

Chem. J. Chinese Universities ›› 2020, Vol. 41 ›› Issue (12): 2749.doi: 10.7503/cjcu20200563
• Organic Chemistry • Previous Articles Next Articles
LIN Junjie, WANG Shuang, LI Weiqiang, CUI Xin, HUANG Chao( )
)
Received:2020-08-14
Online:2020-12-10
Published:2020-12-09
Contact:
HUANG Chao
E-mail:huangchao@ynu.edu.cn
Supported by:CLC Number:
TrendMD:
LIN Junjie, WANG Shuang, LI Weiqiang, CUI Xin, HUANG Chao. Efficient Synthesis of Pyridine [2,3-d]pyrimidine Derivatives by Catalyst-free Tandem Cyclization Under Microwave Irradiation[J]. Chem. J. Chinese Universities, 2020, 41(12): 2749.
| Compd. | Appearance | Yield*(%) | m. p./℃(ref.) | HRMS, m/z[M+H]+ | IR(KBr), |
|---|---|---|---|---|---|
| 3a | White solid | 88 | 245—246(262—264)[ | 344.1398 | 3067, 2906, 1705, 1663, 1555, 1420, 1363, 1004, 771, 701 |
| 3b | White solid | 85 | 220—223 | 358.1557 | 3327, 2988, 1658, 1550, 1364, 1092, 821, 777, 698 |
| 3c | White solid | 83 | 220—222 | 358.1555 | 3335, 2978, 1701, 1658, 1553, 1364, 1121, 747, 609 |
| 3d | Yellow solid | 85 | 260—262 | 371.1711 | 3376, 2977, 1702, 1655, 1553, 1417, 1095, 747, 610 |
| 3e | White solid | 79 | 231—233 | 358.1555 | 3284, 2980, 1704, 1657, 1364, 1095, 699, 608 |
| 3f | White solid | 78 | 232—234 | 358.1557 | 3384, 2987, 1605, 1690, 1655, 1369, 1193, 812, 748 |
| 3g | White solid | 81 | 214—216(217—219)[ | 372.1713 | 3379, 2988, 1705, 1658, 1655, 1363, 1093, 802, 749 |
| 3h | White solid | 78 | 253—254 | 374.1504 | 3356, 2976, 1655, 1551, 1416, 1367, 1243, 1178, 608, 569 |
| 3i | White solid | 82 | 201—203 | 388.1661 | 3357, 2989, 1706, 1678, 1261, 1083, 800, 608 |
| 3j | White solid | 81 | 228—231 | 404.1609 | 3354, 2968, 1704, 1654, 1600, 1089, 999, 787, 609 |
| 3k | White solid | 80 | 241—243(259—260)[ | 388.1661 | 3368, 2879, 1706, 1674, 1603, 1089, 998, 786, 605 |
| 3l | White solid | 88 | 241—243 | 362.1306 | 3389, 2989, 1708, 1661, 1550, 1361, 1159, 1001, 608, 562 |
| 3m | White solid | 85 | 251—253 | 378.1008 | 3354, 2968, 1655, 1567, 1365, 1094, 752, 609 |
| 3n | White solid | 85 | 231—233 | 422.0505 | 3375, 2968, 1655, 1549, 1360, 1112, 1001, 751, 609 |
| 3o | White solid | 84 | 235—237 | 376.1463 | 3389, 2988, 1666, 1567, 1546, 1153, 1096, 840, 799, 607 |
| 3p | White solid | 84 | 232—233(238—239)[ | 392.1167 | 3387, 2999, 1701, 1667, 1590, 1545, 1366, 1091, 799, 610 |
| 3q | White solid | 82 | 230—232 | 436.0662 | 3375, 2976, 1705, 1592, 1543, 1361, 1086, 1003, 823, 750, 609 |
| 3r | Yellow solid | 79 | 240—242 | 389.1252 | 3369, 2968, 1706, 1670, 1550, 1342, 1109, 521, 694, 609 |
| 3s | White solid | 77 | 214—215 | 369.1351 | 3378, 2899, 2025, 1704, 1360, 1120, 608, 536 |
| 3t | White solid | 78 | 215—217 | 360.1349 | 3397, 3066, 2948, 1710, 1670, 1551, 1367, 1224, 770, 746 |
| 3u | White solid | 84 | 220—222 | 383.1509 | 3389, 2967, 1633, 1556, 1367, 1128, 999, 671 |
| 3v | Yellow solid | 80 | 201—202 | 394.1555 | 3398, 2977, 1705, 1663, 1552, 1348, 1090, 767, 609 |
| 3w | Purple solid | 81 | 238—240 | 350.0964 | 3386, 2988, 1709, 1656, 1415, 1097, 730, 607 |
| 3x | Yellow solid | 81 | 217—218(213—215)[ | 350.0965 | 3389, 2977, 2025, 1656, 1424, 1094, 800, 608 |
| 5a | White solid | 70 | 165—166(171—178)[ | 268.1083 | 3367, 2980, 1705, 1655, 1122, 608 |
| 5b | White solid | 73 | 184—186 | 282.1235 | 3358, 2966, 1706, 1657, 1551, 1416, 1375, 754, 697, 550 |
| 5c | White solid | 71 | 186—188 | 296.1941 | 3378, 2980, 2025, 1702, 1667, 2593, 1419, 753, 608 |
| 5d | White solid | 78 | 185—186(180—182)[ | 298.1193 | 3301, 3129, 2987, 1626, 1504, 1155, 1063, 754, 539 |
| Compd. | Appearance | Yield*(%) | m. p./℃(ref.) | HRMS, m/z[M+H]+ | IR(KBr), |
|---|---|---|---|---|---|
| 3a | White solid | 88 | 245—246(262—264)[ | 344.1398 | 3067, 2906, 1705, 1663, 1555, 1420, 1363, 1004, 771, 701 |
| 3b | White solid | 85 | 220—223 | 358.1557 | 3327, 2988, 1658, 1550, 1364, 1092, 821, 777, 698 |
| 3c | White solid | 83 | 220—222 | 358.1555 | 3335, 2978, 1701, 1658, 1553, 1364, 1121, 747, 609 |
| 3d | Yellow solid | 85 | 260—262 | 371.1711 | 3376, 2977, 1702, 1655, 1553, 1417, 1095, 747, 610 |
| 3e | White solid | 79 | 231—233 | 358.1555 | 3284, 2980, 1704, 1657, 1364, 1095, 699, 608 |
| 3f | White solid | 78 | 232—234 | 358.1557 | 3384, 2987, 1605, 1690, 1655, 1369, 1193, 812, 748 |
| 3g | White solid | 81 | 214—216(217—219)[ | 372.1713 | 3379, 2988, 1705, 1658, 1655, 1363, 1093, 802, 749 |
| 3h | White solid | 78 | 253—254 | 374.1504 | 3356, 2976, 1655, 1551, 1416, 1367, 1243, 1178, 608, 569 |
| 3i | White solid | 82 | 201—203 | 388.1661 | 3357, 2989, 1706, 1678, 1261, 1083, 800, 608 |
| 3j | White solid | 81 | 228—231 | 404.1609 | 3354, 2968, 1704, 1654, 1600, 1089, 999, 787, 609 |
| 3k | White solid | 80 | 241—243(259—260)[ | 388.1661 | 3368, 2879, 1706, 1674, 1603, 1089, 998, 786, 605 |
| 3l | White solid | 88 | 241—243 | 362.1306 | 3389, 2989, 1708, 1661, 1550, 1361, 1159, 1001, 608, 562 |
| 3m | White solid | 85 | 251—253 | 378.1008 | 3354, 2968, 1655, 1567, 1365, 1094, 752, 609 |
| 3n | White solid | 85 | 231—233 | 422.0505 | 3375, 2968, 1655, 1549, 1360, 1112, 1001, 751, 609 |
| 3o | White solid | 84 | 235—237 | 376.1463 | 3389, 2988, 1666, 1567, 1546, 1153, 1096, 840, 799, 607 |
| 3p | White solid | 84 | 232—233(238—239)[ | 392.1167 | 3387, 2999, 1701, 1667, 1590, 1545, 1366, 1091, 799, 610 |
| 3q | White solid | 82 | 230—232 | 436.0662 | 3375, 2976, 1705, 1592, 1543, 1361, 1086, 1003, 823, 750, 609 |
| 3r | Yellow solid | 79 | 240—242 | 389.1252 | 3369, 2968, 1706, 1670, 1550, 1342, 1109, 521, 694, 609 |
| 3s | White solid | 77 | 214—215 | 369.1351 | 3378, 2899, 2025, 1704, 1360, 1120, 608, 536 |
| 3t | White solid | 78 | 215—217 | 360.1349 | 3397, 3066, 2948, 1710, 1670, 1551, 1367, 1224, 770, 746 |
| 3u | White solid | 84 | 220—222 | 383.1509 | 3389, 2967, 1633, 1556, 1367, 1128, 999, 671 |
| 3v | Yellow solid | 80 | 201—202 | 394.1555 | 3398, 2977, 1705, 1663, 1552, 1348, 1090, 767, 609 |
| 3w | Purple solid | 81 | 238—240 | 350.0964 | 3386, 2988, 1709, 1656, 1415, 1097, 730, 607 |
| 3x | Yellow solid | 81 | 217—218(213—215)[ | 350.0965 | 3389, 2977, 2025, 1656, 1424, 1094, 800, 608 |
| 5a | White solid | 70 | 165—166(171—178)[ | 268.1083 | 3367, 2980, 1705, 1655, 1122, 608 |
| 5b | White solid | 73 | 184—186 | 282.1235 | 3358, 2966, 1706, 1657, 1551, 1416, 1375, 754, 697, 550 |
| 5c | White solid | 71 | 186—188 | 296.1941 | 3378, 2980, 2025, 1702, 1667, 2593, 1419, 753, 608 |
| 5d | White solid | 78 | 185—186(180—182)[ | 298.1193 | 3301, 3129, 2987, 1626, 1504, 1155, 1063, 754, 539 |
| Compd. | 1H NMR(400 MHz), δ | 13C NMR(100 MHz), δ |
|---|---|---|
| 3a | 8.13—8.15(m, 2H), 7.52—7.50(m, 3H), 7.48—7.46(m, 4H), 7.37—7.33(m, 2H), 3.89(s, 3H), 3.39(s, 3H) | 159.5, 158.2, 154.2, 150.8, 150.6, 138.6, 136.2, 129.7, 127.9, 127.2, 126.8, 126.5, 117.4, 105.5, 29.1, 27.4 |
3b | 8.25—8.28(s, 1H), 7.57—7.50(m, 3H), 7.30—7.28(m, 2H), 7.24—7.22(m, 2H), 3.72(s, 3H), 3.19(s, 3H), 2.38(s, 3H) | 159.8, 157.7, 154.4, 151.5, 151.0, 137.2, 136.8, 136.4, 130.7, 129.0, 128.3, 128.0, 127.5, 117.8, 106.4, 99.5, 29.8, 28.0, 20.9 |
| 3c | 8.18—8.16(s, 1H), 7.57(s, 1H), 7.43—7.33(m, 7H), 3.72(s, 3H), 3.19(s, 3H), 2.38(s, 3H) | 159.5, 158.2, 154.1, 150.8, 150.6, 140.2, 138.7, 133.5, 128.7, 127.1, 126.8, 126.5, 117.0, 105.6, 29.1, 27.4, 20.4 |
3d | 7.90—7,87 (m, 2H), 7.48—7.44(m, 4H), 7.36—7.34(m, 2H), 7.27(s, 1H), 3.89(s, 3H), 3.39(s, 3H), 2.37—2.34(m, 6H) | 159.6, 158.4, 154.0, 150.7, 150.6, 138.9, 138.7, 136.2, 133.9, 129.3, 127.6, 127.1, 126.8, 126.8, 124.1, 117.1, 105.2, 29.1, 27.4, 19.0, 18.8 |
3e | 8.23—8.26(m, 3H), 7.58(d,J=8.0 Hz, 1H), 7.52—7.53(m, 3H), 7.29—7.27(m, 2H), 3.71(s, 3H), 3.19(s, 3H), 2.38(s, 3H) | 159.5, 158.1, 154.4, 150.8, 150.6, 137.1, 136.3, 135.6, 129.7, 127.9, 127.6, 126.8, 126.5, 117.5, 105.6, 29.1, 27.4, 20.0 |
3f | 8.15—8.13(m, 2H), 7.52—7.50(m, 3H), 7.47(s, 1H), 7.30—7.24(m, 3H), 7.24(s, 1H), 3.89(s, 3H), 3.40(s, 3H), 2.44(s, 3H) | 159.5, 158.1, 154.4, 150.8, 150.6, 137.1, 136.3, 135.6, 129.7, 127.9, 127.6, 126.8, 126.5, 117.5, 105.6, 29.1, 27.4, 20.4 |
3g | 8.02—8.04(m, 2H), 7.44(s, 1H), 7.32—7.27(m, 5H), 7.25(d, J=6.1 Hz, 1H), 3.88(s, 3H), 3.39(s, 3H), 2.44(d, J=3.8 Hz, 6H) | 159.6, 158.1, 154.2, 150.8, 150.6, 140.1, 137.0, 135.7, 133.5, 128.7, 127.8, 126.8, 126.4, 117.1, 105.3, 29.1, 27.4, 20.4, 20.4 |
3h | 8.26(d, J=9.0 Hz, 2H), 7.56(s, 1H), 7.43—7.41(m, 5H), 7.08(d, J=9.0 Hz, 2H), 3.84(s, 3H), 3.73(s, 3H), 3.19(s, 3H) | 160.9, 159.5, 157.8, 154.0 150.8, 150.6, 138.7, 128.7, 128.1, 127.1, 126.8, 126.8, 116.5, 113.3, 104.8, 54.4, 29.1, 27.4 |
3i | 8.04(d, J=8.2 Hz, 2H), 7.43(s, 1H), 7.31(d, J=8.7 Hz, 4H), 7.00(d, J=8.8 Hz, 2H), 3.88(d, J=0.5 Hz, 6H), 3.40(s, 3H), 2.43(s, 3H) | 159.7, 158.7, 158.1, 153.9, 150.9, 150.6, 140.1, 133.6, 130.7, 128.8, 128.4, 126.4, 117.3, 112.3, 105.2, 54.3, 29.1, 27.4, 20.4 |
3j | 8.10(d, J=8.9 Hz, 2H), 8.10(d, J=8.9 Hz, 2H), 7.38(s, 1H), 7.30(d, J=8.7 Hz, 2H), 7.00(m, 4H), 3.88—3.86(m, 8H), 3.39(s, 3H) | 160.9, 159.6, 158.7, 157.6, 153.8, 150.9, 150.6, 130.8, 128.8, 128.4, 128.0, 116.8, 113.3, 112.3, 104.8, 54.4, 54.3, 29.1, 27.4 |
3k | 8.10(d, J=8.9 Hz, 2H), 7.39(s, 1H), 7.28(d, J=8.1 Hz, 2H), 7.25(m, 2H), 7.01(d, J=8.9 Hz, 2H), 3.88(d, J=7.4 Hz, 6H), 3.39(s, 3H), 2.44(s, 3H) | 160.9, 159.6, 157.7, 154.1, 150.8, 150.6, 137.0, 135.8, 128.8, 128.0, 127.6, 126.8, 116.6, 113.3, 104.9, 54.4, 29.1, 27.4, 20.4 |
3l | 8.17—8.13(m, 2H), 7.49—7.48(m, 3H), 7.42(s, 1H), 7.34(m, 2H), 7.19(t, J=8.7 Hz, 2H), 3.88(s, 3H), 3.39(s, 3H) | 164.8, 162.3, 159.4, 157.1, 154.4, 150.8, 150.5, 138.5, 132.4, 132.4, 128.6, 128.5, 127.2, 126.9, 126.7, 117.0, 115.1, 114.9, 105.5, 29.1, 27.4 |
| 3m | 8.09—8.07(m, 2H), 7.48—7.47(m, 5H), 7.46—7.44(s, 1H), 7.35—7.33(m, 2H), 3.87(s, 3H), 3.39(s, 3H) | 159.4 156.9, 154.5, 150.8, 150.5, 138.4, 136.0, 134.7, 128.2, 127.8, 127.3, 126.9, 126.7, 117.1, 105.7, 29.2, 27.5 |
3n | 8.02—8.00(m, 2H), 7.65—7.63(m, 2H), 7.48—7.47(m, 3H), 7.44(s, 1H), 7.35—7.33(m, 2H), 3.87(s, 3H), 3.39(s, 3H) | 159.4, 157.0, 154.5, 150.8, 150.5, 138.4, 135.1, 131.2, 128.0, 127.3, 126.7, 126.7, 124.5, 117.1, 105.8, 29.2, 27.5 |
3o | 8.16—8.12(m, 2H), 7.41(s, 1H), 7.28(d, J=8.1 Hz, 2H), 7.23—7.17(m, 4H), 3.87(s, 3H), 3.39(s, 3H), 2.44(s, 3H) | 164.7, 162.2, 159.5, 157.0, 154.6, 150.8, 150.5, 137.2, 135.5, 132.5, 132.5, 128.6, 128.5, 127.6, 126.8, 117.1, 115.1, 114.9, 105.5, 29.1, 27.4, 20.4 |
| Compd. | 1H NMR(400 MHz), δ | 13C NMR(100 MHz), δ |
3p | 8.07(d, J=8.7 Hz, 2H), 7.47(d, J=8.7 Hz, 2H), 7.43(s, 1H), 7.28(d, J=8.0 Hz, 2H), 7.25—7.25(m, 2H), 3.87(s, 3H), 3.39(s, 3H), 2.44(s, 3H) | 159.4, 156.8, 154.6, 150.9, 150.5, 137.2, 136.0, 135.4, 134.7, 128.2, 127.7, 127.6, 126.8, 117.2, 105.8, 29.2, 27.5, 20.4 |
3q | 8.00(d, J=8.7 Hz, 2H), 7.63(d, J=8.7 Hz, 2H), 7.43(s, 1H), 7.28(d, J=8.0 Hz, 2H), 7.25—7.23(m, 2H), 3.87(s, 3H), 3.39(s, 3H), 2.44(s, 3H) | 159.4, 156.9, 154.7, 150.9, 150.5, 137.2, 135.4, 135.2, 131.1, 128.0, 127.6, 126.8, 124.4, 117.2, 105.8, 29.2, 27.5, 20.4 |
| 3r | 8.55(d, J=9.0 Hz, 2H), 8.34(d, J=9.0 Hz, 2H), 7.79(s, 1H), 7.45—7.41(m, 5H), 3.74(s, 3H), 3.20(s, 3H) | 159.6, 155.3, 154.8, 151.6, 150.9, 148.5, 142.6, 139.1, 128.8, 128.3, 128.0, 127.5, 124.0, 119.0, 107.5, 29.9, 28.1 |
3s | 8.26—8.24(m, 2H), 7.81—7.79(m, 2H), 7.50—7.46(m, 4H), 7.35—7.35(m, 2H), 3.88(s, 3H), 3.39(s, 3H) | 159.2, 155.8, 154.9, 150.9, 150.3, 140.3, 138.1, 131.7, 127.5, 127.0, 127.0, 126.7, 117.8, 117.4, 113.0, 106.5, 29.2, 27.5 |
3t | 13.20(s, 1H), 7.85—7.82(m, 1H), 7.57(s, 1H), 7.52—7.47(m, 1H), 7.44—7.38(m, 1H), 7.35(s, 1H), 7.07—7.05(m, 1H), 6.97—6.91(m, 1H), 3.82(s, 3H), 3.39(s, 3H) | 159.2, 158.7, 154.8, 140.0, 139.4, 138.0, 132.6, 127.5, 127.0, 126.6, 118.6 117.8, 116.8, 116.4, 104.9, 29.5, 27.6 |
3u | 8.25(d, J=8.4 Hz, 2H), 7.80(d, J=8.4 Hz, 2H), 7.50(s, 1H), 7.30—7.26(m, 2H), 7.24(s, 1H), 3.88(s, 3H), 3.40(s, 3H), 2.45(s, 3H) | 159.2, 155.7, 155.1, 151.0, 150.4, 140.3, 137.5, 135.1, 131.7, 127.7, 127.0, 126.7, 118.0, 117.4, 113.0, 106.6, 29.2, 27.5, 20.4 |
3v | 8.59(s, 1H), 8.27—8.25(m, 1H), 7.96—7.88(m, 3H), 7.62(s, 1H), 7.57—7.54(m, 2H), 7.51—7.49(m, 3H), 7.40—7.38(m, 2H), 3.93(s, 3H), 3.40(s, 3H) | 159.5, 158.0, 154.2, 150.8, 150.5, 138.6, 133.5, 133.4, 132.2, 128.0, 127.7, 126.8, 126.7,126.7, 126.5, 125.6, 123.3, 117.5 105.5, 29.2, 27.4 |
3w | 8.14—8.12(m, 2H), 7.60(s, 1H), 7.60—7.50(m, 4H), 7.26—7.24(m, 1H), 7.16—7.14(m, 1H), 3.87(s, 3H), 3.43(s, 3H) | 159.3, 158.2, 151.0, 150.4, 146.71, 138.3, 136.0, 129.8, 127.9, 127.4, 126.5, 126.2, 125.9, 118.3, 105.7, 29.2, 27.5 |
3x | 7.73—7.72(m, 1H), 7.56—7.54(m, 1H), 7.48—7.46(m, 3H), 7.35—7.33(m, 3H), 7.16—7.14(m, 1H), 3.83(s, 3H), 3.37(s, 3H) | 159.3, 154.1, 153.3, 150.8, 150.5, 142.3, 138.4, 129.5, 127.5, 127.2, 126.8, 126.7, 126.4, 115.7, 105.1, 29.1, 27.4 |
| 5a | 8.60(d, J=4.9 Hz, 1H), 7.46—7.44(m, 3H), 7.30—7.26(m, 2H), 7.01(d, J=4.9 Hz, 1H), 3.78(s, 3H), 3.37(s, 3H) | 159.5, 153.5, 151.0, 150.3, 138.1, 127.2, 126.8, 121.0, 107.1, 29.2, 27.5 |
| 5b | 7.44—7.42(m, 3H), 7.29—7.26(m, 2H), 6.87(s, 1H), 3.77(s, 3H), 3.36(s, 3H), 2.61(s, 3H) | 161.6, 159.6, 153.4, 150.5, 138.4, 127.0, 126.7, 120.7, 104.5, 29.0, 27.3, 23.9 |
| 5c | 7.25—7.23(m, 4H), 6.85(s, 1H), 3.76(s, 3H), 3.36(s, 3H), 2.60(s, 3H), 2.41(s, 3H) | 161.5, 159.7, 153.6, 150.6, 150.6, 136.9, 135.4, 127.5, 126.7, 120.8, 104.6, 29.0, 27.4, 23.9, 20.3 |
5d | 13.23(s, 1H), 7.52(s, 1H), 7.39—7.37(m, 1H), 7.04—7.02(m, 1H), 6.98—6.94(m, 1H), 3.74(s, 3H), 3.47(s, 3H), 2.91(s, 3H) | 160.1, 159.1, 158.5, 153.7, 145.0, 149.3, 132.3, 126.3, 118.5, 117.7, 116.7, 116.2, 106.2, 29.4, 27.5, 22.1 |
| Compd. | 1H NMR(400 MHz), δ | 13C NMR(100 MHz), δ |
|---|---|---|
| 3a | 8.13—8.15(m, 2H), 7.52—7.50(m, 3H), 7.48—7.46(m, 4H), 7.37—7.33(m, 2H), 3.89(s, 3H), 3.39(s, 3H) | 159.5, 158.2, 154.2, 150.8, 150.6, 138.6, 136.2, 129.7, 127.9, 127.2, 126.8, 126.5, 117.4, 105.5, 29.1, 27.4 |
3b | 8.25—8.28(s, 1H), 7.57—7.50(m, 3H), 7.30—7.28(m, 2H), 7.24—7.22(m, 2H), 3.72(s, 3H), 3.19(s, 3H), 2.38(s, 3H) | 159.8, 157.7, 154.4, 151.5, 151.0, 137.2, 136.8, 136.4, 130.7, 129.0, 128.3, 128.0, 127.5, 117.8, 106.4, 99.5, 29.8, 28.0, 20.9 |
| 3c | 8.18—8.16(s, 1H), 7.57(s, 1H), 7.43—7.33(m, 7H), 3.72(s, 3H), 3.19(s, 3H), 2.38(s, 3H) | 159.5, 158.2, 154.1, 150.8, 150.6, 140.2, 138.7, 133.5, 128.7, 127.1, 126.8, 126.5, 117.0, 105.6, 29.1, 27.4, 20.4 |
3d | 7.90—7,87 (m, 2H), 7.48—7.44(m, 4H), 7.36—7.34(m, 2H), 7.27(s, 1H), 3.89(s, 3H), 3.39(s, 3H), 2.37—2.34(m, 6H) | 159.6, 158.4, 154.0, 150.7, 150.6, 138.9, 138.7, 136.2, 133.9, 129.3, 127.6, 127.1, 126.8, 126.8, 124.1, 117.1, 105.2, 29.1, 27.4, 19.0, 18.8 |
3e | 8.23—8.26(m, 3H), 7.58(d,J=8.0 Hz, 1H), 7.52—7.53(m, 3H), 7.29—7.27(m, 2H), 3.71(s, 3H), 3.19(s, 3H), 2.38(s, 3H) | 159.5, 158.1, 154.4, 150.8, 150.6, 137.1, 136.3, 135.6, 129.7, 127.9, 127.6, 126.8, 126.5, 117.5, 105.6, 29.1, 27.4, 20.0 |
3f | 8.15—8.13(m, 2H), 7.52—7.50(m, 3H), 7.47(s, 1H), 7.30—7.24(m, 3H), 7.24(s, 1H), 3.89(s, 3H), 3.40(s, 3H), 2.44(s, 3H) | 159.5, 158.1, 154.4, 150.8, 150.6, 137.1, 136.3, 135.6, 129.7, 127.9, 127.6, 126.8, 126.5, 117.5, 105.6, 29.1, 27.4, 20.4 |
3g | 8.02—8.04(m, 2H), 7.44(s, 1H), 7.32—7.27(m, 5H), 7.25(d, J=6.1 Hz, 1H), 3.88(s, 3H), 3.39(s, 3H), 2.44(d, J=3.8 Hz, 6H) | 159.6, 158.1, 154.2, 150.8, 150.6, 140.1, 137.0, 135.7, 133.5, 128.7, 127.8, 126.8, 126.4, 117.1, 105.3, 29.1, 27.4, 20.4, 20.4 |
3h | 8.26(d, J=9.0 Hz, 2H), 7.56(s, 1H), 7.43—7.41(m, 5H), 7.08(d, J=9.0 Hz, 2H), 3.84(s, 3H), 3.73(s, 3H), 3.19(s, 3H) | 160.9, 159.5, 157.8, 154.0 150.8, 150.6, 138.7, 128.7, 128.1, 127.1, 126.8, 126.8, 116.5, 113.3, 104.8, 54.4, 29.1, 27.4 |
3i | 8.04(d, J=8.2 Hz, 2H), 7.43(s, 1H), 7.31(d, J=8.7 Hz, 4H), 7.00(d, J=8.8 Hz, 2H), 3.88(d, J=0.5 Hz, 6H), 3.40(s, 3H), 2.43(s, 3H) | 159.7, 158.7, 158.1, 153.9, 150.9, 150.6, 140.1, 133.6, 130.7, 128.8, 128.4, 126.4, 117.3, 112.3, 105.2, 54.3, 29.1, 27.4, 20.4 |
3j | 8.10(d, J=8.9 Hz, 2H), 8.10(d, J=8.9 Hz, 2H), 7.38(s, 1H), 7.30(d, J=8.7 Hz, 2H), 7.00(m, 4H), 3.88—3.86(m, 8H), 3.39(s, 3H) | 160.9, 159.6, 158.7, 157.6, 153.8, 150.9, 150.6, 130.8, 128.8, 128.4, 128.0, 116.8, 113.3, 112.3, 104.8, 54.4, 54.3, 29.1, 27.4 |
3k | 8.10(d, J=8.9 Hz, 2H), 7.39(s, 1H), 7.28(d, J=8.1 Hz, 2H), 7.25(m, 2H), 7.01(d, J=8.9 Hz, 2H), 3.88(d, J=7.4 Hz, 6H), 3.39(s, 3H), 2.44(s, 3H) | 160.9, 159.6, 157.7, 154.1, 150.8, 150.6, 137.0, 135.8, 128.8, 128.0, 127.6, 126.8, 116.6, 113.3, 104.9, 54.4, 29.1, 27.4, 20.4 |
3l | 8.17—8.13(m, 2H), 7.49—7.48(m, 3H), 7.42(s, 1H), 7.34(m, 2H), 7.19(t, J=8.7 Hz, 2H), 3.88(s, 3H), 3.39(s, 3H) | 164.8, 162.3, 159.4, 157.1, 154.4, 150.8, 150.5, 138.5, 132.4, 132.4, 128.6, 128.5, 127.2, 126.9, 126.7, 117.0, 115.1, 114.9, 105.5, 29.1, 27.4 |
| 3m | 8.09—8.07(m, 2H), 7.48—7.47(m, 5H), 7.46—7.44(s, 1H), 7.35—7.33(m, 2H), 3.87(s, 3H), 3.39(s, 3H) | 159.4 156.9, 154.5, 150.8, 150.5, 138.4, 136.0, 134.7, 128.2, 127.8, 127.3, 126.9, 126.7, 117.1, 105.7, 29.2, 27.5 |
3n | 8.02—8.00(m, 2H), 7.65—7.63(m, 2H), 7.48—7.47(m, 3H), 7.44(s, 1H), 7.35—7.33(m, 2H), 3.87(s, 3H), 3.39(s, 3H) | 159.4, 157.0, 154.5, 150.8, 150.5, 138.4, 135.1, 131.2, 128.0, 127.3, 126.7, 126.7, 124.5, 117.1, 105.8, 29.2, 27.5 |
3o | 8.16—8.12(m, 2H), 7.41(s, 1H), 7.28(d, J=8.1 Hz, 2H), 7.23—7.17(m, 4H), 3.87(s, 3H), 3.39(s, 3H), 2.44(s, 3H) | 164.7, 162.2, 159.5, 157.0, 154.6, 150.8, 150.5, 137.2, 135.5, 132.5, 132.5, 128.6, 128.5, 127.6, 126.8, 117.1, 115.1, 114.9, 105.5, 29.1, 27.4, 20.4 |
| Compd. | 1H NMR(400 MHz), δ | 13C NMR(100 MHz), δ |
3p | 8.07(d, J=8.7 Hz, 2H), 7.47(d, J=8.7 Hz, 2H), 7.43(s, 1H), 7.28(d, J=8.0 Hz, 2H), 7.25—7.25(m, 2H), 3.87(s, 3H), 3.39(s, 3H), 2.44(s, 3H) | 159.4, 156.8, 154.6, 150.9, 150.5, 137.2, 136.0, 135.4, 134.7, 128.2, 127.7, 127.6, 126.8, 117.2, 105.8, 29.2, 27.5, 20.4 |
3q | 8.00(d, J=8.7 Hz, 2H), 7.63(d, J=8.7 Hz, 2H), 7.43(s, 1H), 7.28(d, J=8.0 Hz, 2H), 7.25—7.23(m, 2H), 3.87(s, 3H), 3.39(s, 3H), 2.44(s, 3H) | 159.4, 156.9, 154.7, 150.9, 150.5, 137.2, 135.4, 135.2, 131.1, 128.0, 127.6, 126.8, 124.4, 117.2, 105.8, 29.2, 27.5, 20.4 |
| 3r | 8.55(d, J=9.0 Hz, 2H), 8.34(d, J=9.0 Hz, 2H), 7.79(s, 1H), 7.45—7.41(m, 5H), 3.74(s, 3H), 3.20(s, 3H) | 159.6, 155.3, 154.8, 151.6, 150.9, 148.5, 142.6, 139.1, 128.8, 128.3, 128.0, 127.5, 124.0, 119.0, 107.5, 29.9, 28.1 |
3s | 8.26—8.24(m, 2H), 7.81—7.79(m, 2H), 7.50—7.46(m, 4H), 7.35—7.35(m, 2H), 3.88(s, 3H), 3.39(s, 3H) | 159.2, 155.8, 154.9, 150.9, 150.3, 140.3, 138.1, 131.7, 127.5, 127.0, 127.0, 126.7, 117.8, 117.4, 113.0, 106.5, 29.2, 27.5 |
3t | 13.20(s, 1H), 7.85—7.82(m, 1H), 7.57(s, 1H), 7.52—7.47(m, 1H), 7.44—7.38(m, 1H), 7.35(s, 1H), 7.07—7.05(m, 1H), 6.97—6.91(m, 1H), 3.82(s, 3H), 3.39(s, 3H) | 159.2, 158.7, 154.8, 140.0, 139.4, 138.0, 132.6, 127.5, 127.0, 126.6, 118.6 117.8, 116.8, 116.4, 104.9, 29.5, 27.6 |
3u | 8.25(d, J=8.4 Hz, 2H), 7.80(d, J=8.4 Hz, 2H), 7.50(s, 1H), 7.30—7.26(m, 2H), 7.24(s, 1H), 3.88(s, 3H), 3.40(s, 3H), 2.45(s, 3H) | 159.2, 155.7, 155.1, 151.0, 150.4, 140.3, 137.5, 135.1, 131.7, 127.7, 127.0, 126.7, 118.0, 117.4, 113.0, 106.6, 29.2, 27.5, 20.4 |
3v | 8.59(s, 1H), 8.27—8.25(m, 1H), 7.96—7.88(m, 3H), 7.62(s, 1H), 7.57—7.54(m, 2H), 7.51—7.49(m, 3H), 7.40—7.38(m, 2H), 3.93(s, 3H), 3.40(s, 3H) | 159.5, 158.0, 154.2, 150.8, 150.5, 138.6, 133.5, 133.4, 132.2, 128.0, 127.7, 126.8, 126.7,126.7, 126.5, 125.6, 123.3, 117.5 105.5, 29.2, 27.4 |
3w | 8.14—8.12(m, 2H), 7.60(s, 1H), 7.60—7.50(m, 4H), 7.26—7.24(m, 1H), 7.16—7.14(m, 1H), 3.87(s, 3H), 3.43(s, 3H) | 159.3, 158.2, 151.0, 150.4, 146.71, 138.3, 136.0, 129.8, 127.9, 127.4, 126.5, 126.2, 125.9, 118.3, 105.7, 29.2, 27.5 |
3x | 7.73—7.72(m, 1H), 7.56—7.54(m, 1H), 7.48—7.46(m, 3H), 7.35—7.33(m, 3H), 7.16—7.14(m, 1H), 3.83(s, 3H), 3.37(s, 3H) | 159.3, 154.1, 153.3, 150.8, 150.5, 142.3, 138.4, 129.5, 127.5, 127.2, 126.8, 126.7, 126.4, 115.7, 105.1, 29.1, 27.4 |
| 5a | 8.60(d, J=4.9 Hz, 1H), 7.46—7.44(m, 3H), 7.30—7.26(m, 2H), 7.01(d, J=4.9 Hz, 1H), 3.78(s, 3H), 3.37(s, 3H) | 159.5, 153.5, 151.0, 150.3, 138.1, 127.2, 126.8, 121.0, 107.1, 29.2, 27.5 |
| 5b | 7.44—7.42(m, 3H), 7.29—7.26(m, 2H), 6.87(s, 1H), 3.77(s, 3H), 3.36(s, 3H), 2.61(s, 3H) | 161.6, 159.6, 153.4, 150.5, 138.4, 127.0, 126.7, 120.7, 104.5, 29.0, 27.3, 23.9 |
| 5c | 7.25—7.23(m, 4H), 6.85(s, 1H), 3.76(s, 3H), 3.36(s, 3H), 2.60(s, 3H), 2.41(s, 3H) | 161.5, 159.7, 153.6, 150.6, 150.6, 136.9, 135.4, 127.5, 126.7, 120.8, 104.6, 29.0, 27.4, 23.9, 20.3 |
5d | 13.23(s, 1H), 7.52(s, 1H), 7.39—7.37(m, 1H), 7.04—7.02(m, 1H), 6.98—6.94(m, 1H), 3.74(s, 3H), 3.47(s, 3H), 2.91(s, 3H) | 160.1, 159.1, 158.5, 153.7, 145.0, 149.3, 132.3, 126.3, 118.5, 117.7, 116.7, 116.2, 106.2, 29.4, 27.5, 22.1 |
| Compd. | Product | Yieldb(%) | Time/min | Compd. | Product | Yieldb(%) | Time/min |
|---|---|---|---|---|---|---|---|
| 3a | 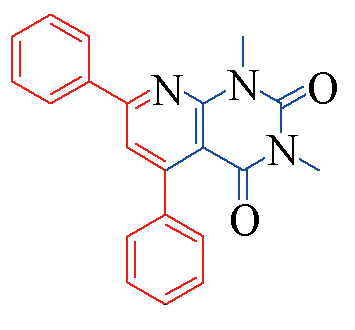 | 88 | 10 | 3h | 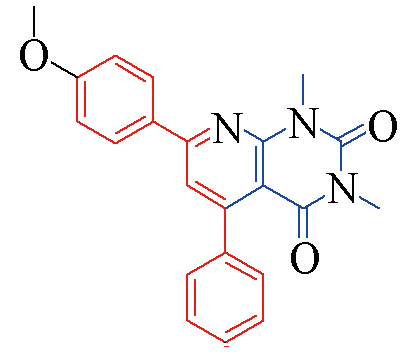 | 78 | 13 |
| 3b | 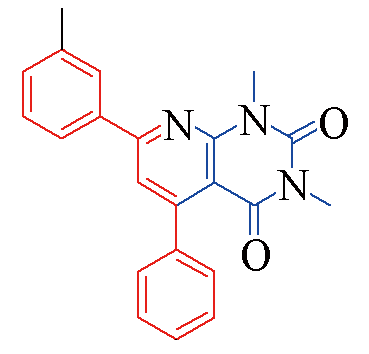 | 85 | 11 | 3i | 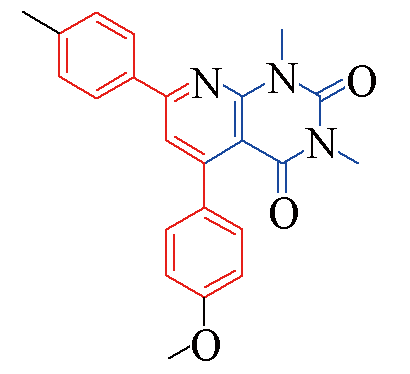 | 82 | 15 |
| 3c | 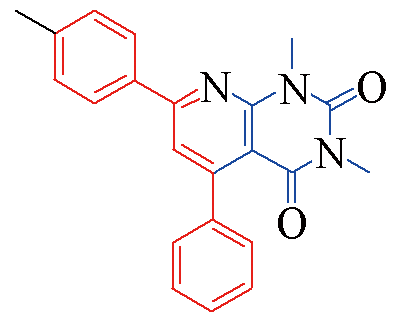 | 83 | 11 | 3j | 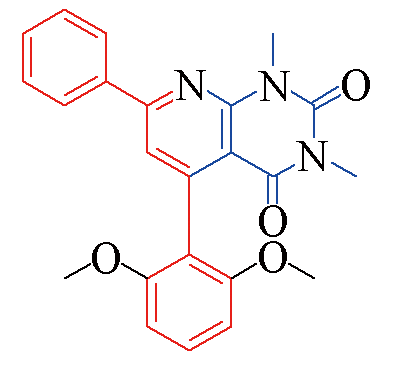 | 81 | 15 |
| 3d | 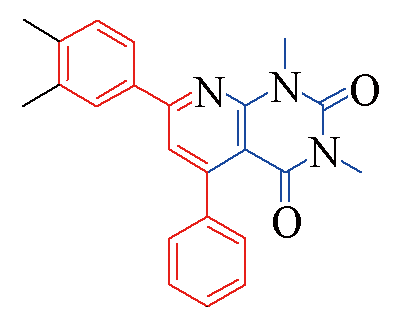 | 85 | 12 | 3k | 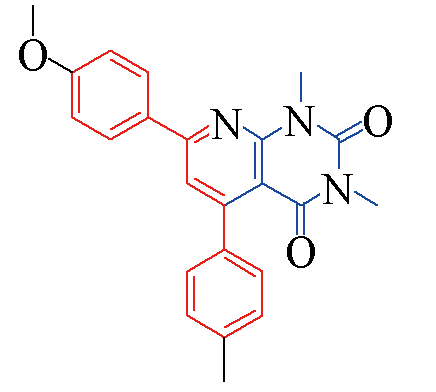 | 80 | 13 |
| 3e | 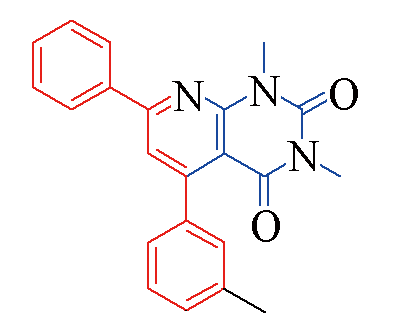 | 79 | 10 | 3l | 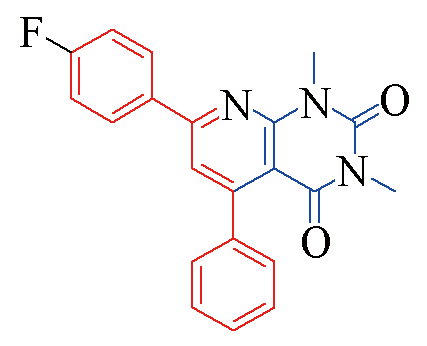 | 88 | 10 |
| 3f | 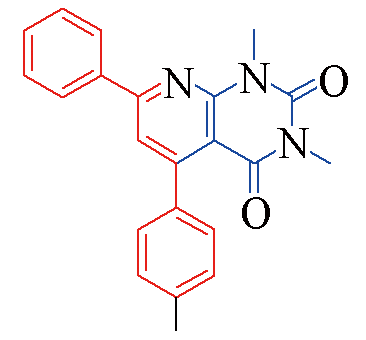 | 78 | 11 | 3m | 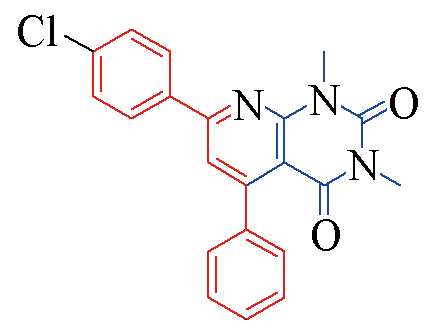 | 85 | 10 |
| 3g | 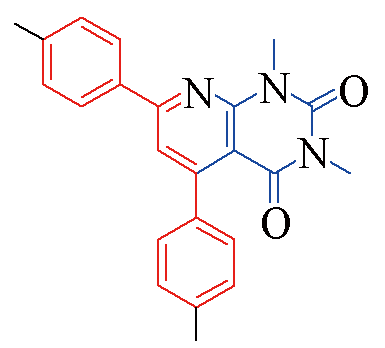 | 81 | 14 | 3n | 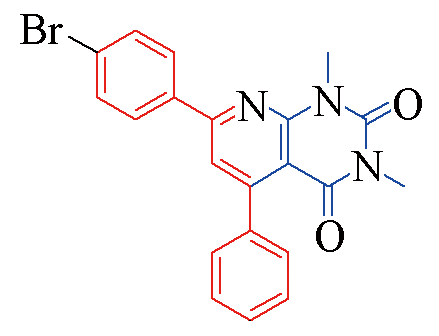 | 85 | 11 |
| Compd. | Product | Yieldb(%) | Time/min | Compd. | Product | Yieldb(%) | Time/min |
| 3o | 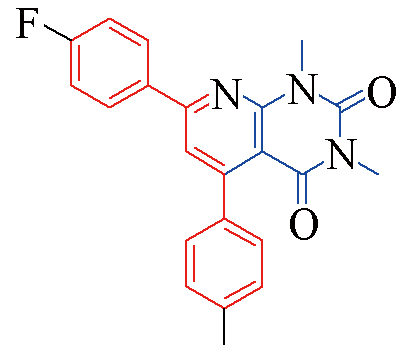 | 84 | 12 | 3t | 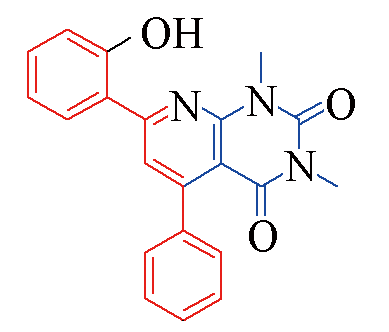 | 78 | 15 |
| 3p | 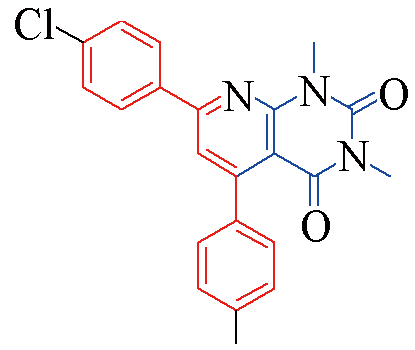 | 81 | 13 | 3u | 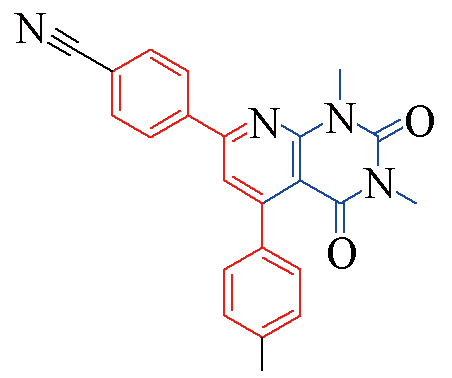 | 84 | 13 |
| 3q | 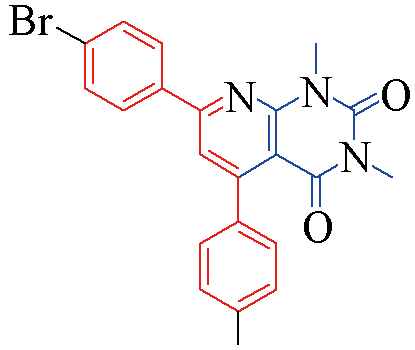 | 82 | 12 | 3v | 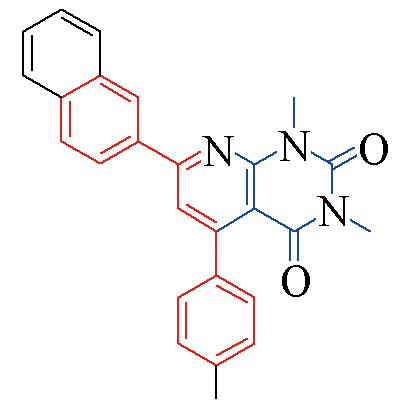 | 80 | 13 |
| 3r | 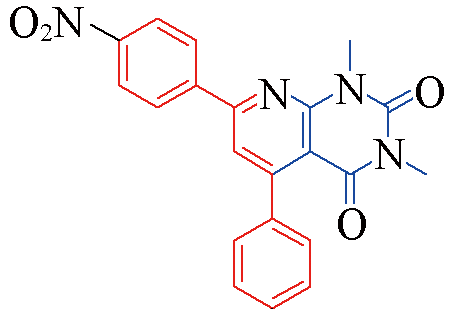 | 79 | 12 | 3w | 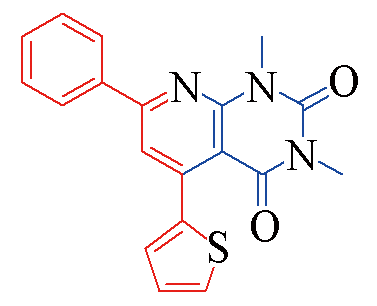 | 81 | 11 |
| 3s | 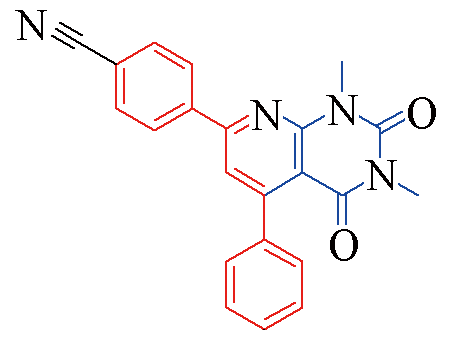 | 77 | 11 | 3x | 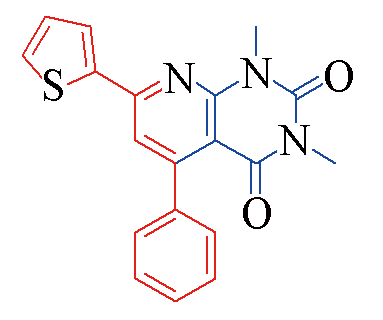 | 83 | 10 |
| Compd. | Product | Yieldb(%) | Time/min | Compd. | Product | Yieldb(%) | Time/min |
|---|---|---|---|---|---|---|---|
| 3a |  | 88 | 10 | 3h |  | 78 | 13 |
| 3b |  | 85 | 11 | 3i |  | 82 | 15 |
| 3c |  | 83 | 11 | 3j |  | 81 | 15 |
| 3d |  | 85 | 12 | 3k |  | 80 | 13 |
| 3e |  | 79 | 10 | 3l |  | 88 | 10 |
| 3f |  | 78 | 11 | 3m |  | 85 | 10 |
| 3g |  | 81 | 14 | 3n |  | 85 | 11 |
| Compd. | Product | Yieldb(%) | Time/min | Compd. | Product | Yieldb(%) | Time/min |
| 3o |  | 84 | 12 | 3t |  | 78 | 15 |
| 3p |  | 81 | 13 | 3u |  | 84 | 13 |
| 3q |  | 82 | 12 | 3v |  | 80 | 13 |
| 3r |  | 79 | 12 | 3w |  | 81 | 11 |
| 3s |  | 77 | 11 | 3x |  | 83 | 10 |
| 1 | Zhang H. J., Wang S. B., Wen X., Li J. Z., Quan Z. S., Med. Chem. Res., 2016, 25(7), 1287—1298 |
| 2 | Dongre R. S., Meshram J. S., Selokar R. S., Almalki F. A., Ben H. T., New J. Chem., 2018, 42(19), 15610—15617 |
| 3 | Gao X. G., Cen L. Q., Li F. Y., Wen R., Yan H. R., Yao H., Zhu S. L., Biochem. Biophys. Res. Commun., 2018, 505(3), 761—767 |
| 4 | Buron F., Merour J. Y., Akssira M., Guillaumet G., Routier S., Eur. J. Med. Chem., 2015, 95, 76—95 |
| 5 | Shimizu M., Takase Y., Nakamura S., Katae H., Minami A., Antimicrob.Agents Chemother., 1975, 8(2), 132—138 |
| 6 | Acosta P., Insuasty B., Ortiz A., Abonia R., Sortino M., Zacchino S. A., Quiroga J., Arabian J. Chem., 2016, 9(3), 481—492 |
| 7 | Veeraswamy B., Madhu D., Dev G. J., Poornachandra Y., Kumar G. S., Kumar C. G., Narsaiah B., Bioorg. Med. Chem. Lett., 2018, 28(9), 1670—1675 |
| 8 | Fang F., Xue L. M., Cong J., Tian C., Wang X. W., Liu J., Zhang Z. L., Chem. J. Chinese Universities, 2019, 40(10), 2111—2120 (方芳, 薛良敏, 丛 婧, 田 超, 王孝伟, 刘俊义, 张志丽. 高等学校化学学报, 2019, 40(10), 2111—2120) |
| 9 | Abbas S. E. S., George R. F., Samir E. M., Aref M. M. A., Abdel⁃Aziz H. A., Future Med. Chem., 2019, 11(18), 2395—2414 |
| 10 | Cheung A. W. H., BanneB R., Bose J., Kim K., Li S., Marcopulos N., Orzechowski L., Sergi J. A., Thakkar K. C., Wang B. B., Yun W., Zwingelstein C., Berthel S., Olivier A. R., Bioorg. Med. Chem. Lett., 2012, 22(24), 7518—7522 |
| 11 | Lacbay C. M., Mancuso J., Lin Y. S., Bennett N., G€otte M., Tsantrizos Y. S., J. Med. Chem., 2014, 57(17), 7435—7449 |
| 12 | Wawzonek S., J. Org. Chem., 1976, 41(19), 3149—3151 |
| 13 | Zhang F. R., Li C. M., Liang X. Z., Green Chem., 2018, 20(9), 2057—2063 |
| 14 | Shi D. Q., Zhou Y., Liu H., J. Heterocycl. Chem., 2010, 47, 131—135 |
| 15 | Wang Z. S., Wang M. Y., Xu S. S., Gu M. M., Meng Z. Y., Li C., Cai P. J., Rong L. C., Synth. Commun., 2016, 46(23), 1887—1892 |
| 16 | Farahmand T., Hashemian S., Sheibani A., J. Mol. Struct., 2020, 1206, 1—7 |
| 17 | Upadhyay A., Sharma L. K., Singh V. K., Singh R. K. P., Tetrahedron Lett., 2016, 57(50), 5599—5604 |
| 18 | Chate A. V., Kulkarni A. S., Jadhav C. K., Nipte A. S., Bondle G. M., J. Heterocycl. Chem., 2020, 57(5), 2184—2193 |
| 19 | Mamaghani M., Sheykhan M., Sadeghpour M., Tavakoli F., Monatsh Chem., 2018, 149(8), 1437—1446 |
| 20 | Jiang L., Ye W., Su W., Yu C., Chem. Res. Chinese Universities, 2019, 35(1), 21—25 |
| 21 | Kappe C. O., van der Eycken E., Chem. Soc. Rev., 2010, 39(4), 1280—1290 |
| 22 | Polshettiwar V., Varma R. S., Chem. Soc. Rev., 2008, 37(8) , 1546—1557 |
| 23 | Appukkuttan P., Mehta V. P., van der Eycken E., Chem. Soc. Rev., 2010, 39(5), 1467—1477 |
| 24 | Sharma A., Appukkuttan P., van der Eycken E., Chem. Commun., 2012, 48(11), 1623—1637 |
| 25 | Jiang Z., Liu J., Zhou F., Zhang J., Liu Z., Zhang C., Chen H., Chem. Res. Chinese Universities, 2018, 34(6), 918—922 |
| 26 | Sun H. S., Wang J. Q., Guo C., Shen L. J., Chin. J. Org. Chem., 2013, 33(10), 2220—2225(孙宏顺, 王建强, 郭成, 沈临江. 有机化学, 2013, 33(10), 2220—2225) |
| 27 | Polshettiwar V., Varma R. S., Acc.Chem. Res., 2008, 41(5), 629—639 |
| 28 | Kokel A., Schafer C., Torok B., Green Chem., 2017, 19(16), 3729—3751 |
| 29 | Bai H. R., Sun R. R., Chen X. B., Yang L. J., Huang C., ChemistrySelect, 2018, 3(17), 4635—4638 |
| 30 | Bai H., Sun R., Liu S., Yang L., Chen X., Huang C., J. Org. Chem., 2018, 83(20), 12535—12548 |
| 31 | Li J., Duan W., Pan X., Ye Y., Huang C., ChemistrySelect, 2019, 4(12), 3281—3285 |
| 32 | Li J. S., Li X. H., Duan W. W., Huang C., Jouranl of Yunnan Minzu Universtiy(Natural Sciences Edition), 2019, 117(05), 444—451 (李济森, 李新汉, 段文文, 黄超. 云南民族大学学报(自然科学版), 2019, 117(05), 444—451) |
| 33 | Majumdar K., Ponra S., Ghosh D., Synthesis, 2011, 2011(7), 1132—1136 |
| 34 | Abdelrazek F. M., Gomha S. M., Abdel⁃aziz H. M., Farghaly M. S., Metz P., Abdel⁃Shafy A., J. Heterocycl. Chem., 2020, 57(4), 1759—1769 |
| [1] | YU Jing, WU Chao, LI Chenyang, CHEN Danfeng, DING Liuyue, MA Xiantao. Catalyst-free and Highly Efficient O-Silylation of Alcohols and Phenols [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210588. |
| [2] | PU Weiwen, SHI Yongsen, LIU Jianfeng, KE Dehong, WU Xiaolan, XU Sheng. Synthesis of (E)-α-Hydroxyethyl-α,β-unsaturated Aldehydes with Tetrahydrofuran as Carbonyl Source [J]. Chem. J. Chinese Universities, 2021, 42(12): 3641. |
| [3] | LI Chen, LI Yuesheng. Living Ring-opening Polymerization of O-Carboxyanhydrides Catalyzed by Pyridine Derivatives [J]. Chem. J. Chinese Universities, 2021, 42(10): 3203. |
| [4] | ZHANG Weiguo, FAN Songhua, WANG Hongzhi, YAO Suwei. Synthesis of Self-assembled α-Fe2O3/Graphene Hydrogel for Supercapacitors with Promising Electrochemical Properties [J]. Chem. J. Chinese Universities, 2020, 41(8): 1850. |
| [5] | CHEN Xiangyun, ZHU Benqiang, YUAN Bing, YU Fengli, XIE Congxia, YU Shitao. Hydrogenation of α-Pinene Catalyzed by Ru Nanoparticles Stabilized by Magnetic Alkali Lignin Amine [J]. Chem. J. Chinese Universities, 2020, 41(8): 1826. |
| [6] | HU Zhiyuan, WAN Qiuxiang, ZHOU Hang, SONG Chuanjun, CHANG Junbiao. Synthesis of 6β-Hydroxy-5,5,8aβ-trimethyloctahydronaphthalen-1(2H)-one Toward the Establishment of the Scaffold of Phenylspirodrimane Meroterpenoid Natural Products † [J]. Chem. J. Chinese Universities, 2020, 41(5): 955. |
| [7] | WU Tao,MU Xiaoqing,NIE Yao,XU Yan. Improving Catalytic Efficiency of Bacillus Cereus Amine Dehydrogenase for Acetophenone Reduction by Iterative Saturation Mutagenesis † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1018. |
| [8] | WANG Biwen, DU Tian, TANG Weijun. Synthesis and Application of Chiral Ru Pincer Catalysts on the Hydrogenation of α-Hydroxy Esters by Dynamic Kinetic Resolution† [J]. Chem. J. Chinese Universities, 2020, 41(10): 2256. |
| [9] | TANG Yucai,QU Huang,ZHANG Wenxi,WANG Feifei,WANG Gang. Synthesis of α-Sulfonyl Ketones via I2/TBHP Promoted Radical Sulfonylation of Silyl Enol Ethers with Sulfohydrazides under Mild Conditions † [J]. Chem. J. Chinese Universities, 2020, 41(1): 118. |
| [10] | REN Jun, WANG Dong-Lei, PEI Yong-Li, QIN Zhi-Feng, LIN Jian-Ying, LI Zhong. Effects of Lithium Content on the Structural Properties and Catalytic Activities of CuLi/AC Catalysts in the Oxidative Carbonylation of Methanol to Dimethyl Carbonate [J]. Chem. J. Chinese Universities, 2013, 34(11): 2594. |
| [11] | ZHANG Zhao, WANG Qi, WU Qiong, HU Xiao-Ying, WANG Cheng-Xi, MEI Wen-Jie, TAO Yun-Yi, WU Wei-Li, ZHENG Wen-Jie. Microwave-assisted Synthesis of Imidazole[4,5f][1,10]phenanthroline Derivatives and Microwave Nonthermal Effect [J]. Chem. J. Chinese Universities, 2012, 33(11): 2441. |
| [12] | ZHANG Xin-Rong, YAO Cheng-Zhang, WANG Lu-Cun, CAO Yong, WU Dong, SUN Yu-Han, DAI Wei-Lin, FAN Kang-Nian. Microwave-irradiation Promoted Cu/ZnO/Al2O3 Catalyst for Hydrogen Production from Steam Reforming of Methanol [J]. Chem. J. Chinese Universities, 2005, 26(6): 1137. |
| [13] | LIU Li-Xin, LIU Ruo-Wang, ZHANG Dong, AN Li-Jia, WANG Yu. Effects of Crystallinity on Hydrolytic Depolymerization of PET Under Microwave Irradiation [J]. Chem. J. Chinese Universities, 2005, 26(12): 2394. |
| [14] | LIU Li-Xin,LIU Ruo-Wang,ZHANG Dong,AN Li-Jia,WANG Yu . Effects of Crystallinity on Hydrolytic Depolymerization of PET Under Microwave Irradiation [J]. Chem. J. Chinese Universities, 2005, 26(12): 2394. |
| [15] | LAO Wen-Jian, YOU Jing, LU Hao-Jie, CHEN Shu-Lian, SHEN Xue-Jiao, OU Qing-Yu . Studies on the Synthesis, Properties and Biological Activities of (9-Carbazoly)-carboxylic Acids [J]. Chem. J. Chinese Universities, 2001, 22(6): 955. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||