

Chem. J. Chinese Universities ›› 2023, Vol. 44 ›› Issue (8): 20230059.doi: 10.7503/cjcu20230059
• Physical Chemistry • Previous Articles Next Articles
LI Zipeng1,2, GENG Jiaxin2, LIU Yingya1,2( ), SUN Zhichao1,2, WANG Yao2, WANG Anjie1,2(
), SUN Zhichao1,2, WANG Yao2, WANG Anjie1,2( )
)
Received:2023-02-14
Online:2023-08-10
Published:2023-04-14
Contact:
LIU Yingya, WANG Anjie
E-mail:yingya.liu@dlut.edu.cn;ajwang@dlut.edu.cn
Supported by:CLC Number:
TrendMD:
LI Zipeng, GENG Jiaxin, LIU Yingya, SUN Zhichao, WANG Yao, WANG Anjie. Effect of Acid Property Regulation on the Performance of Pd/MIL-100(Cr) Catalyzed One-step Oxidation of Benzyl Alcohol to Acetal[J]. Chem. J. Chinese Universities, 2023, 44(8): 20230059.
| Entry | Reaction | Catalyst d | Conversion(%) | Selectivity(%) | ||
|---|---|---|---|---|---|---|
| Toluene | Benzaldehyde | Acetal | ||||
| 1 a | 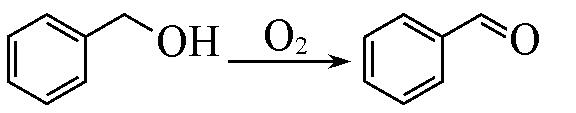 | Pd/MIL⁃100(Cr) | 90.1 | 0.8 | 78.5 | — |
| 2 a | MIL⁃100(Cr) | 5.1 | Trace | Trace | — | |
| 3 b | 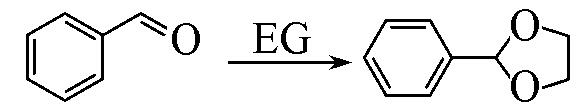 | Pd/MIL⁃100(Cr) | 54.0 | — | — | 99.9 |
| 4 b | MIL⁃100(Cr) | 72.0 | — | — | 99.9 | |
| 5 c | 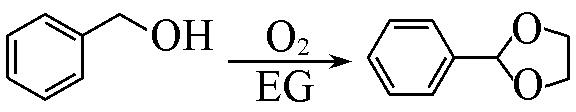 | Pd/MIL⁃100(Cr) | 99.9 | 11.3 | 13.8 | 72.9 |
Table 1 Stepwise reaction versus tandem reaction
| Entry | Reaction | Catalyst d | Conversion(%) | Selectivity(%) | ||
|---|---|---|---|---|---|---|
| Toluene | Benzaldehyde | Acetal | ||||
| 1 a | 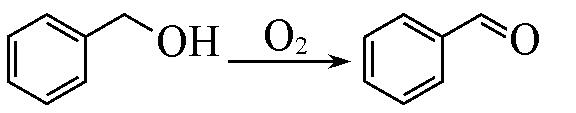 | Pd/MIL⁃100(Cr) | 90.1 | 0.8 | 78.5 | — |
| 2 a | MIL⁃100(Cr) | 5.1 | Trace | Trace | — | |
| 3 b | 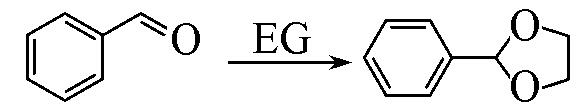 | Pd/MIL⁃100(Cr) | 54.0 | — | — | 99.9 |
| 4 b | MIL⁃100(Cr) | 72.0 | — | — | 99.9 | |
| 5 c | 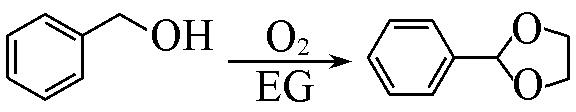 | Pd/MIL⁃100(Cr) | 99.9 | 11.3 | 13.8 | 72.9 |
| Cycle | Conversion(%) | Selectivity(%) | ||
|---|---|---|---|---|
| Toluene | Benzaldehyde | Acetal | ||
| 1 | 99.9 | 16.9 | 6.0 | 76.2 |
| 2 b | 99.5 | 9.7 | 82.9 | 3.5 |
| 2 c | 99.1 | 10.4 | 85.3 | 4.3 |
Table 2 Recycling test of the Pd/MIL-100(Cr) a
| Cycle | Conversion(%) | Selectivity(%) | ||
|---|---|---|---|---|
| Toluene | Benzaldehyde | Acetal | ||
| 1 | 99.9 | 16.9 | 6.0 | 76.2 |
| 2 b | 99.5 | 9.7 | 82.9 | 3.5 |
| 2 c | 99.1 | 10.4 | 85.3 | 4.3 |
| 1 | Ajaikumar S., Pandurangan A., J. Mol. Catal. A: Chem., 2008, 290(1), 35—43 |
| 2 | Climent M. J., Velty A., Corma A., Green Chem., 2002, 4(6), 565—569 |
| 3 | Abouakil N., Rogalska E., Bonicel J., Lombardo D., Biochim. Biophys. Acta, 1988, 961(3), 299—308 |
| 4 | Meskens F., Synthesis⁃Stuttgart, 1981, 7, 501—522 |
| 5 | Miao J., Wan H., Shao Y., Guan G., Xu B., J. Mol. Catal. A: Chem., 2011, 348(1), 77—82 |
| 6 | Gong J., Wang Y., Qian H., Fine Chemicals, 2004, 21(12), 920—922 |
| 7 | Tian J., Li B., Liu W., Chemical World, 2007, 48(11), 668—670, 674 |
| 8 | Ren L., Zhang X., Gao W., Petrochem. Technol., 2008, 37(4), 373—377 |
| 9 | Guo Y., Liang X., Shen D., Qi C., Petrochem. Technol., 2011, 40(8), 869—873 |
| 10 | Nasrollahzadeh M., Sajjadi M., Shokouhimehr M., Varma R. S., Coordin. Chem. Rev., 2019, 397, 54—75 |
| 11 | Nikbakht E., Yadollahi B., Farsani M. R., Inorg. Chem. Commun., 2015, 55, 135—138 |
| 12 | Song H. B., Liu Z., Wang Y. J., Zhang N., Qu X. F., Guo K., Xiao M., Gai H. J., Green Energy Environ., 2019, 4(3), 278—286 |
| 13 | Ndolomingo M. J., Meijboom R., Appl. Surf. Sci., 2017, 398, 19—32 |
| 14 | Feng T. K., Zhang S. W., Li C. E., Li T., Green Chem., 2022, 24(4), 1474—1480 |
| 15 | Chen J. Z., Wang S. P., Huang J., Chen L. M., Ma L. L., Huang X., ChemSusChem, 2013, 6(8), 1545—1555 |
| 16 | Bétard A., Fischer R. A., Chem. Rev., 2012, 112(2), 1055—1083 |
| 17 | Getman R. B., Bae Y. S., Wilmer C. E., Snurr R. Q., Chem. Rev., 2012, 112(2), 703—723 |
| 18 | Li X., Goh T. W., Li L., Xiao C., Guo Z., Zeng X. C., Huang W., ACS Catal., 2016, 6(6), 3461—3468 |
| 19 | Wang P. C., Shan L., Fan Y., Wang L., Xu J. N., Wu S. J., Chem. J. Chinese Universities, 2019, 40(8), 1655—1661 |
| 王鹏程, 单梁, 范勇, 王莉, 徐家宁, 吴淑杰. 高等学校化学学报, 2019, 40(8), 1655—1661 | |
| 20 | Jiang H. R., Xue C. H., Sun W. J., Gong Z. X., Yuan X., Catal. Lett., 2022, 1—14 |
| 21 | Chen J. W., Qi L., Zhang B. Y., Chen M. D., Kobayashi T., Bao Z. B., Yang Q. W., Ren Q. L., Huang W. Y., Zhang Z. G., Catal. Sci. Technol., 2021, 11(13), 4332 |
| 22 | Ren H. X., Li C., Yin D. D., Liu J. X., Liang C. H., RSC Adv., 2016, 6(88), 85659—85665 |
| 23 | Chen J. Z., Wang S. P., Huang J., Chen L. M., Ma L. L., Huang X., ChemSusChem, 2013, 6(8), 1545—1555 |
| 24 | Aijaz A., Karkamkar A., Choi Y. J., Tsumori N., Ronnebro E., Autrey T., Shioyama H., Xu Q., J. Am. Chem. Soc., 2012, 134(34), 13926—13929 |
| 25 | Sepehrmansouri H., Zarei M., Zolfigol M. A., Moosavi⁃Zare A. R., Rostamnia S., Moradi S., Mol. Catal., 2020, 481, 110303 |
| 26 | Yang S. D., Dong J., Yao Z. H., Shen C. M., Shi X. Z., Tian Y., Lin S. X., Zhang X. G., Sci. Rep.⁃UK, 2014, 4, 4501 |
| 27 | Vimont A., Goupil J. M., Lavalley J. C., Daturi M., Surble S., Serre C., Millange F., Ferey G., Audebrand N., J. Am. Chem. Soc., 2006, 128(10), 3218—3227 |
| 28 | Enache D. I., Barker D. P., Edwards J. K., Taylor S. H., Knight D. W., Carley A. F., Hutchings G. J., Catal. Today, 2007, 122, 407—411 |
| 29 | Sankar M., Nowicka E., Tiruvalam R., He Q., Taylor S. H., Kiely C. J., Bethell D., Knight D. W., Hutchings G. J., Chem. Eur. J., 2011, 17(23), 6524—6532 |
| 30 | Hall J. N., Bollini P., ACS Catal., 2020, 10(6), 3750—3763 |
| 31 | Nowicka E., Althahban S., Leah T. D., Shaw G., Morgan D., Kiely C. J., Roldan A., Hutchings G. J., Sci. Technol. Adv. Mat., 2019, 20(1), 367—378 |
| 32 | Hara T., Ishikawa M., Sawada J., Ichikuni N., Shimazu S., Green Chem., 2009, 11(12), 2034—2040 |
| 33 | Dayan S., Cetin A., Arslan N. B., Ozpozan N. K., Ozdemir N., Dayan O., Polyhedron, 2015, 85, 748—753 |
| 34 | Wang X. M., Wu G. J., Guan N. J., Li L. D., Appl. Catal. B: Environ., 2012, 115, 7—15 |
| 35 | Wang D., Ke Y., Guo D., Guo H., Chen J., Weng W., Sens. Actuators B: Chem., 2015, 216, 504—510 |
| 36 | Choe K., Zheng F. B., Wang H., Yuan Y., Zhao W. S., Xue G. X., Qiu X. Y., Ri M., Shi X. H., Wang Y. L., Li G. D., Tang Z. Y., Angew. Chem. Int. Ed., 2020, 59(9), 3650—3657 |
| [1] | HOU Junying, HOU Chuanyuan, HAO Jianjun, LI Jianchang, WANG Yaya. Fabrication of Copper-doped Porous NiO Heterogeneous Catalyst and Its Application in Aerobic Oxidation of Benzyl Alcohol [J]. Chem. J. Chinese Universities, 2023, 44(6): 20230042. |
| [2] | SONG Jiaxin, CUI Jing, FAN Xiaoqiang, KONG Lian, XIAO Xia, XIE Zean, ZHAO Zhen. Preparation of Mesoporous Silica Supported Highly Dispersed Vanadium Catalyst and Their Catalytic Performance for Selective Oxidation of Ethane [J]. Chem. J. Chinese Universities, 2023, 44(2): 20220532. |
| [3] | LIN Junxu, XI Zhiwei, LI Zhiping, WANG Yingchun. Palladium Catalyzed Selective Synthesis of Pyrrolofuran Derivatives and Carbamates from Propargylic Alcohols and tert⁃Butyl Isonitrile [J]. Chem. J. Chinese Universities, 2023, 44(2): 20220473. |
| [4] | DENG Yuan, WANG Si, FENG Haisong, ZHANG Xin. Theoretical Calculation of Solvent Dependence in Pd-catalyzed Hydrogenation of Furfural [J]. Chem. J. Chinese Universities, 2023, 44(2): 20220486. |
| [5] | TAN Yan, YU Shen, LYU Jiamin, LIU Zhan, SUN Minghui, CHEN Lihua, SU Baolian. Efficient Preparation of Mesoporous γ-Al2O3 Microspheres and Performance of Pd-loaded Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220133. |
| [6] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| [7] | WANG Mingzhi, ZHENG Yanping, WENG Weizheng. Catalytic Methane Combustion over CeO2 Supported PdO and Ce1‒x Pd x O2‒δ Species [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210816. |
| [8] | ZHAO Wanjun, LI Xiao, Dang Hui, WANG Yongzhao, ZHAO Yongxiang. Preparation of Supported Pd-Cu Catalyst and Its Preferential Oxidation of CO Under Hydrogen-rich Atmosphere [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210754. |
| [9] | JIANG Shan, SHEN Qianqian, LI Qi, JIA Husheng, XUE Jinbo. Pd-loaded Defective TiO2 Nanotube Arrays for Enhanced Photocatalytic Hydrogen Production Performance [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220206. |
| [10] | GUO Yang, LIN Kai, XIE Kaiqiang, LIU Sheng. Novel Approach to Isatins via Pd-Cu Catalyzed Oxidative Transformation [J]. Chem. J. Chinese Universities, 2021, 42(9): 2798. |
| [11] | WEN Wei, HUANG Dading, BAO Jingxiao, ZHANG John Z. H.. Residue Specific Binding Mechanisms of PD-1 to Its Monoclonal Antibodies by Computational Alanine Scanning [J]. Chem. J. Chinese Universities, 2021, 42(7): 2161. |
| [12] | WANG Yuxiang, YU Shen, LIU Zhan, LYU Jiamin, LI Xiaoyun, CHEN Lihua, SU Baolian. One-step Synthesis of Amorphous Silica Aluminum Support Materials with Controllable Acidity and Porosity and Catalytic Performance of Their Pd-based Catalysts [J]. Chem. J. Chinese Universities, 2021, 42(6): 1826. |
| [13] | REN Ying, LI Changhua, WANG Tao, XUE Shanshan, ZHANG Tingting, JIA Jianfeng, WU Haishun. Theoretical Studies on Pd-catalyzed Oxidative N─H Carbonylation to Synthesis of 1,3,4-Oxadiazole-2(3H)-one Heterocyclic Compounds [J]. Chem. J. Chinese Universities, 2021, 42(6): 1793. |
| [14] | LI Xiao,XING Lisha,ZHAO Wanjun,WANG Yongzhao,ZHAO Yongxiang. Preparation and Characterization of Pd-Cu/hydroxyapatite Catalyst and Its Catalytic Performance for Room-temperature CO Oxidation in Humid Circumstances† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1600. |
| [15] | HAO Yan, YANG Hua, WANG Xiang, LI Qingyang, ZHAO Pan, TANG Qinghu, SONG Shili, XI Guoxi. Palladium-based Nanocatalysts Supported on Polybenzoxazine for Aromatic Alcohol Oxidation † [J]. Chem. J. Chinese Universities, 2020, 41(4): 757. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||