

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (6): 1271.doi: 10.7503/cjcu2080706
• Physical Chemistry • Previous Articles Next Articles
YIN Yanhong, LI Ke, DONG Hongyu, JIN Cheng, XIAO Xinglu, GAO Yicong, YANG Shuting( )
)
Received:2018-10-19
Online:2019-06-10
Published:2019-03-27
Supported by:CLC Number:
TrendMD:
YIN Yanhong,LI Ke,DONG Hongyu,JIN Cheng,XIAO Xinglu,GAO Yicong,YANG Shuting. Performance of Ru/graphene/carbon Nanotube Composites with Three-dimensional Network Structure as Positive Electrode Catalysts for Lithium Oxygen Batteries†[J]. Chem. J. Chinese Universities, 2019, 40(6): 1271.
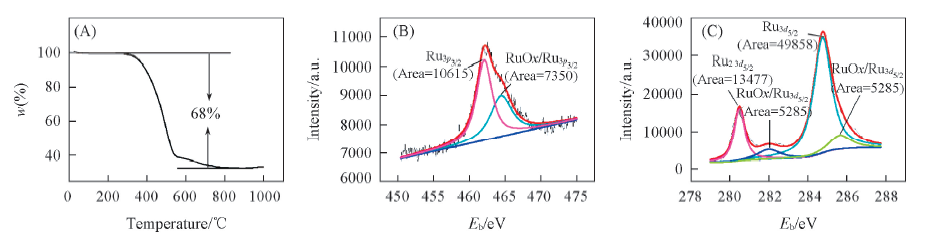
Fig.5 Thermogravimetric curve of RGC-2-500 composites in O2 atmosphere(A) and XPS diagram of RGC-2-500 composites after heat treatment at 1000 ℃(B, C)
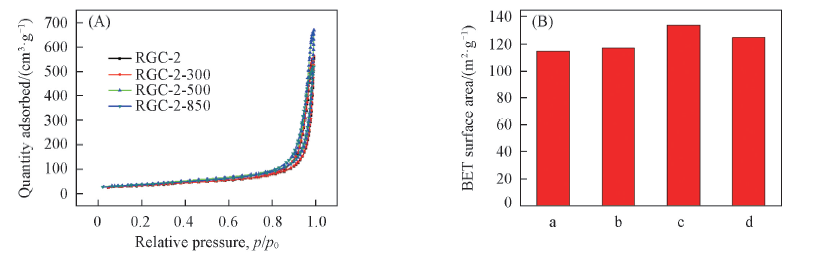
Fig.7 Nitrogen adsorption-desorption isotherms(A) and specific surface area(B) of RGC-2, RGC-2-300, RGC-2-500 and RGC-2-850 a. RGC-2; b. RGC-2-300; c. RGC-2-500; d. RGC-2-850.
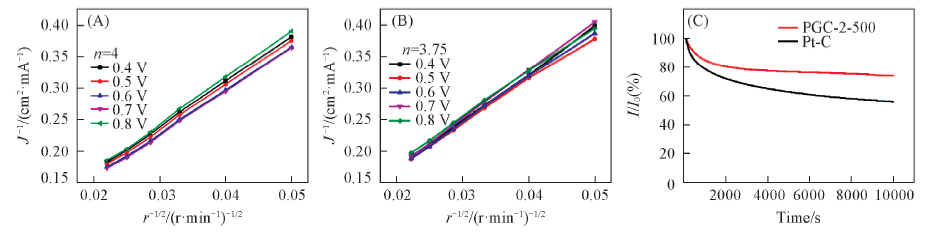
Fig.8 Cyclic voltammograms at a rate of 50 mV/s(A, D), oxygen reduction reaction curves(B, E) and oxygen evolution reaction curves(C, F) at a rotation rate of 1600 r/min and a scanning rate of 10 mV/s
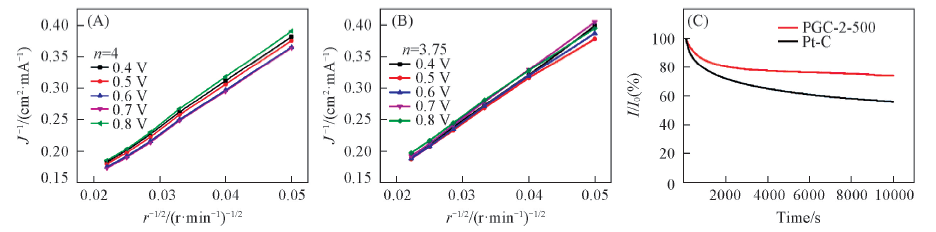
Fig.9 KL curves of RGC-2-500(A), Pt/C(B) during the ORR processes at different rotation rates and chronoamperometric responses for the ORR of RGC-2-500 and Pt/C at a rotation rate of 1600 r/min for 10000 s(C)
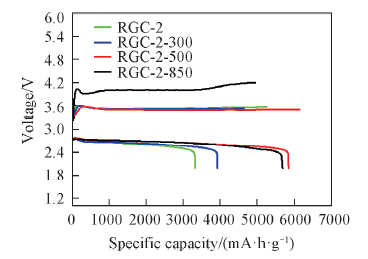
Fig.10 Galvanostatic discharge/charge curves of Li-O2 batteries with RGC-2, RGC-2-300, RGC-2-500 and RGC-2-850 as the cathode catalyst at a current density of 50 mA/g in the voltage range of 2.0—4.2 V(vs. Li+ /Li)
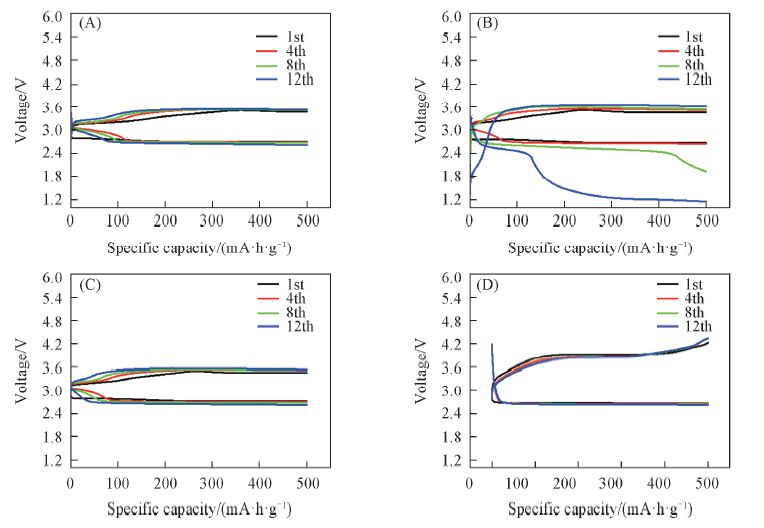
Fig.11 Charge-discharge curves of RGC-2(A), RGC-2-300(B), RGC-2-500(C) and RGC-2-850(D) as cathode catalyst at 50 mA/g with a limited specific capacity of 500 mA·h/g
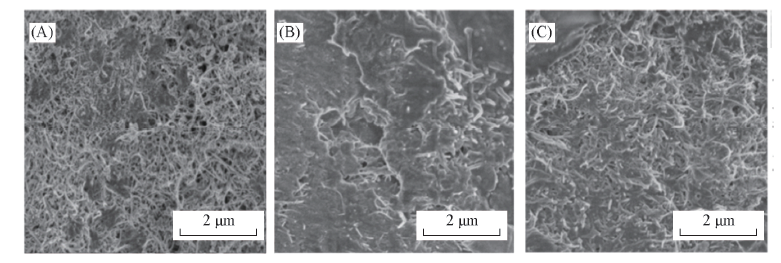
Fig.12 SEM images of the pole piece after charging and discharging with GRC-2-500 composite as cathode electrode catalyst for Li-O2 battery under current density of 50 mA/g and limited capacity of 500 mA·h/g (A) Original cathode electrode; (B) discharged cathode electrode; (C) charged cathode electrode.
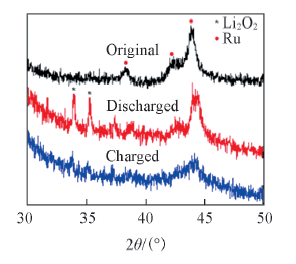
Fig.13 XRD patterns of the pole piece after charging and discharging with GRC-2-500 composite as cathode electrode catalyst for Li-O2 battery under current density of 50 mA/g and limited capacity of 500 mA·h/g
| [1] | Salunkhe R. R., Lee Y. H., Chang K. H., Li J. M., Simon P., Tang J., Torad N. L., Hu C. C., Yamauchi Y., Chemistry-A European Journal,2014, 20(43), 13838-13852 |
| [2] | Salunkhe R. R., Tang J., Kobayashi N., Kim J., Ide Y., Tominaka S., Kim J. H., Yamauchi Y., Chemical Science,2016, 7(9), 5704-5713 |
| [3] | Salunkhe R. R., Hsu S. H., Wu K. C. W., Yamauchi Y., Chem. Sus. Chem.,2014, 7(6), 1551-1556 |
| [4] | Yue H. Y., Wang Q. X., Zhang X., Hua S., Ma H., Yue D. Y., Yang S. T., Chem. J. Chinese Universities,2015, 36(4), 745-750 |
| (岳红云,王秋娴,张雪,华双,马华,岳东媛,杨书廷.高等学校化学学报, 2015, 36(4), 745-750) | |
| [5] | Pramanik M., Tsujimoto Y., Malgras V., Dou S. X., Kim J. H., Yamauchi Y., Chemistry of Materials,2015, 27(3), 1082-1089 |
| [6] | Hwang S. M., Lim Y. G., Kim J. G., Heo Y. U., Lim J. H., Yamauchi Y., Park M. S., Kim Y. J., Dou S. X., Kim J. H., Nano Energy,2014, 10, 53-62 |
| [7] | Xue H., Zhao J., Tang J., Gong H., He P., Zhou H., Yamauchi Y., He J., Chemistry-A European Journal,2016, 22(14), 4915-4923 |
| [8] | Tan G., Chong L., Amine R., Lu J., Liu C., Yuan Y., Wen J., He K., Bi X., Guo Y., Wang H. H., Shahbazian-Yassar R., Al Hallaj S., Miller D. J., Liu D., Amine K., Nano Letters,2017, 17(5), 2959-2966 |
| [9] | Chen Y., Freunberger S. A., Peng Z., Fontaine O., Bruce P. G., Nature Chemistry,2013, 5, 489-494 |
| [10] | Wang Z. L., Xu D., Xu J. J., Zhang L. L., Zhang X. B., Advanced Functional Materials,2012, 22(17), 3699-3705 |
| [11] | Xu J., Ma J., Fan Q., Guo S., Dou S., Advanced Materials,2017, 29(28), 1606454 |
| [12] | Yuan M., Yang Y., Nan C., Sun G., Li H., Ma S., Applied Surface Science,2018, 444, 312-319 |
| [13] | Tian F., Radin M. D., Siegel D. J., Chemistry of Materials,2014, 26(9), 2952-2959 |
| [14] | Guo X., Liu P., Han J., Ito Y., Hirata A., Fujita T., Chen M., Advanced Materials,2015, 27(40), 6137-6143 |
| [15] | Guo Z., Zhou D., Dong X., Qiu Z., Wang Y., Xia Y., Advanced Materials,2013, 25(39), 5668-5672 |
| [16] | Cao Y., Zheng M. S., Cai S., Lin X., Yang C., Hu W., Dong Q. F., Journal of Materials Chemistry A,2014, 2(44), 18736-18741 |
| [17] | Feng J. X., Xu H., Dong Y. T., Ye S. H., Tong Y. X., Li G. R., Angew. Chem. Int. Ed.,2016, 55(11), 3694-3698 |
| [18] | Lu X. F., Gu L. F., Wang J. W., Wu J. X., Liao P. Q., Li G. R., Advanced Materials,2017, 29(3), 1604437 |
| [19] | Feng N., He P., Zhou H., Advanced Energy Materials,2016, 6(9), 1502303 |
| [20] | Zhang W., Zhu J., Ang H., Zeng Y., Xiao N., Gao Y., Liu W., Hng H. H., Yan Q., Nanoscale,2013, 5(20), 9651-9658 |
| [21] | Luo L., Liu B., Song S., Xu W., Zhang J. G., Wang C., Nature Nanotechnology,2017, 12, 535 |
| [22] | Qiu D., Bu G., Zhao B., Lin Z., Pu L., Pan L., Shi Y., Materials Letters,2015, 141, 43-46 |
| [23] | Read J., Journal of the Electrochemical Society, 2002, 149(9), A1190-A1195 |
| [24] | Ishiguro K., Nemori H., Sunahiro S., Nakata Y., Sudo R., Matsui M., Takeda Y., Yamamoto O., Imanishi N., Journal of the Electrochemical Society,2014, 161(5), A668-A674 |
| [25] | Zhang J., Li P., Wang Z., Qiao J., Rooney D., Sun W., Sun K., Journal of Materials Chemistry A,2015, 3(4), 1504-1510 |
| [26] | Feng J. X., Ye S. H., Xu H., Tong Y. X., Li G. R., Advanced Materials,2016, 28(23), 4698-4703 |
| [27] | Lin X., Cao Y., Cai S., Fan J., Li Y., Wu Q.-H., Zheng M., Dong Q., Journal of Materials Chemistry A,2016, 4(20), 7788-7794 |
| [28] | Yi G., Xing B., Zeng H., Wang X., Zhang C., Cao J., Chen L., Journal of Nanomaterials,2017, 2017, 1-10 |
| [29] | Zhao G., Zhang D., Yu J., Xie Y., Hu W., Jiao F., Ceramics International,2017, 43(17), 15080-15088 |
| [30] | Kim M., Nam D. H., Park H. Y., Kwon C., Eom K., Yoo S., Jang J., Kim H. J., Cho E., Kwon H., Journal of Materials Chemistry A,2015, 3(27), 14284-14290 |
| [31] | Cui L., Lv G., He X., Journal of Power Sources,2015, 282, 9-18 |
| [32] | Li F., Chen Y., Tang D. M., Jian Z., Liu C., Golberg D., Yamada A., Zhou H., Energy & Environmental Science,2014, 7(5), 1648-1652 |
| [1] | TENG Zhenyuan, ZHANG Qitao, SU Chenliang. Charge Separation and Surface Reaction Mechanisms for Polymeric Single-atom Photocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220325. |
| [2] | WANG Ruyue, WEI Hehe, HUANG Kai, WU Hui. Freezing Synthesis for Single Atom Materials [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220428. |
| [3] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [4] | FAN Jianling, TANG Hao, QIN Fengjuan, XU Wenjing, GU Hongfei, PEI Jiajing, CEHN Wenxing. Nitrogen Doped Ultra-thin Carbon Nanosheet Composited Platinum-ruthenium Single Atom Alloy Catalyst for Promoting Electrochemical Hydrogen Evolution Process [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220366. |
| [5] | LIN Zhi, PENG Zhiming, HE Weiqing, SHEN Shaohua. Single-atom and Cluster Photocatalysis: Competition and Cooperation [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220312. |
| [6] | CHENG Qian, YANG Bolong, WU Wenyi, XIANG Zhonghua. S-doped Fe-N-C as Catalysts for Highly Reactive Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220341. |
| [7] | CHU Yuyi, LAN Chang, LUO Ergui, LIU Changpeng, GE Junjie, XING Wei. Single-atom Cerium Sites Designed for Durable Oxygen Reduction Reaction Catalyst with Weak Fenton Effect [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220294. |
| [8] | YANG Jingyi, LI Qinghe, QIAO Botao. Synergistic Catalysis Between Ir Single Atoms and Nanoparticles for N2O Decomposition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220388. |
| [9] | LIN Gaoxin, WANG Jiacheng. Progress and Perspective on Molybdenum Disulfide with Single-atom Doping Toward Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220321. |
| [10] | REN Shijie, QIAO Sicong, LIU Chongjing, ZHANG Wenhua, SONG Li. Synchrotron Radiation X-Ray Absorption Spectroscopy Research Progress on Platinum Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220466. |
| [11] | WEI Chunhong, JIANG Qian, WANG Panpan, JIANG Chengfa, LIU Yuefeng. Atomic Scale Investigation of Pt Atoms/clusters Promoted Co-catalyzed Fischer-Tropsch Synthesis [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220074. |
| [12] | ZHANG Xinxin, XU Di, WANG Yanqiu, HONG Xinlin, LIU Guoliang, YANG Hengquan. Effect of Mn Promoter on CuFe-based Catalysts for CO2 Hydrogenation to Higher Alcohols [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220187. |
| [13] | ZHAO Runyao, JI Guipeng, LIU Zhimin. Efficient Electrocatalytic CO2 Reduction over Pyrrole Nitrogen-coordinated Single-atom Copper Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220272. |
| [14] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| [15] | SONG Youwei, AN Jiangwei, WANG Zheng, WANG Xuhui, QUAN Yanhong, REN Jun, ZHAO Jinxian. Effects of Ag,Zn,Pd-doping on Catalytic Performance of Copper Catalyst for Selective Hydrogenation of Dimethyl Oxalate [J]. Chem. J. Chinese Universities, 2022, 43(6): 20210842. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||