1H, CH2, J=13.5, 4.0 Hz), 2.89(t, 1H, CH2, J=12.5 Hz) | 173.03, 163.27, 150.84(dd, JC—F=246.6, 13.25 Hz), 149.65(dd, JC—F=250.00, 13.62 Hz), 138.14, 137.70, 130.53(d, JC—F=8.75 Hz), 128.98, 127.27, 125.06, 124.29(dd, JC—F=6.50, 4.50 Hz), 121.82, 121.50, 118.40(d, JC—F=17.12 Hz), 65.49, 52.67, 35.40 |
| 4ba | 6.99—7.69(m, 7H, PhH), 4.70(dd, 1H, NCH, J=11.0, 4.0 Hz), 4.41(s, 1H, NH), 3.79(s, 3H, OCH3), 3.43(dd, 1H, CH2, J=13.5, 4.0 Hz), 2.85(t, 1H, CH2, J=12.5 Hz) | 173.07, 163.20, 158.84(dd, JC—F=246.6, 13.25 Hz), 156.65(dd, JC—F=250.00, 13.62 Hz), 138.16, 137.73, 129.58(dd, JC—F=14.50, 7.25 Hz), 128.93, 127.27, 121.87, 121.53, 118.15(dd, JC—F=24.62, 9.50 Hz), 117.77(dd, JC—F=26.50, 8.37 Hz), 116.72(dd, JC—F=25.37, 3.62 Hz), 65.59, 52.65, 35.14 |
| 4ca | 6.98—7.99(m, 7H, PhH), 4.55(dd, 1H, NCH, J=11.0, 4.0 Hz), 4.34(s, 1H, NH), 3.82(s, 3H, OCH3), 3.44(dd, 1H, CH2, J=13.5, 4.0 Hz), 2.88(dd, 1H, CH2, J=10.5, 3.0 Hz) | 172.78, 163.20, 150.84(dd, JC—F=246.60, 13.25 Hz), 149.65(dd, JC—F=250.00, 13.62 Hz), 138.79, 137.17, 135.86, 128.80, 126.94, 125.06, 123.34(dd, JC—F=6.50, 4.50 Hz), 122.11, 121.38, 118.40(dd, JC—F=123.25, 17.75 Hz), 65.81, 52.79, 31.70 |
| 4da | 6.97—7.70(m, 7H, PhH), 4.66(dd, 1H, NCH, J=11.0, 4.0 Hz), 4.43(s, 1H, NH), 4.22(q, 2H, OCH2, J=7.0 Hz), 3.41(dd, 1H, CH2, J=13.5, 3.5 Hz), 2.87(t, 1H, CH2, J=12.5 Hz), 1.28(t, 3H, CH3, J=7.5 Hz) | 172.53, 163.39, 150.84(dd, JC—F=246.60, 13.25 Hz), 149.65(dd, JC—F=250.00, 13.62 Hz), 138.14, 137.74, 130.53(d, JC—F=8.75 Hz), 128.98, 127.26, 125.06, 124.29(dd, JC—F=6.50, 4.50 Hz), 120.82, 121.47, 118.40(d, JC—F=17.12 Hz), 65.58, 61.87, 35.41, 14.16 |
| 4ea | 6.97—7.68(m, 7H, PhH), 4.65(dd, 1H, NCH, J=11.0, 4.0 Hz), 4.40(s, 1H, NH), 4.22(q, 2H, OCH2, J=7.0 Hz), 3.42(dd, 1H, CH2, J=13.5, 4.0 Hz), 2.84(t, 1H, CH2, J=12.5 Hz) | 172.58, 163.31, 157.84(dd, JC—F=246.60, 13.25 Hz), 156.65(dd, JC—F=250.00, 13.62 Hz), 138.14, 137.78, 129.68(dd, JC—F=14.50, 7.25 Hz), 128.96, 127.24, 121.83, 121.50, 118.10(dd, JC—F=24.62, 9.50 Hz), 117.75(dd, JC—F=26.50, 8.37 Hz), 116.72(dd, JC—F=25.37, 3.62 Hz), 65.66, 61.85, 35.17, 14.17 |
| 4fa | 6.97—7.78(m, 7H, PhH), 4.55(dd, 1H, NCH, J=11.0, 4.0 Hz), 4.35(s, 1H, NH), 4.25(q, 2H, OCH2, J=7.0 Hz), 3.42(dd, 1H, CH2, J=13.5, 4.0 Hz), 2.88(dd, 1H, CH2, J=10.5, 3.0 Hz) | 172.28, 163.24, 150.64(dd, JC—F=246.60, 13.25 Hz), 149.45(dd, JC—F=250.00, 13.62 Hz), 138.80, 137.27, 135.97, 128.84, 126.92, 123.34(dd, JC—F=6.50, 4.50 Hz), 122.05, 121.39, 118.40(dd, JC—F=123.25, 17.75 Hz), 65.89, 62.01, 31.76, 14.24 |
| 5ab | 6.79—7.68(m, 7H, PhH), 5.42—5.45(m, 1H, NCH), 4.92(s, 1H, NH), 4.11(dd, 1H, NCH, J=10.0, 5.0 Hz), 3.67(s, 3H, OCH3), 2.53—2.59(m, 1H, CH2), 2.35—2.40(m, 1H, CH2) | 173.03, 163.27, 150.84(dd, JC—F=246.60, 13.25 Hz), 149.65(dd, JC—F=250.00, 13.62 Hz), 149.51, 128.98, 132.83, 128.72, 124.69(dd, JC—F=6.50, 4.50 Hz), 124.07, 120.86, 120.08, 116.40(d, JC—F=17.12 Hz), 51.73, 50.64, 43.17, 37.05 |
| 5bb | 6.79—7.68(m, 7H, PhH), 5.42—5.45(m, 1H, NCH), 4.92(s, 1H, NH), 4.11(dd, 1H, NCH, J=10.0, 5.0 Hz), 3.67(s, 3H, OCH3), 2.53—2.59(m, 1H, CH2), 2.35—2.40(m, 1H, CH2) | 173.03, 163.27, 150.84(dd, JC—F=246.60, 13.25 Hz), 149.65(dd, JC—F=250.00, 13.62 Hz), 149.51, 128.98, 132.83, 128.72, 124.69(dd, JC—F=6.50, 4.50 Hz), 124.07, 120.86, 120.08, 116.40(d, JC—F=17.12 Hz), 51.73, 50.64, 43.17, 37.05 |
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
| 5cb | 6.78—7.62(m, 7H, PhH), 5.06—5.09(m, 1H, NCH), 4.88(s, 1H, NH), 3.98(dd, 1H, NCH, J=10.0, 5.0 Hz), 3.66(s, 3H, OCH3), 2.43—2.49(m, 1H, CH2), 2.34—2.40(m, 1H, CH2) | 171.26, 150.55(dd, JC—F=244.37, 12.50 Hz), 149.34(dd, JC—F=250.00, 13.62 Hz), 149.18, 141.20, 133.04, 128.83, 123.73(dd, JC—F=6.50, 4.50 Hz), 120.77, 120.03, 119.86, 117.03(dd, JC—F=123.25, 17.75 Hz), 56.33, 51.73, 42.68, 38.14 |
| 5db | 6.81—7.69(m, 7H, PhH), 5.43—5.46(m, 1H, NCH), 4.91(s, 1H, NH), 4.11(dd, 1H, NCH, J=10.0, 5.0 Hz), 4.12(q, 2H, OCH2, J=7.0 Hz), 2.55—2.60(m, 1H, CH2), 2.37—2.42(m, 1H, CH2), 1.23(t, 3H, CH3, J=7.5 Hz) | 170.57, 163.27, 150.64(dd, JC—F=246.60, 13.25 Hz), 149.44(dd, JC—F=250.00, 13.62 Hz), 149.32, 132.94, 132.70, 128.72, 124.67(dd, JC—F=6.50, 4.50 Hz), 124.04, 120.91, 120.26, 120.15, 116.19(d, JC—F=17.12 Hz), 60.91, 50.67, 43.38, 37.18, 13.56 |
| 5eb | 6.79—7.68(m, 7H, PhH), 5.42—5.45(m, 1H, NCH), 4.92(s, 1H, NH), 4.11(dd, 1H, NCH, J=10.0, 5.0 Hz), 4.12(q, 2H, OCH2, J=7.0 Hz), 2.53—2.59(m, 1H, CH2), 2.35—2.40(m, 1H, CH2), 1.23(t, 3H, CH3, J=7.5 Hz) | 173.03, 163.27, 150.64(dd, JC—F=246.60, 13.25 Hz), 149.65(dd, JC—F=250.00, 13.62 Hz), 149.51, 128.88, 132.53, 127.72, 124.59(dd, JC—F=6.50, 4.50 Hz), 124.07, 120.86, 120.28, 116.43(d, JC—F=17.12 Hz), 62.63, 52.64, 42.67, 37.65, 13.79 |
| 5fb | 6.78—7.62(m, 7H, PhH), 5.06—5.09(m, 1H, NCH), 4.88(s, 1H, NH), 3.98(dd, 1H, NCH, J=10.0, 5.0 Hz), 4.12(q, 2H, OCH2, J=7.0 Hz), 2.43—2.49(m, 1H, CH2), 2.34—2.40(m, 1H, CH2), 1.23(t, 3H, CH3, J=7.5 Hz) | 171.26, 150.55(dd, JC—F=244.37, 12.50 Hz), 149.34(dd, JC—F=250.00, 13.62 Hz), 149.18, 141.20, 133.04, 128.83, 123.73(dd, JC—F=6.50, 4.50 Hz), 120.77, 120.03, 119.86, 117.03(dd, JC—F=123.25, 17.75 Hz), 66.30, 51.73, 42.68, 37.14, 13.46 |
| 6aa | 7.10—7.82(m, 7H, PhH), 3.69(t, 2H, SCH2, J=7.0 Hz), 2.93(t, 2H, SCCH2, J=7.0 Hz) | 168.57, 151.86, 150.95(dd, JC—F=247.12, 13.37 Hz), 149.70(dd, JC—F=251.00, 13.87 Hz), 134.84, 129.65, 129.37(d, JC—F=8.75 Hz), 125.53, 125.28, 125.10, 124.28(dd, JC—F=6.50, 4.50 Hz), 123.88, 119.03(d, JC—F=17.12 Hz), 41.25, 33.01 |
| 6ba | 7.10—7.92(m, 7H, PhH), 3.68(t, 2H, SCH2, J=7.0 Hz), 2.94(t, 2H, SCCH2, J=7.0 Hz) | 168.59, 151.79, 150.90(dd, JC—F=247.10, 13.35 Hz), 149.70(dd, JC—F=251.00, 13.87 Hz), 134.83, 129.59, 129.36(d, JC—F=8.75 Hz), 125.51, 125.29, 125.05, 124.22(dd, JC—F=6.50, 4.50 Hz), 123.88, 119.00(d, JC—F=17.12 Hz), 41.05, 32.01 |
| 6ca | 7.10—8.00(m, 7H, PhH), 3.66(t, 2H, SCH2, J=7.0 Hz), 2.96(t, 2H, SCCH2, J=7.0 Hz) | 168.61, 152.33(dd, JC—F=247.12, 13.37 Hz), 150.95(dd, JC—F=247.12, 13.37 Hz), 149.70(dd, JC—F=251.00, 13.87 Hz), 134.84, 129.65, 129.37(d, JC—F=8.75 Hz), 125.53, 125.28, 125.10, 124.28(dd, JC—F=6.50, 4.50 Hz), 123.88, 119.03(d, JC—F=17.12 Hz), 41.53, 29.49 |
Table 4 1H NMR and 13C NMR data for compounds 3—6
| Compd. | 1H NMR(500 MHz), δ | 13C NMR(125 MHz), δ |
|---|---|---|
| 3aa | 6.93—7.81(m, 7H, PhH), 5.81(dd, 1H, OCH, J=9.5, 2.5 Hz), 3.69(s, 3H, OCH3), 2.88(dd, 1H, CH2, J=15.5, 9.5 Hz), 2.62(d, 1H, CH2, J=16.0 Hz) | 169.67, 156.46, 150.92(dd, JC—F=246.62, 12.62 Hz), 149.55(dd, JC—F=251.62, 13.62 Hz), 144.01, 132.82, 129.97, 127.92, 125.70, 125.01, 124.65(dd, JC—F=10.87, 4.37 Hz), 122.67, 119.45(d, JC—F=17.00 Hz), 116.87, 69.83, 52.08, 34.87 |
| 3ba | 6.93—7.84(m, 7H, PhH), 5.84(dd, 1H, OCH, J=9.5, 2.5 Hz), 3.70(s, 3H, OCH3), 2.88(dd, 1H, CH2, J=15.5, 9.5 Hz), 2.62(dd, 1H, CH2, J=15.5, 2.5 Hz) | 169.79, 158.98(dd, JC—F=243.75, 3.37 Hz), 157.25(dd, JC—F=247.00, 3.87 Hz), 156.13, 144.01, 132.80, 129.95, 127.90, 124.79(dd, JC—F=13.37, 8.37 Hz), 122.67, 119.42(t, JC—F=17.00 Hz), 117.31(dd, JC—F=23.50, 11.25 Hz), 116.86, 116.39(t, JC—F=22.50 Hz), 69.73, 52.07, 34.79 |
| 3ca | 6.95—8.00(m, 7H, PhH), 5.89(dd, 1H, OCH, J=9.5, 2.5 Hz), 3.76(s, 3H, OCH3), 2.88(dd, 1H, CH2, J=15.5, 9.5 Hz), 2.62(dd, 1H, CH2, J=16.0, 3.0 Hz) | 169.82, 156.62, 150.92(dd, JC—F=246.62, 12.62 Hz), 149.55(dd, JC—F=251.62, 13.62 Hz), 143.42, 132.77, 129.60, 127.79, 123.08, 122.80, 116.95(dd, JC—F=10.87, 4.37 Hz), 116.88, 67.75, 52.27, 35.26 |
| 3da | 6.93—7.81(m, 7H, PhH), 5.81(dd, 1H, OCH, J=9.5, 2.5 Hz), 4.14(q, 2H, OCH2, J=7.0 Hz), 2.86(dd, 1H, CH2, J=16.0, 9.5 Hz), 2.61(d, 1H, CH2, J=15.5 Hz), 1.24(t, 3H, CH3, J=7.0 Hz) | 169.21, 156.62, 150.92(dd, JC—F=246.62, 12.62 Hz), 149.55(dd, JC—F=251.62, 13.62 Hz), 144.10, 132.84, 129.94, 127.92, 125.78, 125.03, 124.65(dd, JC—F=6.50, 4.37 Hz), 122.62, 119.43(d, JC—F=17.00 Hz), 116.84, 69.91, 61.09, 35.20, 14.10 |
| 3ea | 6.93—7.83(m, 7H, PhH), 5.84(dd, 1H, OCH, J=9.5, 2.5 Hz), 4.14(q, 2H, OCH2, J=7.0 Hz), 2.85(dd, 1H, CH2, J=15.5, 9.5 Hz), 2.61(dd, 1H, CH2, J=15.5, 2.5 Hz), 1.24(t, 3H, CH3, J=7.0 Hz) | 169.35, 158.98(dd, JC—F=243.75, 3.37 Hz), 157.25(dd, JC—F=247.00, 3.87 Hz), 156.23, 144.10, 132.82, 129.92, 127.90, 124.84(dd, JC—F=13.37, 8.37 Hz), 122.63, 119.42(dd, JC—F=17.00, 10.00 Hz), 117.31(dd, JC—F=23.50, 11.25 Hz), 116.84, 116.39(dd, JC—F=22.50, 5.00 Hz), 69.79, 61.09, 35.12, 14.13 |
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
| 3fa | 6.95—8.00(m, 7H, PhH), 5.89(dd, 1H, OCH, J=9.5, 2.5 Hz), 4.14(q, 2H, OCH2, J=7.0 Hz), 2.88(dd, 1H, CH2, J=15.5, 9.5 Hz), 2.62(dd, 1H, CH2, J=16.0, 3.0 Hz), 1.24(t, 3H, CH3, J=7.0 Hz) | 169.38, 156.73, 152.92(dd, JC—F=246.62, 12.62 Hz), 150.45(dd, JC—F=251.62, 13.62 Hz), 143.52, 132.81, 131.84, 129.55, 127.78, 123,12(dd, JC—F=65.00, 3.50 Hz), 122.74, 116.95(dd, JC—F=10.87, 4.37 Hz), 116.86, 67.84, 61.30, 35.56, 14.15 |
| 4aa | 6.99—7.12(m, 7H, PhH), 4.70(dd, 1H, NCH, J=11.0, 4.0 Hz), 4.43(s, 1H, NH), 3.78(s, 3H, OCH3), 3.42(dd, 1H, CH2, J=13.5, 4.0 Hz), 2.89(t, 1H, CH2, J=12.5 Hz) | 173.03, 163.27, 150.84(dd, JC—F=246.6, 13.25 Hz), 149.65(dd, JC—F=250.00, 13.62 Hz), 138.14, 137.70, 130.53(d, JC—F=8.75 Hz), 128.98, 127.27, 125.06, 124.29(dd, JC—F=6.50, 4.50 Hz), 121.82, 121.50, 118.40(d, JC—F=17.12 Hz), 65.49, 52.67, 35.40 |
| 4ba | 6.99—7.69(m, 7H, PhH), 4.70(dd, 1H, NCH, J=11.0, 4.0 Hz), 4.41(s, 1H, NH), 3.79(s, 3H, OCH3), 3.43(dd, 1H, CH2, J=13.5, 4.0 Hz), 2.85(t, 1H, CH2, J=12.5 Hz) | 173.07, 163.20, 158.84(dd, JC—F=246.6, 13.25 Hz), 156.65(dd, JC—F=250.00, 13.62 Hz), 138.16, 137.73, 129.58(dd, JC—F=14.50, 7.25 Hz), 128.93, 127.27, 121.87, 121.53, 118.15(dd, JC—F=24.62, 9.50 Hz), 117.77(dd, JC—F=26.50, 8.37 Hz), 116.72(dd, JC—F=25.37, 3.62 Hz), 65.59, 52.65, 35.14 |
| 4ca | 6.98—7.99(m, 7H, PhH), 4.55(dd, 1H, NCH, J=11.0, 4.0 Hz), 4.34(s, 1H, NH), 3.82(s, 3H, OCH3), 3.44(dd, 1H, CH2, J=13.5, 4.0 Hz), 2.88(dd, 1H, CH2, J=10.5, 3.0 Hz) | 172.78, 163.20, 150.84(dd, JC—F=246.60, 13.25 Hz), 149.65(dd, JC—F=250.00, 13.62 Hz), 138.79, 137.17, 135.86, 128.80, 126.94, 125.06, 123.34(dd, JC—F=6.50, 4.50 Hz), 122.11, 121.38, 118.40(dd, JC—F=123.25, 17.75 Hz), 65.81, 52.79, 31.70 |
| 4da | 6.97—7.70(m, 7H, PhH), 4.66(dd, 1H, NCH, J=11.0, 4.0 Hz), 4.43(s, 1H, NH), 4.22(q, 2H, OCH2, J=7.0 Hz), 3.41(dd, 1H, CH2, J=13.5, 3.5 Hz), 2.87(t, 1H, CH2, J=12.5 Hz), 1.28(t, 3H, CH3, J=7.5 Hz) | 172.53, 163.39, 150.84(dd, JC—F=246.60, 13.25 Hz), 149.65(dd, JC—F=250.00, 13.62 Hz), 138.14, 137.74, 130.53(d, JC—F=8.75 Hz), 128.98, 127.26, 125.06, 124.29(dd, JC—F=6.50, 4.50 Hz), 120.82, 121.47, 118.40(d, JC—F=17.12 Hz), 65.58, 61.87, 35.41, 14.16 |
| 4ea | 6.97—7.68(m, 7H, PhH), 4.65(dd, 1H, NCH, J=11.0, 4.0 Hz), 4.40(s, 1H, NH), 4.22(q, 2H, OCH2, J=7.0 Hz), 3.42(dd, 1H, CH2, J=13.5, 4.0 Hz), 2.84(t, 1H, CH2, J=12.5 Hz) | 172.58, 163.31, 157.84(dd, JC—F=246.60, 13.25 Hz), 156.65(dd, JC—F=250.00, 13.62 Hz), 138.14, 137.78, 129.68(dd, JC—F=14.50, 7.25 Hz), 128.96, 127.24, 121.83, 121.50, 118.10(dd, JC—F=24.62, 9.50 Hz), 117.75(dd, JC—F=26.50, 8.37 Hz), 116.72(dd, JC—F=25.37, 3.62 Hz), 65.66, 61.85, 35.17, 14.17 |
| 4fa | 6.97—7.78(m, 7H, PhH), 4.55(dd, 1H, NCH, J=11.0, 4.0 Hz), 4.35(s, 1H, NH), 4.25(q, 2H, OCH2, J=7.0 Hz), 3.42(dd, 1H, CH2, J=13.5, 4.0 Hz), 2.88(dd, 1H, CH2, J=10.5, 3.0 Hz) | 172.28, 163.24, 150.64(dd, JC—F=246.60, 13.25 Hz), 149.45(dd, JC—F=250.00, 13.62 Hz), 138.80, 137.27, 135.97, 128.84, 126.92, 123.34(dd, JC—F=6.50, 4.50 Hz), 122.05, 121.39, 118.40(dd, JC—F=123.25, 17.75 Hz), 65.89, 62.01, 31.76, 14.24 |
| 5ab | 6.79—7.68(m, 7H, PhH), 5.42—5.45(m, 1H, NCH), 4.92(s, 1H, NH), 4.11(dd, 1H, NCH, J=10.0, 5.0 Hz), 3.67(s, 3H, OCH3), 2.53—2.59(m, 1H, CH2), 2.35—2.40(m, 1H, CH2) | 173.03, 163.27, 150.84(dd, JC—F=246.60, 13.25 Hz), 149.65(dd, JC—F=250.00, 13.62 Hz), 149.51, 128.98, 132.83, 128.72, 124.69(dd, JC—F=6.50, 4.50 Hz), 124.07, 120.86, 120.08, 116.40(d, JC—F=17.12 Hz), 51.73, 50.64, 43.17, 37.05 |
| 5bb | 6.79—7.68(m, 7H, PhH), 5.42—5.45(m, 1H, NCH), 4.92(s, 1H, NH), 4.11(dd, 1H, NCH, J=10.0, 5.0 Hz), 3.67(s, 3H, OCH3), 2.53—2.59(m, 1H, CH2), 2.35—2.40(m, 1H, CH2) | 173.03, 163.27, 150.84(dd, JC—F=246.60, 13.25 Hz), 149.65(dd, JC—F=250.00, 13.62 Hz), 149.51, 128.98, 132.83, 128.72, 124.69(dd, JC—F=6.50, 4.50 Hz), 124.07, 120.86, 120.08, 116.40(d, JC—F=17.12 Hz), 51.73, 50.64, 43.17, 37.05 |
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
| 5cb | 6.78—7.62(m, 7H, PhH), 5.06—5.09(m, 1H, NCH), 4.88(s, 1H, NH), 3.98(dd, 1H, NCH, J=10.0, 5.0 Hz), 3.66(s, 3H, OCH3), 2.43—2.49(m, 1H, CH2), 2.34—2.40(m, 1H, CH2) | 171.26, 150.55(dd, JC—F=244.37, 12.50 Hz), 149.34(dd, JC—F=250.00, 13.62 Hz), 149.18, 141.20, 133.04, 128.83, 123.73(dd, JC—F=6.50, 4.50 Hz), 120.77, 120.03, 119.86, 117.03(dd, JC—F=123.25, 17.75 Hz), 56.33, 51.73, 42.68, 38.14 |
| 5db | 6.81—7.69(m, 7H, PhH), 5.43—5.46(m, 1H, NCH), 4.91(s, 1H, NH), 4.11(dd, 1H, NCH, J=10.0, 5.0 Hz), 4.12(q, 2H, OCH2, J=7.0 Hz), 2.55—2.60(m, 1H, CH2), 2.37—2.42(m, 1H, CH2), 1.23(t, 3H, CH3, J=7.5 Hz) | 170.57, 163.27, 150.64(dd, JC—F=246.60, 13.25 Hz), 149.44(dd, JC—F=250.00, 13.62 Hz), 149.32, 132.94, 132.70, 128.72, 124.67(dd, JC—F=6.50, 4.50 Hz), 124.04, 120.91, 120.26, 120.15, 116.19(d, JC—F=17.12 Hz), 60.91, 50.67, 43.38, 37.18, 13.56 |
| 5eb | 6.79—7.68(m, 7H, PhH), 5.42—5.45(m, 1H, NCH), 4.92(s, 1H, NH), 4.11(dd, 1H, NCH, J=10.0, 5.0 Hz), 4.12(q, 2H, OCH2, J=7.0 Hz), 2.53—2.59(m, 1H, CH2), 2.35—2.40(m, 1H, CH2), 1.23(t, 3H, CH3, J=7.5 Hz) | 173.03, 163.27, 150.64(dd, JC—F=246.60, 13.25 Hz), 149.65(dd, JC—F=250.00, 13.62 Hz), 149.51, 128.88, 132.53, 127.72, 124.59(dd, JC—F=6.50, 4.50 Hz), 124.07, 120.86, 120.28, 116.43(d, JC—F=17.12 Hz), 62.63, 52.64, 42.67, 37.65, 13.79 |
| 5fb | 6.78—7.62(m, 7H, PhH), 5.06—5.09(m, 1H, NCH), 4.88(s, 1H, NH), 3.98(dd, 1H, NCH, J=10.0, 5.0 Hz), 4.12(q, 2H, OCH2, J=7.0 Hz), 2.43—2.49(m, 1H, CH2), 2.34—2.40(m, 1H, CH2), 1.23(t, 3H, CH3, J=7.5 Hz) | 171.26, 150.55(dd, JC—F=244.37, 12.50 Hz), 149.34(dd, JC—F=250.00, 13.62 Hz), 149.18, 141.20, 133.04, 128.83, 123.73(dd, JC—F=6.50, 4.50 Hz), 120.77, 120.03, 119.86, 117.03(dd, JC—F=123.25, 17.75 Hz), 66.30, 51.73, 42.68, 37.14, 13.46 |
| 6aa | 7.10—7.82(m, 7H, PhH), 3.69(t, 2H, SCH2, J=7.0 Hz), 2.93(t, 2H, SCCH2, J=7.0 Hz) | 168.57, 151.86, 150.95(dd, JC—F=247.12, 13.37 Hz), 149.70(dd, JC—F=251.00, 13.87 Hz), 134.84, 129.65, 129.37(d, JC—F=8.75 Hz), 125.53, 125.28, 125.10, 124.28(dd, JC—F=6.50, 4.50 Hz), 123.88, 119.03(d, JC—F=17.12 Hz), 41.25, 33.01 |
| 6ba | 7.10—7.92(m, 7H, PhH), 3.68(t, 2H, SCH2, J=7.0 Hz), 2.94(t, 2H, SCCH2, J=7.0 Hz) | 168.59, 151.79, 150.90(dd, JC—F=247.10, 13.35 Hz), 149.70(dd, JC—F=251.00, 13.87 Hz), 134.83, 129.59, 129.36(d, JC—F=8.75 Hz), 125.51, 125.29, 125.05, 124.22(dd, JC—F=6.50, 4.50 Hz), 123.88, 119.00(d, JC—F=17.12 Hz), 41.05, 32.01 |
| 6ca | 7.10—8.00(m, 7H, PhH), 3.66(t, 2H, SCH2, J=7.0 Hz), 2.96(t, 2H, SCCH2, J=7.0 Hz) | 168.61, 152.33(dd, JC—F=247.12, 13.37 Hz), 150.95(dd, JC—F=247.12, 13.37 Hz), 149.70(dd, JC—F=251.00, 13.87 Hz), 134.84, 129.65, 129.37(d, JC—F=8.75 Hz), 125.53, 125.28, 125.10, 124.28(dd, JC—F=6.50, 4.50 Hz), 123.88, 119.03(d, JC—F=17.12 Hz), 41.53, 29.49 |
| Compd. | C. albicans ATCC10231 | C. Neofonmans | Compd. | C. albicans ATCC10231 | C. Neofonmans | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | ||||||||
| 2a | 9.4 | 24.3 | 24.5 | 2o | 6.0 | 17.3 | 21.2 | ||||
| 2b | 6.0 | 21.4 | 24.0 | 2p | 6.0 | 6.0 | 6.0 | ||||
| 2c | 6.0 | 21.2 | 24.0 | 2q | 6.0 | 6.0 | 6.0 | ||||
| 2d | 6.0 | 22.5 | 20.9 | 2r | 6.0 | 6.0 | 6.0 | ||||
| 2e | 6.0 | 23.4 | 23.3 | 2s | 6.0 | 6.0 | 6.0 | ||||
| 2f | 6.0 | 23.8 | 19.9 | 2t | 6.0 | 15.3 | 15.3 | ||||
| 2g | 6.0 | 6.0 | 6.0 | 2u | 6.0 | 13.8 | 13.7 | ||||
| 2h | 6.0 | 15.3 | 17.8 | 2v | 6.0 | 10.3 | 10.2 | ||||
| 2i | 6.0 | 6.0 | 6.0 | 2w | 6.0 | 6.0 | 6.0 | ||||
| 2j | 6.0 | 13.8 | 13.1 | 2x | 6.0 | 6.0 | 6.0 | ||||
| 2k | 6.0 | 6.0 | 6.0 | 2y | 6.0 | 6.0 | 6.0 | ||||
| 2l | 6.0 | 6.0 | 6.0 | 2z | 6.0 | 6.0 | 6.0 | ||||
| 2m | 6.0 | 6.0 | 6.0 | DMSO | 6.0 | 6.0 | 6.0 | ||||
| 2n | 6.0 | 6.0 | 6.0 | Ab | 6.3 | 22.5 | 22.6 | ||||
Table 5 Zone(mm) of growth inhibition of compounds 2a
| Compd. | C. albicans ATCC10231 | C. Neofonmans | Compd. | C. albicans ATCC10231 | C. Neofonmans | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | ||||||||
| 2a | 9.4 | 24.3 | 24.5 | 2o | 6.0 | 17.3 | 21.2 | ||||
| 2b | 6.0 | 21.4 | 24.0 | 2p | 6.0 | 6.0 | 6.0 | ||||
| 2c | 6.0 | 21.2 | 24.0 | 2q | 6.0 | 6.0 | 6.0 | ||||
| 2d | 6.0 | 22.5 | 20.9 | 2r | 6.0 | 6.0 | 6.0 | ||||
| 2e | 6.0 | 23.4 | 23.3 | 2s | 6.0 | 6.0 | 6.0 | ||||
| 2f | 6.0 | 23.8 | 19.9 | 2t | 6.0 | 15.3 | 15.3 | ||||
| 2g | 6.0 | 6.0 | 6.0 | 2u | 6.0 | 13.8 | 13.7 | ||||
| 2h | 6.0 | 15.3 | 17.8 | 2v | 6.0 | 10.3 | 10.2 | ||||
| 2i | 6.0 | 6.0 | 6.0 | 2w | 6.0 | 6.0 | 6.0 | ||||
| 2j | 6.0 | 13.8 | 13.1 | 2x | 6.0 | 6.0 | 6.0 | ||||
| 2k | 6.0 | 6.0 | 6.0 | 2y | 6.0 | 6.0 | 6.0 | ||||
| 2l | 6.0 | 6.0 | 6.0 | 2z | 6.0 | 6.0 | 6.0 | ||||
| 2m | 6.0 | 6.0 | 6.0 | DMSO | 6.0 | 6.0 | 6.0 | ||||
| 2n | 6.0 | 6.0 | 6.0 | Ab | 6.3 | 22.5 | 22.6 | ||||
| Compd. | MIC | MIC80 | MFC | |||
|---|---|---|---|---|---|---|
| C. Neofonmans | C. Neofonmans | C. Neofonmans | ||||
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | |
| 2a | 1.0 | 0.8 | 2.0 | 2.0 | 10.0 | 9.0 |
| 2b | 1.5 | 1.5 | 2.0 | 2.0 | 12.0 | 10.0 |
| 2c | 4.5 | 3.0 | 2.0 | 2.0 | 17.0 | 16.0 |
| 2d | 2.0 | 2.0 | 1.5 | 1.5 | 12.0 | 12.0 |
| 2e | 3.0 | 3.0 | 1.5 | 1.5 | 12.0 | 10.0 |
| 2f | 5.0 | 4.0 | 2.0 | 2.0 | 15.0 | 16.0 |
| Fluconazole | >128.0 | >128.0 | 2.0 | 3.0 | >128.0 | >128.0 |
Table 6 MIC, MIC80 and MFC values(μg/mL) for compounds 2a—2f and fluconazole
| Compd. | MIC | MIC80 | MFC | |||
|---|---|---|---|---|---|---|
| C. Neofonmans | C. Neofonmans | C. Neofonmans | ||||
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | |
| 2a | 1.0 | 0.8 | 2.0 | 2.0 | 10.0 | 9.0 |
| 2b | 1.5 | 1.5 | 2.0 | 2.0 | 12.0 | 10.0 |
| 2c | 4.5 | 3.0 | 2.0 | 2.0 | 17.0 | 16.0 |
| 2d | 2.0 | 2.0 | 1.5 | 1.5 | 12.0 | 12.0 |
| 2e | 3.0 | 3.0 | 1.5 | 1.5 | 12.0 | 10.0 |
| 2f | 5.0 | 4.0 | 2.0 | 2.0 | 15.0 | 16.0 |
| Fluconazole | >128.0 | >128.0 | 2.0 | 3.0 | >128.0 | >128.0 |
| Compd. | C. Neofonmans | Compd. | C. Neofonmans | ||
|---|---|---|---|---|---|
| ATCC34874 | Clinical isolate | ATCC34874 | Clinical isolate | ||
| 3a—3f | 6.0 | 6.0 | 5a—5f | 6.0 | 6.0 |
| 4a | 13.4 | 13.5 | 6a—6c | 6.0 | 6.0 |
| 4b | 16.3 | 16.5 | Ab | 22.5 | 22.6 |
| 4c—4f | 6.0 | 6.0 | |||
Table 7 Zone(mm) of growth inhibition of compounds 3—6a
| Compd. | C. Neofonmans | Compd. | C. Neofonmans | ||
|---|---|---|---|---|---|
| ATCC34874 | Clinical isolate | ATCC34874 | Clinical isolate | ||
| 3a—3f | 6.0 | 6.0 | 5a—5f | 6.0 | 6.0 |
| 4a | 13.4 | 13.5 | 6a—6c | 6.0 | 6.0 |
| 4b | 16.3 | 16.5 | Ab | 22.5 | 22.6 |
| 4c—4f | 6.0 | 6.0 | |||
| [1] | Wang J., Liu H., Chin. J. Org. Chem., 2011, 31(11), 1785—1798 |
| (王江, 柳红. 有机化学, 2011, 31(11), 1785—1798) | |
| [2] | Qing F. X., Chin. J. Org. Chem., 2012, 32(5), 815—824 |
| (卿凤翔. 有机化学, 2012, 32(5), 815—824) | |
| [3] | Zhang W.S., Li A. L., Medicinal Chemistry, Higher Education Press, Beijing, 1999, 545—553 |
| (仉文升, 李安良. 药物化学, 北京:高等教育出版社, 1999, 545—553) | |
| [4] | Song Y., Wang J. C., Xu H., Du Z. W., Zhang G. Z., Selim H. A., Li G. S., Wang Q., Gao Z. L., Chem. Res. Chinese Universities,2013, 29(2), 263—269 |
| [5] | Chandrasekar P. H., Cutright J., Manavathu E., J. Antimicrob. Chemother., 2000, 45, 673—676 |
| [6] | Laszlo R., Menzel K. A., Bentz K., Schreiner B., Kettering K., Eick C., Schreieck J., Life Sci., 2010, 87, 507—513 |
| [7] | Tang L. J., Zhao G. Y., Huang Z. L., Wang N. N., Guo J. J., Chem. Res. Chinese Universities,2013, 29(2), 214—217 |
| [8] | Hagmann W. K., J. Med. Chem., 2008, 51(15), 4359—4369 |
| [9] | Kirk K., J. Fluorine Chem., 2006, 127, 1013—1029 |
| [10] | Wan K., Zhang Y. Y., Zhou C. H., Zhou X. D., Geng R. X., Ji Q. G., Chin. J. Antibiot., 2012, 37(1), 8—15 |
| (万昆, 张奕奕, 周成合, 周向东, 耿蓉霞, 吉庆刚. 中国抗生素杂志, 2012, 37(1), 8—15) | |
| [11] | Jiang X. L., Cui Y. B., Cao S. H., Chin. J. Antibiot., 2011, 36(4), 255—263 |
| (蒋晓磊, 崔玉彬, 曹胜华. 中国抗生素杂志, 2011, 36(4), 255—263) | |
| [12] | Bariwal J. B., Upadhyay K. D., Manvar A. T., Trivedi J. C., Singh J. S., Jain K. S., Shah A. K., Eur. J. Med. Chem., 2008, 43(11), 2279—2290 |
| [13] | Morton G. C., Salvino J. M., Labaudinie`re R. F., Herpin T. F., Tetrahedron Lett., 2000, 41, 3029—3033 |
| [14] | Nagao T., Sato M., Iwasawa Y., Takada T., Ishida R., Jpn. J. Pharmacol., 1972, 22, 467—478 |
| [15] | Nagao T., Sato M., Nakajima H., Kiyomoto A., Chem. Pharm. Bull., 1973, 21(1), 92—97 |
| [16] | Yamamoto H., Asai H., Chem. Pharm. Bull., 1986, 34(9), 3844—3853 |
| [17] | Asano T., Okumura T., Hirano K., Adachi T., Sugiura M., Chem. Pharm. Bull., 1986, 34(10), 4238—4243 |
| [18] | Kang W., Bu H. J., Li W. H., Li Y., Chem. J. Chinese Universities,2014, 35(4), 766—775 |
| (康旺, 卜辉娟, 李文红, 李媛. 高等学校化学学报, 2014, 35(4), 766—775 | |
| [19] | Dandia A., Singh R., Singh D., Laxkar A., Sivpuri A., Phosphorus Sulfur Silicon Relat. Elem., 2010, 185, 2472—2479 |
| [20] | Yan Y. H., Yang X. J., Wu L. Q., Phosphorus Sulfur Silicon Relat. Elem., 2012, 187, 573—579 |
| [21] | Mor S., Pahal P., Narasimhan B., Eur. J. Med. Chem., 2012, 57, 196—210 |
| [22] | Phippen C. B. W., McErlean C. S. P., Tetrahedron Lett., 2011, 52(13), 1490—1492 |
| [23] | Ayral E., Gloanec P., Bergé G., de Nanteuil G., Mennecier P., Rupin A.,Verbeuren T. J., Fulcrand P., Martinez J., Hernandez J. F., Bioorg. Med. Chem., 2009, 19(5), 1386—1391 |
| [24] | Akbarzadeh R., Amanpour T., Khavasi H. R., Bazgir A., Tetrahedron,2014, 70(2), 169—175 |
| [25] | Kang W., Du X. Q., Wang L. Z., Hu L. J., Dong Y. H., Bian Y. Q., Li Y., Chin. J. Chem., 2013, 31, 1305—1314 |
| [26] | Fan S. L., Zhang B., Gao L. Y., Wang L. Z., Bian Y. Q., Li Y., Chem. J. Chinese Universities,2014, 35(9), 1912—1918 |
| (范世丽, 张博, 高丽叶, 王兰芝, 边艳青, 李媛. 高等学校化学学报, 2014, 35(9), 1912—1918) |
| [1] | ZHAO Yingzhe, ZHANG Jianling. Applications of Metal-organic Framework-based Material in Carbon Dioxide Photocatalytic Conversion [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220223. |
| [2] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [3] | ZUO Huailong, LEI Simin, ZHANG Rui, LI Yuxin, CHEN Wei. Design, Synthesis and Antifungal Activity of Novel Isoquinoline Derivatives [J]. Chem. J. Chinese Universities, 2021, 42(9): 2766. |
| [4] | HOU Hua, WANG Baoshan. Group Additivity Theoretical Model for the Prediction of Dielectric Strengths of the Alternative Gases to SF6 [J]. Chem. J. Chinese Universities, 2021, 42(12): 3709. |
| [5] | YE Xiaodong, QI Guodong, XU Jun, DENG Feng. Glucose Oxidation on Au-supported SBA-15 Molecular Sieve † [J]. Chem. J. Chinese Universities, 2020, 41(5): 960. |
| [6] | CHANG Junpeng,ZHAO Jiarui,CHEN Sijia,MENG Kai,SHI Weini,LI Ruifang. Structure-activity Relationship of Antimicrobial Peptide SAMP1 and Its Analog Peptides† [J]. Chem. J. Chinese Universities, 2019, 40(4): 705. |
| [7] | HE Feng,BAI Jinhai,CHEN Shuxian,TAN Xiaobei. Antifungal Activity and Mechanism of an Essential Oil from Eremothecium ashbyii† [J]. Chem. J. Chinese Universities, 2019, 40(2): 272. |
| [8] | YU Min, HUANG Jingjing, MA Min, FU Ruiyan, YAN Yan, ZHANG Fusheng, YIN Junfeng, XIE Ningning. Zinc Chelating Activity and Quantitative Structure-activity Relationship of Tripeptides† [J]. Chem. J. Chinese Universities, 2018, 39(2): 234. |
| [9] | HOU Hua, YU Xiaojuan, ZHOU Wenjun, LUO Yunbai, WANG Baoshan. Theoretical Investigations on the Structure-activity Relationship to the Dielectric Strength of the Insulation Gases† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2477. |
| [10] | WANG Lei, ZHENG Guojun, JI Qi, CHEN Bo, GONG Longlong, GAO Congmin, DU Zhenjian, ZHANG Xingmin. Synthesis and Biological Activity of Novel PI3K/mTOR Inhibitors† [J]. Chem. J. Chinese Universities, 2017, 38(9): 1590. |
| [11] | LIU Yuming, TIAN Lijun, HU Dong, NIE Jianbing. yntheses and Anti-cholinesterase Activity of 4-N-Phenylaminoquinoline Derivatives † [J]. Chem. J. Chinese Universities, 2017, 38(3): 392. |
| [12] | MU Boshuai, CHANG Suna, BIAN Yanqing, LI Yuan. Asymmetric Synthesis and Antibacterial Activity of Chiral 2-Methoxycarbonyl-4-fluorophenyl-1,5-benzothiazepines† [J]. Chem. J. Chinese Universities, 2017, 38(3): 413. |
| [13] | LIU Benguo, LIU Jiangwei, LI Jiaqi, GENG Sheng, MO Haizhen, LIANG Guizhao. 3D-QSAR and Interaction Mechanism of Flavonoids s P-glycoprotein Inhibitors† [J]. Chem. J. Chinese Universities, 2017, 38(1): 41. |
| [14] | GUO Liang, CAO Rihui, FAN Wenxi, GAN Ziyun, MA Qin. Design, Synthesis and in vitro Antitumor Activities of Novel Bivalent β-Carbolines† [J]. Chem. J. Chinese Universities, 2016, 37(6): 1093. |
| [15] | ZHANG Jie, ZHOU Changjian, XIE Jianwei, DAI Bin. Synthesis and Antitumor Activities of Rhein-Valine Adducts† [J]. Chem. J. Chinese Universities, 2016, 37(12): 2159. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||