

Chem. J. Chinese Universities ›› 2020, Vol. 41 ›› Issue (3): 530.doi: 10.7503/cjcu20190516
• Physical Chemistry • Previous Articles Next Articles
ZHUO Mengning1,LI Fei2,JIANG Hao3,CHEN Qianwen1,LI Peng3,WANG Lizhang1,*
Received:2019-10-10
Online:2020-02-26
Published:2019-12-25
Contact:
Lizhang WANG
Supported by:CLC Number:
TrendMD:
ZHUO Mengning,LI Fei,JIANG Hao,CHEN Qianwen,LI Peng,WANG Lizhang. Preparation of SnO2/GDE Cathodes and Their Electrocatalytic Reduction of CO2 to Produce Formic Acid †[J]. Chem. J. Chinese Universities, 2020, 41(3): 530.
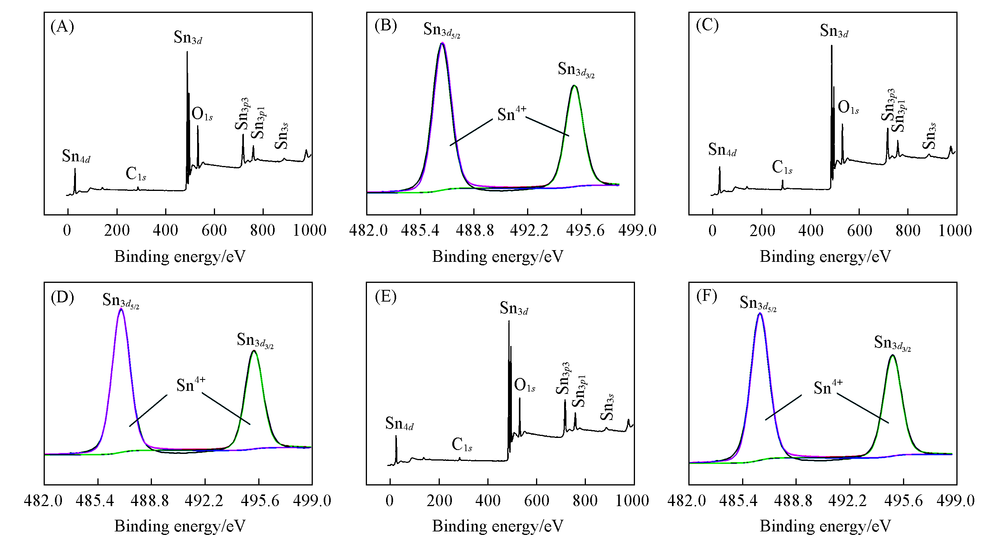
Fig.3 XPS survey(A, C, E) and high resolution spectra of Sn3d(B, D, F) of SnO2-T/GDE (A), (B) SnO2-60/GDE; (C), (D) SnO2-75/GDE; (E), (F) SnO2-100/GDE.
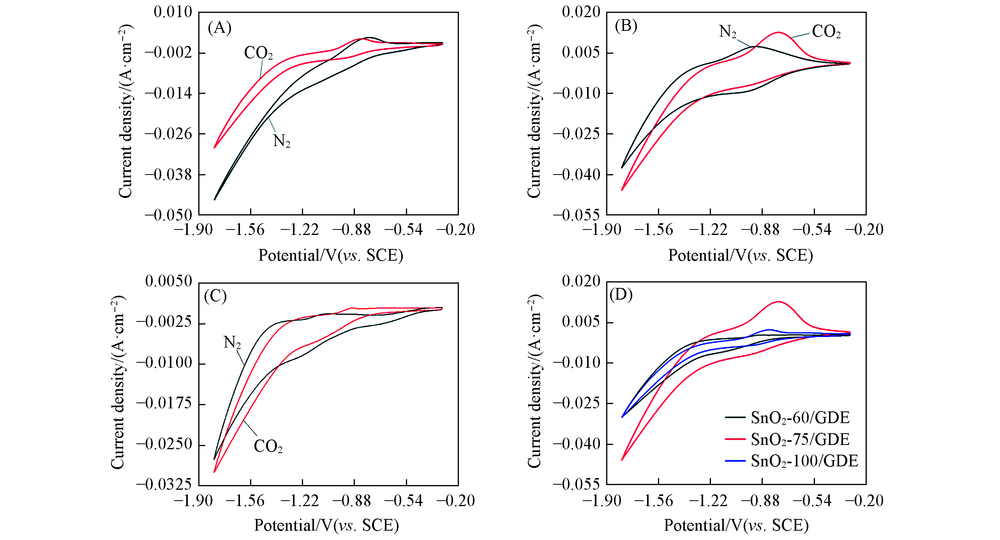
Fig.4 Cyclic voltammetric scan of SnO2-T/GDE in N2 and CO2 environments Scanning rate: 50 mV/s; potential window: -0.3—1.8 V(vs. SCE); electrolyte: 0.1 mol/L KHCO3 solution. (A) SnO2-60/GDE; (B) SnO2-75/GDE; (C) SnO2-100/GDE; (D) SnO2-T/GDE in the CO2 environment.
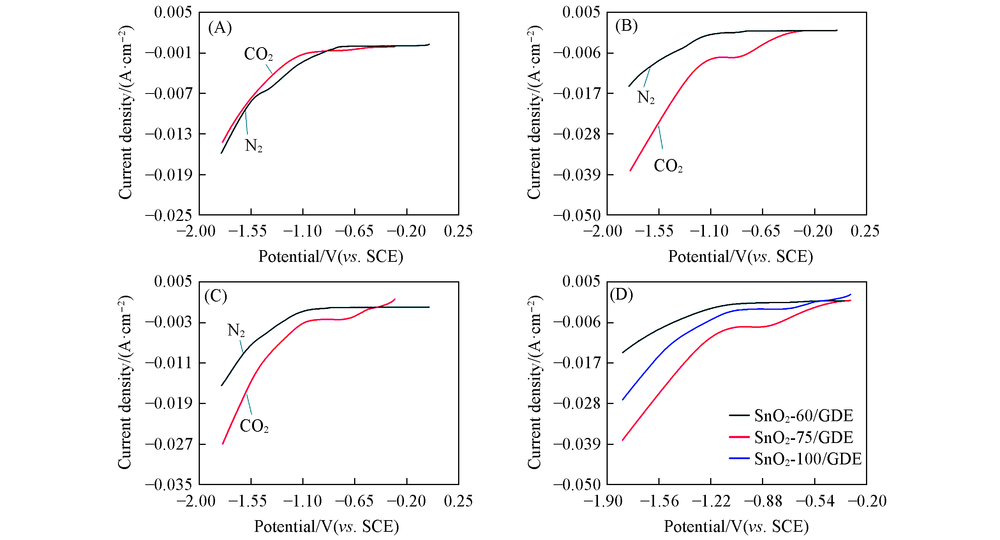
Fig.5 Linear sweep voltammetry of SnO2-T/GDE in N2 and CO2 environments Scanning rate: 50 mV/s; Potential window: -0.3—1.8 V vs. SCE; electrolyte: 0.1 mol/L KHCO3 solution. (A) SnO2-60/GDE; (B) SnO2-75/GDE; (C) SnO2-100/GDE; (D) SnO2-T/GDE in the CO2 environment.
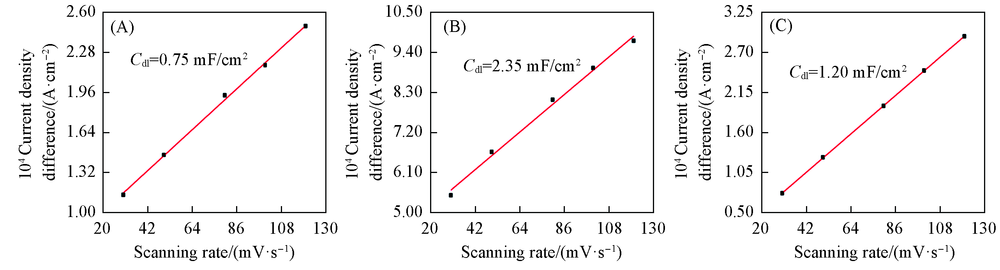
Fig.6 Linear relationship between current density difference and scan rate of SnO2-T/GDE at -0.4 V(vs. SCE) Potential window: -0.3—-0.5 V(vs. SCE); electrolyte: N2 satarated 0.1 mol/L KHCO3 solution. (A) SnO2-60/GDE; (B) SnO2-75/GDE; (C) SnO2-100/GDE.
| Electrode | Rs/(Ω·cm2) | Rct/(Ω·cm2) | Error(%) |
|---|---|---|---|
| SnO2-60/GDE | 4.8 | 8.5 | 1.6 |
| SnO2-75/GDE | 4.8 | 3.9 | 1.3 |
| SnO2-100/GDE | 4.5 | 6.6 | 1.5 |
| Electrode | Rs/(Ω·cm2) | Rct/(Ω·cm2) | Error(%) |
|---|---|---|---|
| SnO2-60/GDE | 4.8 | 8.5 | 1.6 |
| SnO2-75/GDE | 4.8 | 3.9 | 1.3 |
| SnO2-100/GDE | 4.5 | 6.6 | 1.5 |
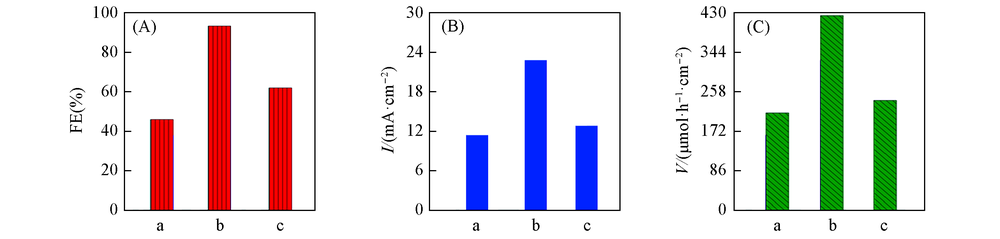
Fig.8 Faradic efficiency(A), current density(B) and production yield(C) of formic acid in CO2 saturared 0.1 mol/L KHCO3 solution over SnO2-T/GDE at -1.8 V(vs. SCE) a. SnO2-60/GDE; b. SnO2-75/GDE; c. SnO2-100/GDE.
| Preparation condition | Electrolysis potential/V | Electrolysis time/h | FE(%) | Current density/(mA·cm-2) | Ref. |
|---|---|---|---|---|---|
| 180 ℃, 24 h | -1.7(vs. SHE) a | 1 | 62 | 12.5 | [ |
| 120 ℃, 6 h | -1.8(vs. SCE) a | 1 | 60 | 9 | [ |
| 100 ℃, 8 h | -1.5(vs. SHE) a | 1 | 87.1 | 10 | [ |
| 60 ℃, 10 h | -1.8(vs. SCE) | 1.21 b | 46.5 | 11.4 | This work |
| 75 ℃, 10 h | -1.8(vs. SCE) | 1.76 b | 93.5 | 22.8 | This work |
| 100 ℃, 10 h | -1.8(vs. SCE) | 1.57 b | 62.1 | 12.8 | This work |
| Preparation condition | Electrolysis potential/V | Electrolysis time/h | FE(%) | Current density/(mA·cm-2) | Ref. |
|---|---|---|---|---|---|
| 180 ℃, 24 h | -1.7(vs. SHE) a | 1 | 62 | 12.5 | [ |
| 120 ℃, 6 h | -1.8(vs. SCE) a | 1 | 60 | 9 | [ |
| 100 ℃, 8 h | -1.5(vs. SHE) a | 1 | 87.1 | 10 | [ |
| 60 ℃, 10 h | -1.8(vs. SCE) | 1.21 b | 46.5 | 11.4 | This work |
| 75 ℃, 10 h | -1.8(vs. SCE) | 1.76 b | 93.5 | 22.8 | This work |
| 100 ℃, 10 h | -1.8(vs. SCE) | 1.57 b | 62.1 | 12.8 | This work |
| [1] | Koo Y., Malik R., Alvarez N., White L., Shanov V N., Schulz M., Collins B., Sankar J., Yun Y ., RSC Adv., 2014, 4( 31), 16362— 16367 |
| [2] | Zhang Q., Xu W. T., Liu Y. Y., Zhang J. J ., Chin. J. Nat., 2017, 39( 4), 242— 250 |
| ( 张琪, 许武韬, 刘予宇, 张久俊 . 自然杂志, 2017, 39( 4), 242— 250) | |
| [3] | Zhang S., Kang P., Meyer T. J ., J. Am. Chem. Soc., 2014, 136( 5), 1734— 1737 |
| [4] | Reis A., Mert S ., Int. J. Hydrog. Energy, 2015, 40( 37), 12776— 12783 |
| [5] | Lu X., Leung D., Wang H., Leung M., Xuan J ., ChemElectroChem, 2014, 1( 5), 836— 849 |
| [6] | Bashir S., Hossain M., Hossain S., Rahman S., Ahmed S., Al-Ahmed A ., J. CO2 Util., 2016, 16, 346— 353 |
| [7] | Scialdone O., Galia A., Nero G. L., Proietto F., Sabatino S., Schiavo B ., Electrochim. Acta, 2016, 199( 199), 331— 332 |
| [8] | Lv W., Zhang R., Gao P., Lei L ., J. Power Sources, 2014, 253, 276— 281 |
| [9] | Asadi M., Kumar B., Behranginia A., Rosen B., Baskin A., Repnin N., Pisasale D., Phillips P., Zhu W., Haasch R., Klie R F., Král P., Abiade J., Salehi-Khojin A ., Nat. Commun., 2014, 5, 4470 |
| [10] | Kim D., Resasco J., Yu Y., Asiri A., Yang P ., Nat. Commun., 2014, 5, 5948 |
| [11] | Li Q., Rao X., Sheng J., Xu J., Yi J., Liu Y., Zhang J ., J. CO2 Util., 2018, 27, 48— 59 |
| [12] | Gao D., Zhou H., Wang J., Miao S., Yang F., Wang G., Wang J., Bao X ., J. Am. Chem. Soc., 2015, 137( 13), 4288— 4291 |
| [13] | Xie J., Huang Y., Yu H ., Front. Environ. Sci. Eng., 2015, 9( 5), 861— 866 |
| [14] | Jing Z. G., Fan G., Zhang D. F ., Southern Metals, 2007, 5, 9—11, 17 |
| ( 荆忠国, 樊刚, 张德丰 . 南方金属, 2007, 5, 9—11, 17) | |
| [15] | Vemury S., Pratsinis S., Kibbey L ., J. Mater. Res., 1997, 12( 4), 1031— 1042 |
| [16] | Pan Q. Y., Dong X. W., Zhang J. P ., J. Inorg. Mater., 1997, 12( 4), 494— 498 |
| ( 潘庆谊, 董晓雯, 张剑平 . 无机材料学报, 1997, 12( 4), 494— 498) | |
| [17] | Wang D. X., Zhong J. M., Sun B. S ., Chin. J. Inorga. Chem., 2008, 24( 6), 892— 896 |
| ( 王东新, 钟景明, 孙本双 . 无机化学学报, 2008, 24( 6), 892— 896) | |
| [18] | Zhang J. R., Gao L ., Acta Chimica Sinica, 2003, 12, 1965—1968 |
| ( 张建荣, 高濂 . 化学学报, 2003, 12, 1965—1968) | |
| [19] | Cheng H. M., Ma J. M ., Chem. J. Chinese Universities, 1996, 17( 6), 833— 83 |
| ( 程虎民, 马季铭 . 高等学校化学学报, 1996, 17( 6), 833— 83) | |
| [20] | Hu X. Y., Wang N., Hao Y. T., Xu Z. Q., Wang M. H., Shi G. Q., Yang H. M., Liang Z. H ., Chem. J. Chinese Universities, 2018, 39( 10), 2265— 2271 |
| ( 胡雪艳, 王娜, 郝玉婷, 许志庆, 王明慧, 师改琴, 杨慧敏, 梁镇海 . 高等学校化学学报, 2018, 39( 10), 2265— 2271) | |
| [21] | Fu Y., Li Y., Zhang X., Qiao J ., Chin. J. Cataly, 2016, 7( 37), 1081— 1088 |
| [22] | Li H., Oloman C ., J. Appli. Electrochem., 2005, 35( 10), 955— 965 |
| [23] | Fan M., Bai Z., Zhang Q ., RSC Adv., 2014, 4( 84), 44583— 44591 |
| [24] | Li Y., Qiao J., Zhang X., Lei T., Girma A., Liu Y., Zhang J ., ChemElectroChem, 2016, 3( 10), 1618— 1628 |
| [25] | Zhang Z. N ., J. Zhejiang Univ. Technol., 2002, 1, 33— 37 |
| ( 张泽南 . 浙江工业大学学报, 2002, 1, 33— 37) | |
| [26] | Wang L., Du J., Xing G. J., Xiong Y. H., Yuan P., Yu X. Y., ., Proceedings of the Twelfth Chinese Conference on Solid State Ionology, 2004, 543— 545 |
| ( 王磊, 杜军, 刑光健, 熊玉华, 苑鹏, 尉秀英 . 第十二届中国固态离子学学术会议论文集, 2004, 543— 545) | |
| [27] | Wu J. F., Zhao N., Xu X. H ., China Conference on Functional Materials and Applications, 2007, 81— 85 |
| ( 吴建锋, 赵娜, 徐晓虹 . 中国功能材料及其应用学术会议, 2007, 81— 85) | |
| [28] | Zhang J., Ma Z., Jiang W., Zou Y., Wang Y., Lu C ., J. Electroanaly. Chem., 2016, 767, 49— 55 |
| [29] | Wu J., Risalvato F., Ma S., Zhou X ., J. Mater. Chem. A, 2014, 2( 6), 1647— 1651 |
| [30] | Li C. W., Kanan M ., J. Am. Chem. Soc., 2012, 134( 17), 7231— 7234 |
| [31] | Popczyk M., Serek A., Budniok A ., Nanotechnology, 2003, 14( 2), 341— 346 |
| [32] | Chen W. M., Sun G. Q., Zhao X. S., Sun P. C., Yang S. H., Xin Q ., Chem. J. Chinese Universities, 2007, 28( 5), 928— 931 |
| ( 陈维民, 孙公权, 赵新生, 孙丕昌, 杨少华, 辛勤 . 高等学校化学学报, 2007, 28( 5), 928— 931) | |
| [33] | Zhang H ., Study on the Electrocatalytic Reduction of CO2 to Formic Acid with SnO2 and Bi-Based Catalysts, Central China Normal University, Wuhan, 2014 |
| ( 张慧 . SnO2和Bi基催化剂电催化还原CO2至甲酸的研究, 武汉: 华中师范大学, 2014) | |
| [34] | Wang Q., Dong H., Yu H ., J. Power Sources, 2014, 271, 278— 284 |
| [35] | Li H., Oloman C ., J. Appl. Electrochem., 2006, 36( 10), 1105— 1115 |
| [36] | Zhang R., Lv W., Lei L ., Appl. Surf. Sci., 2015, 356, 24— 29 |
| [37] | Zhang B., Sun L., Wang Y., Chen S., Zhang J ., J. Energy Chem., 2020, 41, 7— 14 |
| [1] | YANG Jingyi, SHI Siqi, PENG Huaitao, YANG Qihao, CHEN Liang. Integration of Atomically Dispersed Ga Sites with C3N4 Nanosheets for Efficient Photo-driven CO2 Cycloaddition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220349. |
| [2] | WANG Xintian, LI Pan, CAO Yue, HONG Wenhao, GENG Zhongxuan, AN Zhiyang, WANG Haoyu, WANG Hua, SUN Bin, ZHU Wenlei, ZHOU Yang. Techno-economic Analysis and Industrial Application Prospects of Single-atom Materials in CO2 Catalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220347. |
| [3] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [4] | DING Yang, WANG Wanhui, BAO Ming. Recent Progress in Porous Framework-immobilized Molecular Catalysts for CO2 Hydrogenation to Formic Acid [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220309. |
| [5] | WANG Ruhan, JIA Shunhan, WU Limin, SUN Xiaofu, HAN Buxing. CO2-involved Electrochemical C—N Coupling into Value-added Chemicals [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220395. |
| [6] | ZHAO Yingzhe, ZHANG Jianling. Applications of Metal-organic Framework-based Material in Carbon Dioxide Photocatalytic Conversion [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220223. |
| [7] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [8] | ZHANG Xinxin, XU Di, WANG Yanqiu, HONG Xinlin, LIU Guoliang, YANG Hengquan. Effect of Mn Promoter on CuFe-based Catalysts for CO2 Hydrogenation to Higher Alcohols [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220187. |
| [9] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [10] | XIA Wu, REN Yingyi, LIU Jing, WANG Feng. Chitosan Encapsulated CdSe QDs Assemblies for Visible Light-induced CO2 Reduction in an Aqueous Solution [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220192. |
| [11] | WANG Zhengwen, GAO Fengxiang, CAO Han, LIU Shunjie, WANG Xianhong, WANG Fosong. Synthesis and Property of CO2 Copolymer⁃based UV-curable Polymer [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220236. |
| [12] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| [13] | ZHANG Xiaoyu, XUE Dongping, DU Yu, JIANG Su, WEI Yifan, YAN Wenfu, XIA Huicong, ZHANG Jianan. MOF-derived Carbon-based Electrocatalysts Confinement Catalyst on O2 Reduction and CO2 Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210689. |
| [14] | ZHOU Ying, HE Peinan, FENG Haisong, ZHANG Xin. Optimal Distribution of Active Sites of CO2 Reduction Reaction Catalyzed by Diatomic Site M-N-C [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210640. |
| [15] | ZHANG Xiang, XIE Xulan, XIONG Likun, PENG Yang. Urchin-like Gold Nanoneedle for Efficient Electrocatalytic CO2 Reduction [J]. Chem. J. Chinese Universities, 2021, 42(9): 2824. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||