

Chem. J. Chinese Universities ›› 2023, Vol. 44 ›› Issue (5): 20220732.doi: 10.7503/cjcu20220732
• Review • Previous Articles Next Articles
YAN Dafeng1( ), XIE Chao2, CHEN Chen3
), XIE Chao2, CHEN Chen3
Received:2022-11-25
Online:2023-05-10
Published:2023-01-03
Contact:
YAN Dafeng
E-mail:dafengyan@hnu.edu.cn
Supported by:CLC Number:
TrendMD:
YAN Dafeng, XIE Chao, CHEN Chen. Recent Progress on Strategies for Electrochemical Hydrogen Production Coupling with Oxidation of Inorganic Chemicals[J]. Chem. J. Chinese Universities, 2023, 44(5): 20220732.
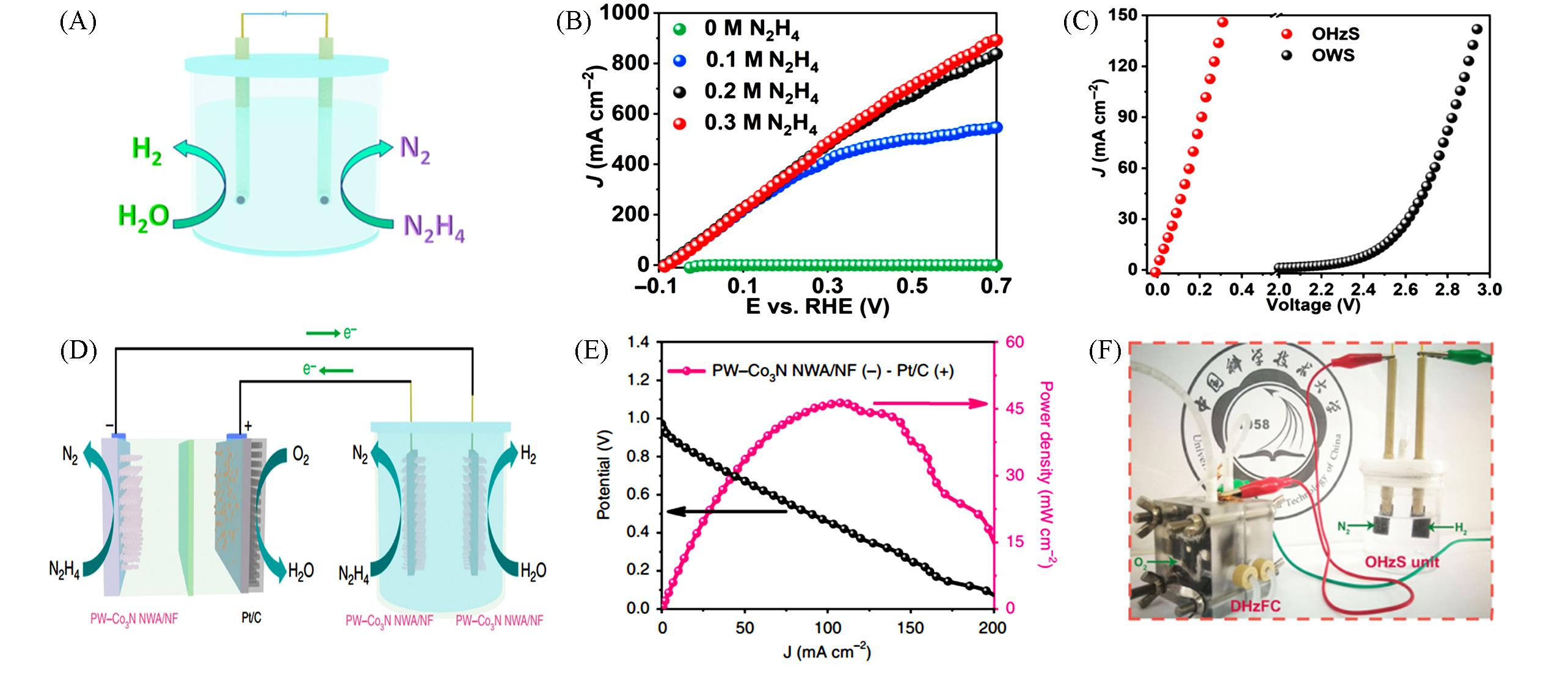
Fig.2 A schematic diagram of the electrochemical N2H4 oxidation coupling with H2 production(A), LSV curves of RuP2⁃CPM in 1.0 mol/L KOH with different concentrations of N2H4(B), comparison of LSV curves for N2H4 oxidation coupling with HER and traditional overall water splitting systems(C) [ 59], schematic illustration of a self⁃powered H2 production system integrating a direct hydrazine fuel cell (DHzFC) and an OHzS(D), the current density⁃voltage and current density⁃power density plots for the designed DHzFC(E), optical image of the designed self⁃powered H2 production system(F) [ 63]
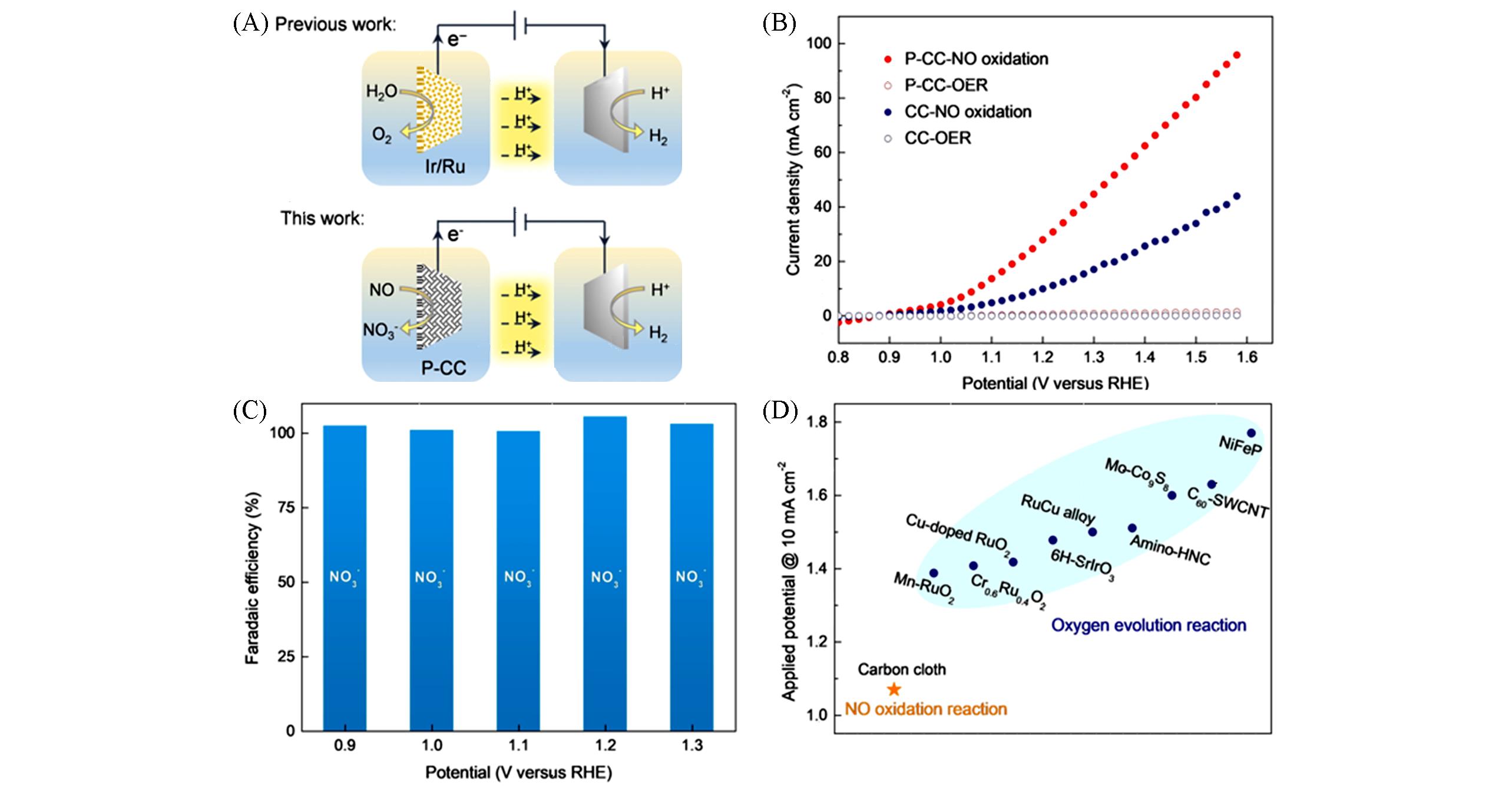
Fig.3 A schematic diagram of the electrochemical NO oxidation coupling with H2 production(A), LSV curves of different samples for electrochemical performance of NO oxidation and OER in 0.5 mol/L H2SO4(B), the Faradaic efficiencies of nitrate for the sample of P⁃CC(C), comparison of the electrochemical NO oxidation with OER(D) [ 70]
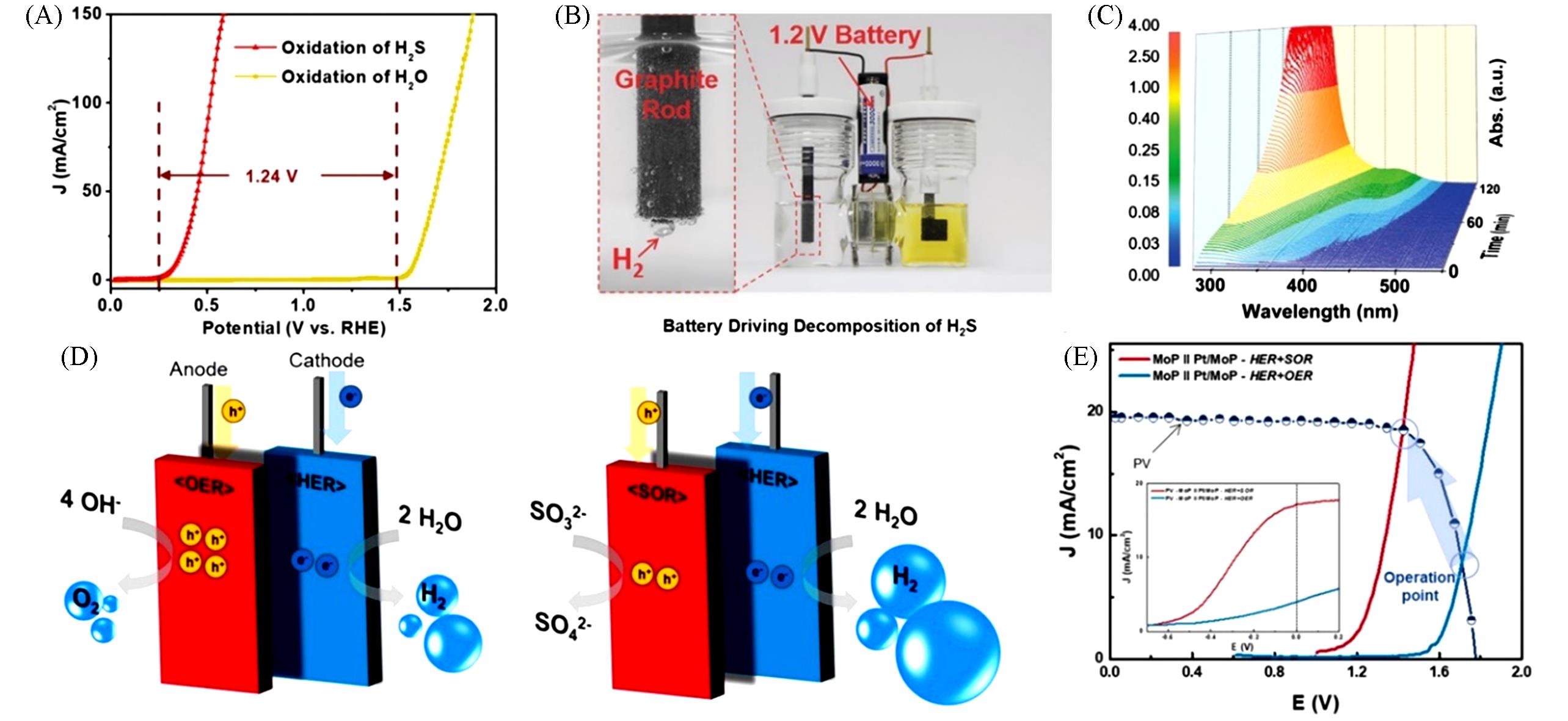
Fig.4 Comparison of SOR and OER polarization curves for CoNi@NGs(A), the photo of a device with a 1.2 V commercial battery directly driving the decomposition of H2S(B), in situ electrochemical UV⁃Vis tests for anodic electrolyte(C) [ 71], scheme for hydrogen production system using SOR instead of OER(D), J⁃ V curve of tandem photovoltaic cell powered electrolytic system for MoP||Pt/MoP catalysts(E) [ 74]
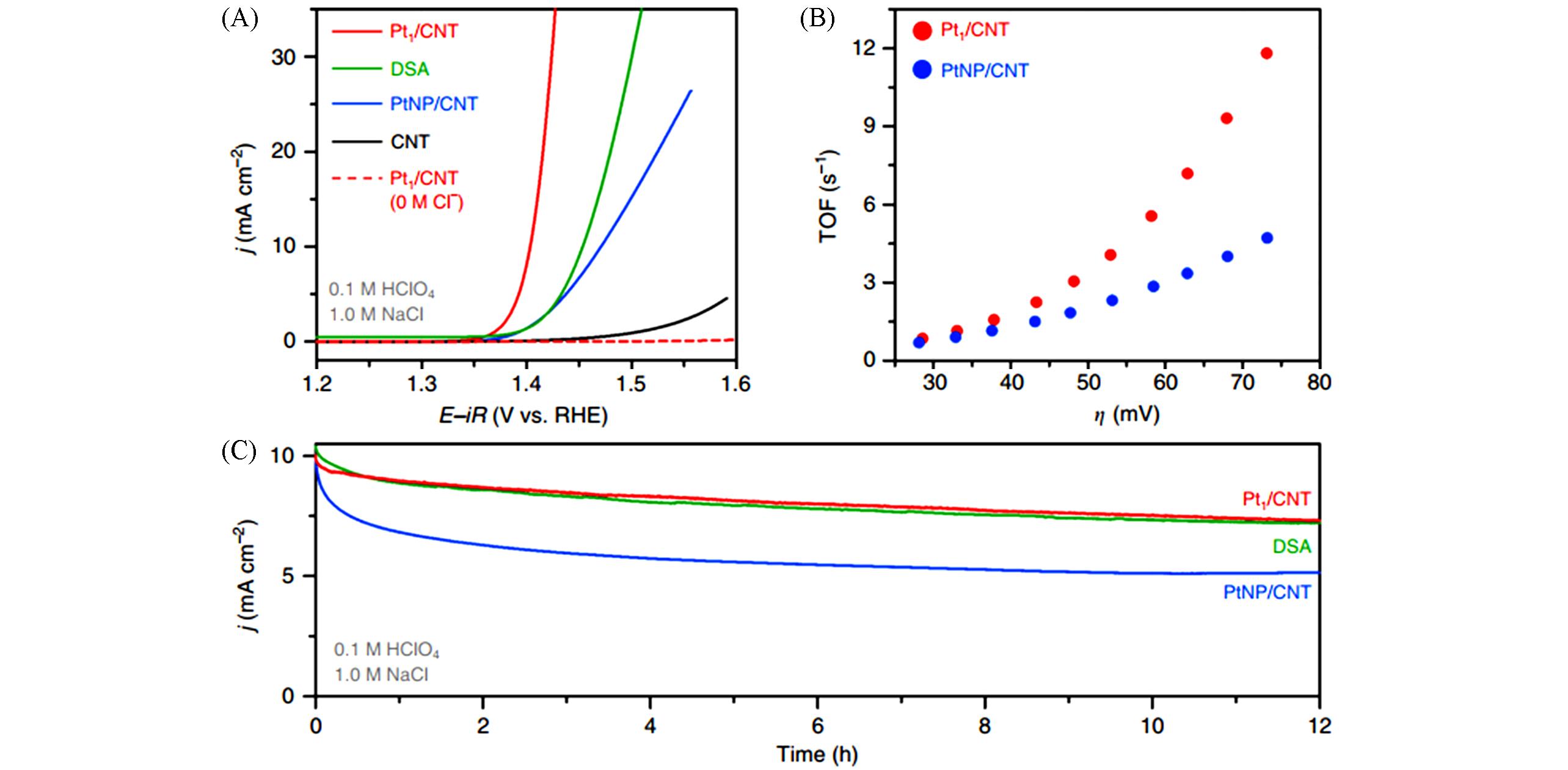
Fig.5 CER polarisation curves of different samples obtained in 0.1 mol/L HClO4+1.0 mol/L NaCl(A), calculated TOFs of Pt1/CNT and PtNP/CNT from the curves of (A)(B), chronoamperograms of Pt1/CNT and PtNP/CNT catalysts measured in 0.1 mol/L HClO4 + 1.0 mol/L NaCl for 12 h(C) [ 76]
| Inorganic chemical | Electrocatalyst | Performance( vs. RHE) | Electrolyte | Reference |
|---|---|---|---|---|
| N2H4 | NiCo/MXene | 43 mV at 500 mA/cm 2 | 1 mol/L KOH+0.5 mol/L N2H4 | [ |
| RuP2 | -70 mV at 10 mA/cm 2 | 1 mol/L KOH+0.3 mol/L N2H4 | [ | |
| Ni/C | -20 mV at 10 mA/cm 2 | 1 mol/L KOH+0.1 mol/L N2H4 | [ | |
| Co3N | -55 mV at 10 mA/cm 2 | 1 mol/L KOH+0.1 mol/L N2H4 | [ | |
| CoSe2 | -17 mV at 10 mA/cm 2 | 1 mol/L KOH+0.5 mol/L N2H4 | [ | |
| NO | Carbon cloth | 1.07 V at 10 mA/cm 2 | 0.5 mol/L H2SO4+ NO | [ |
| H2S | CoNi nanoalloy | 0.25 V at 1 mA/cm 2 | 1 mol/L NaOH+ 1 mol/L Na2S | [ |
| CoFeS2 | 0.6 V at 200 mA/cm 2 | H2S saturated 1 mol/L NaOH | [ | |
| Cu2S | 0.26 V at 10 mA/cm 2 | 1 mol/L NaOH+ 1 mol/L Na2S | [ | |
| Cl2 | Pt1/CNT | 1.4 V at 10 mA/cm 2 | 0.1 mol/L HClO4+ 1 mol/L of Cl - | [ |
| Co3O4 | 1.56 V at 10 mA/cm 2 | Saturated NaCl solution | [ |
Table 1 Summary of recent studies on electrochemical hydrogen production coupling with oxidation of inorganic chemicals
| Inorganic chemical | Electrocatalyst | Performance( vs. RHE) | Electrolyte | Reference |
|---|---|---|---|---|
| N2H4 | NiCo/MXene | 43 mV at 500 mA/cm 2 | 1 mol/L KOH+0.5 mol/L N2H4 | [ |
| RuP2 | -70 mV at 10 mA/cm 2 | 1 mol/L KOH+0.3 mol/L N2H4 | [ | |
| Ni/C | -20 mV at 10 mA/cm 2 | 1 mol/L KOH+0.1 mol/L N2H4 | [ | |
| Co3N | -55 mV at 10 mA/cm 2 | 1 mol/L KOH+0.1 mol/L N2H4 | [ | |
| CoSe2 | -17 mV at 10 mA/cm 2 | 1 mol/L KOH+0.5 mol/L N2H4 | [ | |
| NO | Carbon cloth | 1.07 V at 10 mA/cm 2 | 0.5 mol/L H2SO4+ NO | [ |
| H2S | CoNi nanoalloy | 0.25 V at 1 mA/cm 2 | 1 mol/L NaOH+ 1 mol/L Na2S | [ |
| CoFeS2 | 0.6 V at 200 mA/cm 2 | H2S saturated 1 mol/L NaOH | [ | |
| Cu2S | 0.26 V at 10 mA/cm 2 | 1 mol/L NaOH+ 1 mol/L Na2S | [ | |
| Cl2 | Pt1/CNT | 1.4 V at 10 mA/cm 2 | 0.1 mol/L HClO4+ 1 mol/L of Cl - | [ |
| Co3O4 | 1.56 V at 10 mA/cm 2 | Saturated NaCl solution | [ |
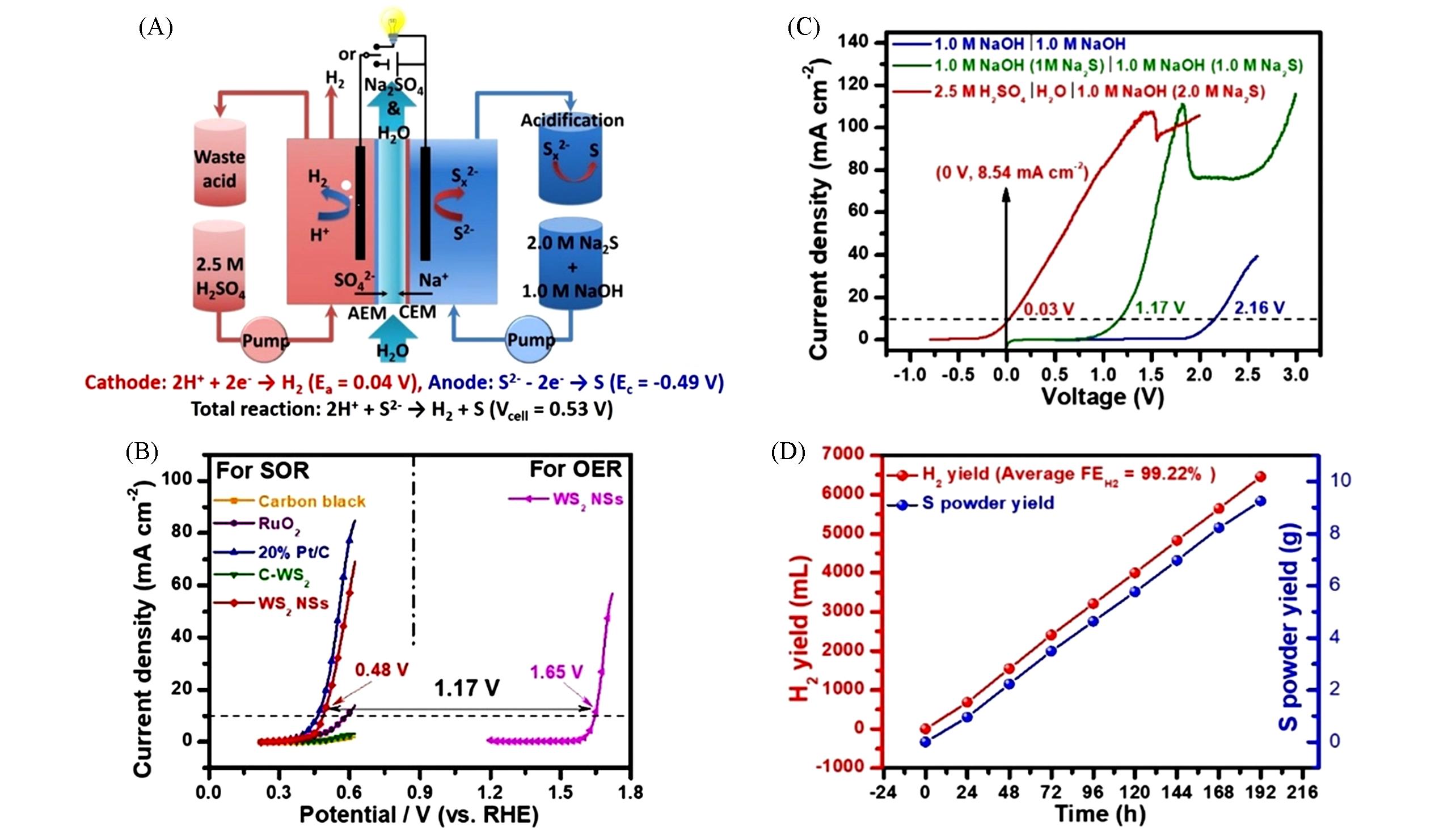
Fig.6 Illustration of the design of the H2 production system coupled with SOR and ENE(A), comparison of LSV curves for different system designs of SOR/HER or OER/HER cell(B), the polarization curves of different samples for electrochemical performance of SOR(C) and the results of yield rates of H2 and sulfur powder(D) [ 55]
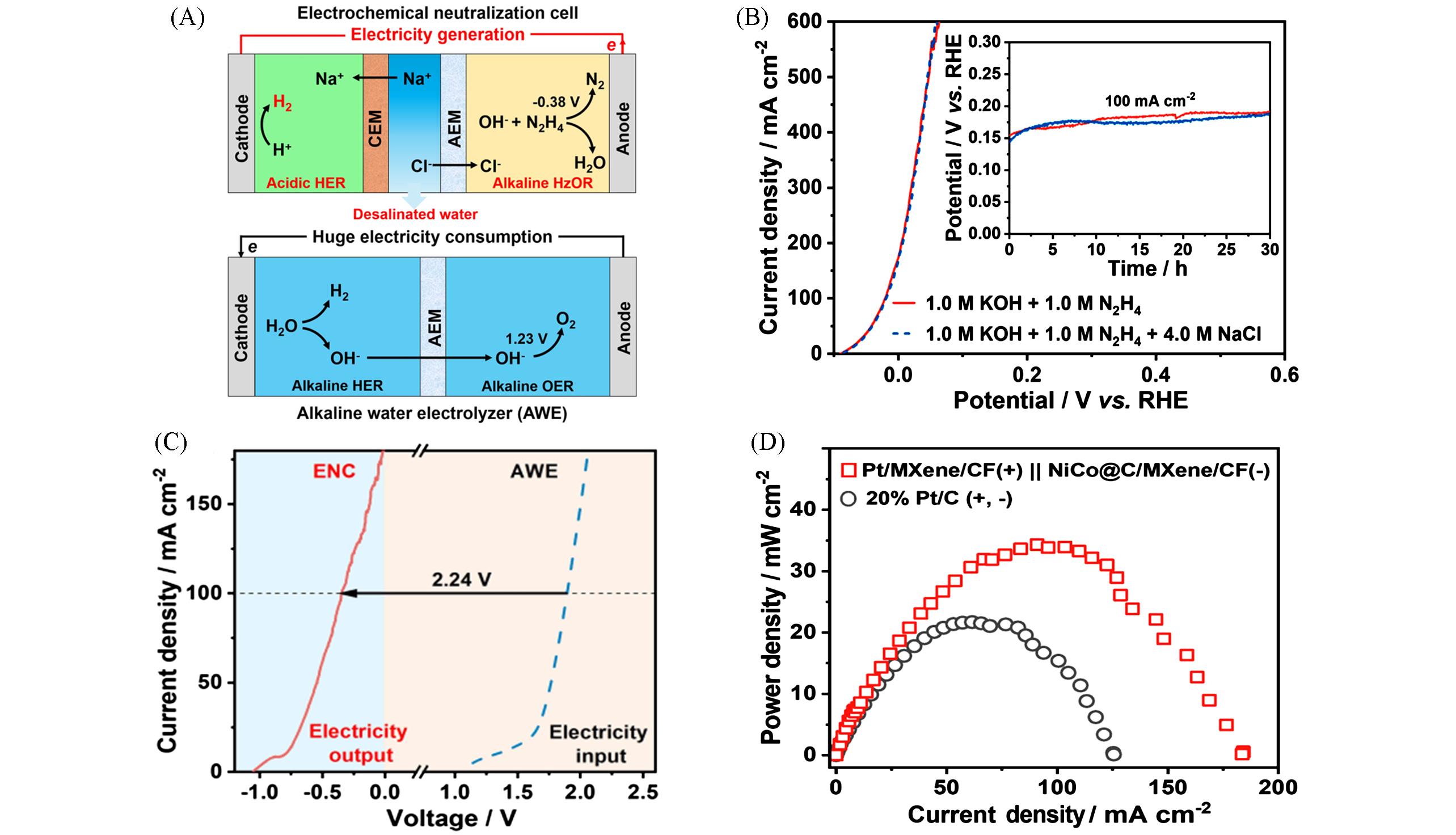
Fig.7 Illustration of the ENE cell for hydrogen production and water desalination with electricity output(A), the LSVs of the electrocatalyst for the HzOR with and without Cl - (B), a comparison of LSVs of electrochemical neutralization cell(ENC) and alkaline water electrolysis(AWE)(C) and the power⁃density curves of the ENC using different samples(D) [ 57](B) Inset are the chronopotentiometric curves at a current density of 100 mA/cm 2 with and without Cl -.Copyright 2022, Wiley-VCH.
| 1 | Turner J. A., Science, 2004, 305, 972—974 |
| 2 | Bogdanov D., Ram M., Aghahosseini A., Gulagi A., Oyewo A. S., Child M., Caldera U., Sadovskaia K., Farfan J., Barbosa L. D. S. N. S., Energy, 2021, 227, 120467 |
| 3 | Jiao Y., Zheng Y., Jaroniec M., Qiao S. Z., Chem. Soc. Rev., 2015, 44, 2060—2086 |
| 4 | Wang Z., Xiao B., Lin Z., Xu Y., Lin Y., Meng F., Zhang Q., Gu L., Fang B., Guo S., Zhong W., Angew. Chem. Int. Ed., 2021, 60, 23388—23393 |
| 5 | Yan D., Dou S., Tao L., Liu Z., Liu Z., Huo J., Wang S., J. Mater. Chem. A, 2016, 4, 13726—13730 |
| 6 | Li M., Zhao Z., Xia Z., Luo M., Zhang Q., Qin Y., Tao L., Yin K., Chao Y., Gu L., Yang W., Yu Y., Lu G., Guo S., Angew. Chem. Int. Ed., 2021, 60, 8243—8250 |
| 7 | Luo Y., Zhang Z., Chhowalla M., Liu B., Adv. Mater., 2022, 34, 2108133 |
| 8 | Xie X. Q., Liu J., Gu C., Li J., Zhao Y., Liu C. S., J. Energy Chem., 2022, 64, 503—510 |
| 9 | Wang Y., Yan D., El Hankari S., Zou Y., Wang S., Adv. Sci., 2018, 5, 1800064 |
| 10 | Kim J. S., Kim B., Kim H., Kang K., Adv. Energy Mater., 2018, 8, 1702774 |
| 11 | Ham K., Hong S., Kang S., Cho K., Lee J., ACS Energy Lett., 2021, 6, 364—370 |
| 12 | Roger I., Shipman M. A., Symes M. D., Nat. Rev. Chem., 2017, 1, 1—13 |
| 13 | Jia Y., Zhang L., Gao G., Chen H., Wang B., Zhou J., Soo M. T., Hong M., Yan X., Qian G., Adv. Mater., 2017, 29, 1700017 |
| 14 | Faber M. S., Jin S., Energy Environ. Sci., 2014, 7, 3519—3542 |
| 15 | Shen Z., Qu M., Shi J., Oropeza F. E., de la Peña O'Shea V. A., Gorni G., Tian C. M., Hofmann J. P., Cheng J., Li J., Zhang K. H. L., J. Energy Chem., 2022, 65, 637—645 |
| 16 | Yan D., Xia C., Zhang W., Hu Q., He C., Xia B. Y., Wang S., Adv. Energy Mater., 2022, 12, 2202317 |
| 17 | You B., Sun Y., Acc. Chem. Res., 2018, 51, 1571—1580 |
| 18 | Yu F., Yu L., Mishra I., Yu Y., Ren Z., Zhou H., Mater. Today Phys., 2018, 7, 121—138 |
| 19 | Xiao Y., Shen Y., Su D., Zhang S., Yang J., Yan D., Fang S., Wang X., J. Mater. Sci. Technol., 2023, 154, 1—8 |
| 20 | Li S., Chen B., Wang Y., Ye M. Y., van Aken P. A., Cheng C., Thomas A., Nat. Mater., 2021, 20, 1240—1247 |
| 21 | Yan D., Li Y., Huo J., Chen R., Dai L., Wang S., Adv. Mater., 2017, 29, 1606459 |
| 22 | Yan D., Li H., Chen C., Zou Y., Wang S., Small Methods, 2019, 3, 1800331 |
| 23 | Xie C., Yan D., Chen W., Zou Y., Chen R., Zang S., Wang Y., Yao X., Wang S., Mater. Today, 2019, 31, 47—68 |
| 24 | Li W., Zhao L., Jiang X., Chen Z., Zhang Y., Wang S., Adv. Funct. Mater., 2022, 2207727 |
| 25 | Xie L., Wang L., Zhao W., Liu S., Huang W., Zhao Q., Nat. Commun., 2021, 12, 5070 |
| 26 | Chen S., Li W. H., Jiang W., Yang J., Zhu J., Wang L., Ou H., Zhuang Z., Chen M., Sun X., Wang D., Li Y., Angew. Chem. Int. Ed., 2022, 61, e202114450 |
| 27 | Yan D., Chen R., Xiao Z., Wang S., Electrochim. Acta, 2019, 303, 316—322 |
| 28 | Yan D., Xia C., He C., Liu Q., Chen G., Guo W., Xia B. Y., Small, 2022, 18, 2106606 |
| 29 | Rausch B., Symes M. D., Chisholm G., Cronin L., Science, 2014, 345, 1326—1330 |
| 30 | Du L., Sun Y., You B., Mater. Rep. Energy, 2021, 1, 100004 |
| 31 | Symes M. D., Cronin L., Nat. Chem., 2013, 5, 403—409 |
| 32 | You B., Liu X., Liu X., Sun Y., ACS Catal., 2017, 7, 4564—4570 |
| 33 | You B., Jiang N., Liu X., Sun Y., Angew. Chem. Int. Ed., 2016, 55, 9913—9917 |
| 34 | Du J., Li F., Sun L., Chem. Soc. Rev., 2021, 50, 2663—2695 |
| 35 | Chen G., Li X., Feng X., Angew. Chem. Int. Ed., 2022, e202209014 |
| 36 | Wang T., Cao X., Jiao L., Angew. Chem. Int. Ed., 2022, e202213328 |
| 37 | Li R., Xiang K., Peng Z., Zou Y., Wang S., Adv. Energy Mater., 2021, 11, 2102292 |
| 38 | Zhang Y., Zhou B., Wei Z., Zhou W., Wang D., Tian J., Wang T., Zhao S., Liu J., Tao L., Wang S., Adv. Mater., 2021, 33, 2104791 |
| 39 | Chen Y., Lavacchi A., Miller H., Bevilacqua M., Filippi J., Innocenti M., Marchionni A., Oberhauser W., Wang L., Vizza F., Nat. Commun., 2014, 5, 4036 |
| 40 | Barwe S., Weidner J., Cychy S., Morales D. M., Dieckhöfer S., Hiltrop D., Masa J., Muhler M., Schuhmann W., Angew. Chem. Int. Ed., 2018, 57, 11460—11464 |
| 41 | Tong Y., Chen P., Zhang M., Zhou T., Zhang L., Chu W., Wu C., Xie Y., ACS Catal., 2018, 8, 1—7 |
| 42 | Huang Y., Chong X., Liu C., Liang Y., Zhang B., Angew. Chem., 2018, 130, 13347—13350 |
| 43 | Zhou B., Li Y., Zou Y., Chen W., Zhou W., Song M., Wu Y., Lu Y., Liu J., Wang Y., Wang S., Angew. Chem. Int. Ed., 2021, 60, 22908—22914 |
| 44 | Chen W., Xu L., Zhu X., Huang Y. C., Zhou W., Wang D., Zhou Y., Du S., Li Q., Xie C., Tao L., Dong C. L., Liu J., Wang Y., Chen R., Su H., Chen C., Zou Y., Li Y., Liu Q., Wang S., Angew. Chem. Int. Ed., 2021, 60, 7297—7307 |
| 45 | Wang T., Huang Z., Liu T., Tao L., Tian J., Gu K., Wei X., Zhou P., Gan L., Du S., Zou Y., Chen R., Li Y., Fu X. Z., Wang S., Angew. Chem. Int. Ed., 2022, 61, e202115636 |
| 46 | Deng C., Toe C. Y., Li X., Tan J., Yang H., Hu Q., He C., Adv. Energy Mater., 2022, 12, 2201047 |
| 47 | Cha H. G., Choi K. S., Nat. Chem., 2015, 7, 328—333 |
| 48 | Lhermitte C. R., Sivula K., ACS Catal., 2019, 9, 2007—2017 |
| 49 | Yan D., Mebrahtu C., Wang S., Palkovits R., Angew. Chem. Int. Ed., 2022. e202214333 |
| 50 | Chen L., Shi J., J. Mater. Chem. A, 2018, 6, 13538—13548 |
| 51 | Luo H., Barrio J., Sunny N., Li A., Steier L., Shah N., Stephens I. E., Titirici M. M., Adv. Energy Mater., 2021, 11, 2101180 |
| 52 | Ifkovits Z. P., Evans J. M., Meier M. C., Papadantonakis K. M., Lewis N. S., Energy Environ. Sci., 2021. 14, 4740—4759 |
| 53 | Li Y., Wei X., Chen L., Shi J., Angew. Chem. Int. Ed., 2021, 60, 19550—19571 |
| 54 | Anantharaj S., Noda S., Jothi V. R., Yi S., Driess M., Menezes P. W., Angew. Chem. Int. Ed., 2021, 60, 18981—19006 |
| 55 | Yi L., Ji Y., Shao P., Chen J., Li J., Li H., Chen K., Peng X., Wen Z., Angew. Chem. Int. Ed., 2021, 60, 21550—21557 |
| 56 | Wang D., He N., Xiao L., Dong F., Chen W., Zhou Y., Chen C., Wang S., Angew. Chem., 2021, 133, 24810—24816 |
| 57 | Sun F., He D., Yang K., Qiu J., Wang Z., Angew. Chem. Int. Ed., 2022, e202203929 |
| 58 | Zhang J. Y., Wang H., Tian Y., Yan Y., Xue Q., He T., Liu H., Wang C., Chen Y., Xia B. Y., Angew. Chem. Int. Ed., 2018, 57, 7649—7653 |
| 59 | Li Y., Zhang J., Liu Y., Qian Q., Li Z., Zhu Y., Zhang G., Sci. Adv., 2020, 6, eabb4197 |
| 60 | Liu X., He J., Zhao S., Liu Y., Zhao Z., Luo J., Hu G., Sun X., Ding Y., Nat. Commun., 2018, 9, 4365 |
| 61 | Sun F., Qin J., Wang Z., Yu M., Wu X., Sun X., Qiu J., Nat. Commun., 2021, 12, 4182 |
| 62 | Zhuang S., Tang Y., Tai X., Huang Q., Wan P., Chen Y., Sun Y., Pan J., Yang X. J., Appl. Catal. B: Environ., 2022, 306, 121132 |
| 63 | Liu Y., Zhang J., Li Y., Qian Q., Li Z., Zhu Y., Zhang G., Nat. Commun., 2020, 11, 1853 |
| 64 | Hu W., Selleri T., Gramigni F., Fenes E., Rout K. R., Liu S., Nova I., Chen D., Gao X., Tronconi E., Angew. Chem., 2021, 133, 7273—7280 |
| 65 | Zhang L., Liang J., Wang Y., Mou T., Lin Y., Yue L., Li T., Liu Q., Luo Y., Li N., Tang B., Liu Y., Gao S., Alshehri A. A., Guo X., Ma D., Sun X., Angew. Chem. Int. Ed., 2021, 60, 25263—25268 |
| 66 | Liu P., Liang J., Wang J., Zhang L., Li J., Yue L., Ren Y., Li T., Luo Y., Li N., Tang B., Liu Q., Asiri A. M., Kong Q., Sun X., Chem. Commun., 2021, 57, 13562—13565 |
| 67 | Ko B. H., Hasa B., Shin H., Zhao Y., Jiao F., J. Am. Chem. Soc., 2022, 144, 1258—1266 |
| 68 | Li Y., Cheng C., Han S., Huang Y., Du X., Zhang B., Yu Y., ACS Energy Lett., 2022, 7, 1187—1194 |
| 69 | Berisha L. S., Kalcher K., Maloku A., Andoni E., Arbneshi T., J. Adv. Chem., 2009, 5, 792—799 |
| 70 | Wang D., He N., Xiao L., Dong F., Chen W., Zhou Y., Chen C., Wang S., Angew. Chem. Int. Ed., 2021, 60, 24605—24611 |
| 71 | Zhang M., Guan J., Tu Y., Chen S., Wang Y., Wang S., Yu L., Ma C., Deng D., Bao X., Energy Environ. Sci., 2020, 13, 119—126 |
| 72 | Kumar M., Nagaiah T. C., J. Mater. Chem. A, 2022, 10, 7048—7057 |
| 73 | Pei Y., Cheng J., Zhong H., Pi Z., Zhao Y., Jin F., Green Chem., 2021, 23, 6975—6983 |
| 74 | Park J., Yoon H., Lee D. Y., Ji S. G., Yang W., Tilley S. D., Sung M. C., Park I. J., Tan J., Lee H., Kim J. Y., Kim D. W., Moon J., Appl. Catal. B: Environ., 2022, 305, 121045 |
| 75 | Zhu X., Wang P., Wang Z., Liu Y., Zheng Z., Zhang Q., Zhang X., Dai Y., Whangbo M. H., Huang B., J. Mater. Chem. A, 2018, 6, 12718—12723 |
| 76 | Lim T., Jung G. Y., Kim J. H., Park S. O., Park J., Kim Y. T., Kang S. J., Jeong H. Y., Kwak S. K., Joo S. H., Nat. Commun., 2020, 11, 412 |
| 77 | Zhu Y., Zhang J., Qian Q., Li Y., Li Z., Liu Y., Xiao C., Zhang G., Xie Y., Angew. Chem. Int. Ed., 2022, 61, e202113082 |
| 78 | Ding Y., Cai P., Wen Z., Chem. Soc. Rev., 2021, 50, 1495—1511 |
| 79 | Zhang M., Chen J., Li H., Cai P., Li Y., Wen Z., Nano Energy, 2019, 61, 576—583 |
| 80 | Bhat Z. M., Pandit D., Ardo S., Thimmappa R., Kottaichamy A. R., Dargily N. C., Devendrachari M. C., Thotiyl M. O., Joule, 2020, 4, 1730—1742 |
| 81 | Zhang M., Li H., Cai P., Chen K., Wen Z., Adv. Funct. Mater., 2021, 31, 2103248 |
| 82 | Wang G., Chen J., Cai P., Jia J., Wen Z., J. Mater. Chem. A, 2018, 6, 17763—17770 |
| [1] | CHEN Wangsong, LUO Lan, LIU Yuguang, ZHOU Hua, KONG Xianggui, LI Zhenhua, DUAN Haohong. Recent Progress in Photoelectrochemical H2 Production Coupled with Biomass-derived Alcohol/aldehyde Oxidation [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210683. |
| [2] | JIANG Shan, SHEN Qianqian, LI Qi, JIA Husheng, XUE Jinbo. Pd-loaded Defective TiO2 Nanotube Arrays for Enhanced Photocatalytic Hydrogen Production Performance [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220206. |
| [3] | LI Shurong, WANG Lin, CHEN Yuzhen, JIANG Hailong. Research Progress of Metal⁃organic Frameworks on Liquid Phase Catalytic Chemical Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210575. |
| [4] | XUE Jinbo, GAO Guoxiang, SHEN Qianqian, LIU Tianwu, LIU Xuguang, JIA Husheng. Construction of a Novel S-scheme CdS-BiVO4 Heterojunction Photoelectrodes and Research on Hydrogen Production [J]. Chem. J. Chinese Universities, 2021, 42(8): 2493. |
| [5] | WU Qiliang, MEI Jinghao, LI Zheng, FAN Haidong, ZHANG Yanwei. Photo-thermal Coupling Water Splitting over Fe-doped TiO2 with Various Nanostructures [J]. Chem. J. Chinese Universities, 2021, 42(6): 1837. |
| [6] | XU Anqi, LI Bin, DU Fanglin. Synthesis of Ordered Mesoporous TiO2 and Their Application for Hydrogen Production from Photocatalytic Water-splitting [J]. Chem. J. Chinese Universities, 2021, 42(4): 978. |
| [7] | WANG Yishu, LI Xue, YAN Li, XU Hongyun, ZHU Yuxin, SONG Yanhua, CUI Yanjuan. Photocatalytic Reduction Performance of Z-scheme Two-dimensional BCN/Sn3O4 Composite Materials [J]. Chem. J. Chinese Universities, 2021, 42(12): 3722. |
| [8] | SUN Dawei,LI Yuejun,CAO Tieping,ZHAO Yanhui,YANG Diankai. Preparation of Dy 3+-doped YVO4/TiO2 Composite Nanofibers with Three-dimensional Net-like Structure and Enhanced Photocatalytic Activity for Hydrogen Evolution † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2348. |
| [9] | DAI Hongyan, YANG Huimin, LIU Xian, JIAN Xuan, GUO Minmin, CAO Lele, LIANG Zhenhai. Preparation and Electrochemical Evaluation of MoS2/graphene as a Catalyst for Hydrogen Evolution in Microbial Electrolysis Cell† [J]. Chem. J. Chinese Universities, 2018, 39(2): 351. |
| [10] | TIAN Yi, LI Yuexiang, PENG Shaoqin. Effect of Y2O3 Supporter on the Catalytic Hydrogen Production from an Aqueous Formaldehyde Solution Catalyzed by Metal Cu Loaded on Y2O3† [J]. Chem. J. Chinese Universities, 2017, 38(10): 1841. |
| [11] | LU Yonghong, WU Pingxiao, HUANG Junyi, TRAN Lytuong, ZHU Nengwu, DANG Zhi. Alkaline-assisted Hydrothermal Fabrication of CdZnS with Enhanced Visible-light Photocatalytic Performance† [J]. Chem. J. Chinese Universities, 2015, 36(8): 1563. |
| [12] | QU Yang, ZHOU Wei, REN Zhiyu, PAN Kai, JIANG Le, FU Honggang. Controllable Preparation of CdTiO3 Nanorods and Their Photocatalytic Properties for Hydrogen Production† [J]. Chem. J. Chinese Universities, 2014, 35(5): 995. |
| [13] | ZHAO Weiliang, LI Cong, HAN Xu, LI Tingting, ZHANG Guiju, GAN Xin, LI Fumin, FU Wenfu. Syntheses, Properties and Photocatalytic Hydrogen Evolution Efficiency of Cyclometalated Pt(Ⅱ) Complexes Bearing S-methylphenyl Group† [J]. Chem. J. Chinese Universities, 2014, 35(10): 2214. |
| [14] | HAO Wei-Chang*, ZHAI Ting-Ting, WANG Xu, WANG Tian-Min. Fabrication and Catalytic Activity of Nickel Core-shell Structure [J]. Chem. J. Chinese Universities, 2010, 31(6): 1213. |
| [15] | ZHANG Xin-Rong, WANG Lu-Cun, YAO Cheng-Zhang, CAO Yong, DAI Wei-Lin, FAN Kang-Nian, WU Dong, SUN Yu-Han . Highly Effective Hydrogen Production from Steam Reforming of CH3OH over Cu/ZnO/Al2O3 Catalysts Promoted by Nanostructured Carbon Materials [J]. Chem. J. Chinese Universities, 2004, 25(11): 2125. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||