

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (12): 2549.doi: 10.7503/cjcu20190382
• Physical Chemistry • Previous Articles Next Articles
Long TIAN1,4,Yan LONG1,2,*( ),Shuyan SONG1,Cheng WANG1,3,*(
),Shuyan SONG1,Cheng WANG1,3,*( )
)
Received:2019-07-10
Online:2019-12-04
Published:2019-12-04
Contact:
Yan LONG,Cheng WANG
E-mail:longyan@sicnu.edu.cn;cwang@tjut.edu.cn
Supported by:CLC Number:
TrendMD:
Long TIAN,Yan LONG,Shuyan SONG,Cheng WANG. Synthesis of Flower-like Structured Mn/CuO-CeO2 and the Catalytic Performance for CO Oxide Reaction †[J]. Chem. J. Chinese Universities, 2019, 40(12): 2549.
| Sample | Molar ratio of Ce/Cu/Mn | Sample | Molar ratio of Ce/Cu/Mn | ||
|---|---|---|---|---|---|
| In precursor | In product | In precursor | In product | ||
| CeO2 | 25:0:0 | 25:0:0 | 0.08Mn/CuO-CeO2 | 25:5:2 | 25:5.18:1.73 |
| CuO-CeO2 | 25:5:0 | 25:5.09:0 | 0.20Mn/CuO-CeO2 | 25:5:5 | 25:5.37:5.10 |
| 0.04Mn/CuO-CeO2 | 25:5:1 | 25:4.89:0.87 | |||
| Sample | Molar ratio of Ce/Cu/Mn | Sample | Molar ratio of Ce/Cu/Mn | ||
|---|---|---|---|---|---|
| In precursor | In product | In precursor | In product | ||
| CeO2 | 25:0:0 | 25:0:0 | 0.08Mn/CuO-CeO2 | 25:5:2 | 25:5.18:1.73 |
| CuO-CeO2 | 25:5:0 | 25:5.09:0 | 0.20Mn/CuO-CeO2 | 25:5:5 | 25:5.37:5.10 |
| 0.04Mn/CuO-CeO2 | 25:5:1 | 25:4.89:0.87 | |||
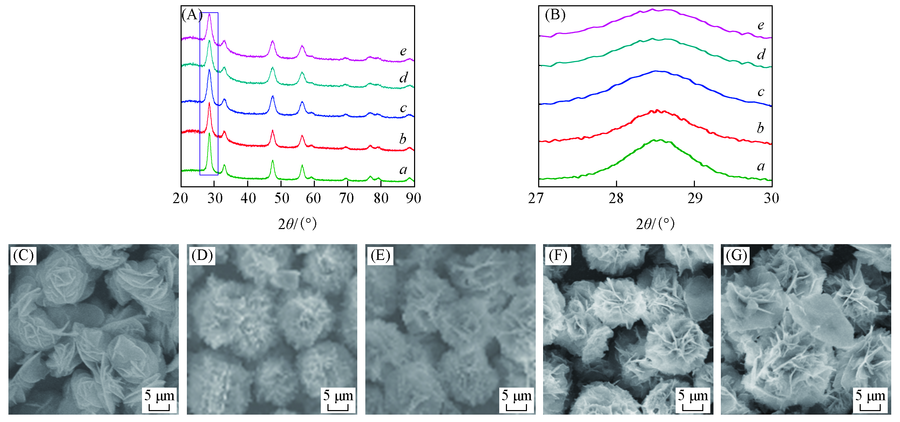
Fig.4 XRD patterns(A, B) and SEM images(C—G) of CeO2, CuO-CeO2 and xMn/CuO-CeO2 a. CeO2; b. CuO-CeO2; c. 0.04Mn/CuO-CeO2; d. 0.08Mn/CuO-CeO2; e. 0.20Mn/CuO-CeO2; (C) CeO2; (D) CuO-CeO2; (E) 0.04Mn/CuO-CeO2; (F) 0.08Mn/CuO-CeO2; (G) 0.20Mn/CuO-CeO2.
| [1] |
Tang W., Li W., Shan X., Wu X., Chen Y ., Mater. Lett., 2015,140, 95— 98
doi: 10.1016/j.matlet.2014.11.011 URL |
| [2] |
Feng Z., Zhang M., Ren Q., Mo S., Peng R., Yan D., Fu M., Chen L., Wu J., Ye D ., Chem. Eng. J., 2019,369, 18— 25
doi: 10.1016/j.cej.2019.03.051 URL |
| [3] |
Long Y., Li J., Wu L., Wang Q., Liu Y., Wang X., Song S., Zhang H ., Nano Res., 2019,12, 869— 875
doi: 10.1007/s12274-018-2315-x URL |
| [4] |
Long Y., Song S., Li J., Wu L., Wang Q., Liu Y., Jin R., Zhang H ., ACS Catal., 2018,8, 8506— 8512
doi: 10.1021/acscatal.8b01851 URL |
| [5] |
Adijanto L., Sampath A., Yu A. S., Cargnello M., Fornasiero P., Gorte R. J., Vohs J. M ., ACS Catal., 2013,3, 1801— 1809
doi: 10.1021/cs4004112 URL |
| [6] |
Montini T., Melchionna M., Monai M., Fornasiero P ., Chem. Rev., 2016,116, 5987— 6041
doi: 10.1021/acs.chemrev.5b00603 URL pmid: 27120134 |
| [7] |
Yang W., Wang X., Song S., Zhang H ., Chem, 2019,5, 1743— 1774
doi: 10.1016/j.chempr.2019.04.009 URL |
| [8] |
Murugan B., Ramaswamy A. V., Srinivas D., Gopinath C. S., Ramaswamy V ., Chem. Mater., 2005,17, 3983— 3993
doi: 10.1021/cm050401j URL |
| [9] |
Wang J., Cheng L., An W., Xu J., Men Y ., Catal. Sci. Technol., 2016,6, 7342— 7350
doi: 10.1039/C6CY01366J URL |
| [10] |
Deng X., Li M., Zhang J., Hu X., Zheng J., Zhang N., Chen B. H., Chem. Eng. J., 2017,313, 544— 555
doi: 10.1016/j.cej.2016.12.088 URL |
| [11] |
Neves T. M., Frantz T. S., Couto do Schenque E. C., Gelesky M. A., Mortola V. B ., Environ. Technol. Innovations, 2017,8, 349— 359
doi: 10.1016/j.eti.2017.08.003 URL |
| [12] |
Yang S., Wang J., Chai W., Zhu J., Men Y ., Catal. Sci. Technol., 2019,9, 1699— 1709
doi: 10.1039/C8CY02605J URL |
| [13] |
Putla S., Amin M. H., Reddy B. M., Nafady A., Al Farhan K. A., Bhargava S. K ., ACS Appl. Mater. Interfaces, 2015,7, 16525— 16535
doi: 10.1021/acsami.5b03988 URL pmid: 26214855 |
| [14] |
Zhai G., Wang J., Chen Z., Yang S., Men Y ., J. Hazard. Mater., 2019,363, 214— 226
doi: 10.1016/j.jhazmat.2018.08.065 URL pmid: 30308360 |
| [15] |
Wang Y., Wang J., Chen H., Yao M., Li Y ., Chem. Eng. Sci., 2015,135, 294— 300
doi: 10.1016/j.ces.2015.03.024 URL |
| [16] |
Du L., Wang W., Yan H., Wang X., Jin Z., Song Q., Si R., Jia C ., J. Rare Earths, 2017,35, 1186— 1196
doi: 10.1016/j.jre.2017.04.005 URL |
| [17] | Xie Y., Wu J., Jing G., Zhang H., Zeng S., Tan X., Zou X., Wen J., Su H., Zhong C. J ., Cui P., Appl. Catal. B, 2018,239, 665— 676 |
| [18] |
Nakagawa K., Ohshima T., Tezuka Y., Katayama M., Katoh M., Sugiyama S ., Catal. Today, 2015,246, 67— 71
doi: 10.1016/j.cattod.2014.08.005 URL |
| [19] | Zhu X., Gao X., Qin R., Zeng Y., Qu R., Zheng C., Tu X ., Appl. Catal. B, 2015,170, 293— 300 |
| [20] |
Jiang Y., Gao J., Zhang Q., Liu Z., Fu M., Wu J., Hu Y., Ye D ., Chem. Eng. J., 2019,371, 78— 87
doi: 10.1016/j.cej.2019.03.233 URL |
| [21] |
Lin X., Li S., He H., Wu Z., Wu J., Chen L., Ye D., Fu M ., Appl. Catal. B, 2018,223, 91— 102
doi: 10.1016/j.apcatb.2017.06.071 URL |
| [22] |
Gandhe A. R., Rebello J. S., Figueiredo J. L., Fernandes J. B ., Appl. Catal. B, 2007,72, 129— 135
doi: 10.1016/j.apcatb.2006.10.017 URL |
| [23] | Li J., Han Y., Zhu Y., Zhou R ., Appl. Catal. B, 2011,108, 72— 80 |
| [24] |
Lu B., Li Z., Kawamoto K ., Mater. Res. Bull., 2013,48, 2504— 2510
doi: 10.1016/j.materresbull.2013.03.016 URL |
| [25] |
Moretti E., Lenarda M., Riello P., Storaro L., Talon A., Frattini R., Reyes-Carmona A., Jimenez-Lopez A., Rodriguez-Castellon E ., Appl. Catal. B, 2013,129, 556— 565
doi: 10.1016/j.apcatb.2012.10.009 URL |
| [26] |
Antonio Cecilia J., Arango-Diaz A., Marrero-Jerez J., Nunez P., Moretti E., Storaro L., Rodriguez-Castellon E ., Catalysts, 2017,7, 160
doi: 10.3390/catal7050160 URL |
| [27] |
Castanet U., Feral-Martin C., Demourgues A., Neale R. L., Sayle D. C., Caddeo F., Flitcroft J. M., Caygill R., Pointon B. J., Molinari M., Majimel J ., ACS Appl. Mater. Interfaces, 2019,11, 11384— 11390
doi: 10.1021/acsami.8b21667 URL pmid: 30843391 |
| [28] |
Arango-Diaz A., Cecilia J. A., Moretti E., Talon A., Nunez P., Marrero-Jerez J., Jimenez-Jimenez J., Jimenez-Lopez A., Rodriguez-Castellon E ., Int. J. Hydrogen Energy, 2014,39, 4102— 4108
doi: 10.1016/j.ijhydene.2013.04.062 URL |
| [29] |
Arango-Diaz A., Cecilia J. A., dos Santos-Gomez L., Marrero-Lopez D., Losilla E. R., Jimenez-Jimenez J., Rodriguez-Castellon E ., Int. J. Hydrogen Energy, 2015,40, 11254— 11260
doi: 10.1016/j.ijhydene.2015.04.094 URL |
| [30] |
Chen H., Sayari A., Adnot A., Larachi F ., Appl. Catal. B, 2001,32, 195— 204
doi: 10.1016/S0926-3373(01)00136-9 URL |
| [31] |
Liu W., Liu X., Feng L., Guo J., Xie A., Wang S., Zhang J., Yang Y ., Nanoscale, 2014,6, 10693— 10700
doi: 10.1039/c4nr02485k URL |
| [32] |
Singhania A., Gupta S. M ., J. Nanosci. Nanotechnol., 2019,19, 5220— 5226
doi: 10.1166/jnn.2019.16825 URL pmid: 30913837 |
| [33] |
Buarod E., Pithakratanayothin S., Naknaka S., Chaiyasith P., Yotkaew T., Tosangthum N., Tongsri R ., Powder Technol., 2015,269, 118— 126
doi: 10.1016/j.powtec.2014.08.052 URL |
| [34] |
Zhao F., Li S., Wu X., Yue R., Li W., Chen Y ., RSC Adv., 2019,9, 2343— 2352
doi: 10.1039/C8RA09626K URL |
| [1] | WANG Mingzhi, ZHENG Yanping, WENG Weizheng. Catalytic Methane Combustion over CeO2 Supported PdO and Ce1‒x Pd x O2‒δ Species [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210816. |
| [2] | ZHAO Mengyang, HUANG Ziyang. Preparation and in vitro Bioactivity of HA/CuO/SrCO3 Gradiently Composite Coating [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210644. |
| [3] | JIN Xin, FENG Xilan, LIU Dapeng, SU Yutong, ZHANG Zheng, ZHANG Yu. Auto-redox Strategy for the Synthesis of Co3O4/CeO2 Nanocomposites and Their Structural Optimization Towards Catalytic CO Oxidation [J]. Chem. J. Chinese Universities, 2020, 41(4): 652. |
| [4] | WANG Yongpeng,XU Zibo,LIU Mengzhu,ZHANG Haibo,JIANG Zhenhua. Non-enzymatic Glucose Sensor Based on the Electrospun Porous Foamy Copper Oxides Micro-nanofibers† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1310. |
| [5] | HUANG Rui,YAO Zhilong,SUN Peiyong,ZHANG Shenghong. Effect of Structure and Properties of CuO-WO3-ZrO2 on Hydrogenation Catalytic of Benzaldehyde† [J]. Chem. J. Chinese Universities, 2019, 40(5): 1005. |
| [6] | JIANG Yilan, YUAN Long, WANG Xiyang, HUANG Keke, FENG Shouhua. Effect of Defect Tuning on the Catalytic Behavior of Perovskite Structure Lanthanum Manganite† [J]. Chem. J. Chinese Universities, 2018, 39(3): 416. |
| [7] | ZHANG Jin, SHI Tiancai, LUO Liwen, LIU Jia, LIU Rong, LIU Le, LIANG Ming, MA Yangmin. One-pot Synthesis of β-Carboline Derivatives Catalyzed by CuO Nanoparticles† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2411. |
| [8] | WANG Mo, LI Xiaowei, SHAO Changlu, ZHAO Yingqian, XIN Jiayu, HAN Chaohan, LI Xinghua, LIU Yichun. Preparation of p-CuO/n-In2O3 Heterojunction Nanofibers and Their Gas Sensing Properties† [J]. Chem. J. Chinese Universities, 2017, 38(9): 1524. |
| [9] | WANG Cheng, GUO Lihong, LI Xingang, MA Kui, DING Tong, WANG Xinlei, CHENG Qingpeng, TIAN Ye. CuO Catalyst Supported on CeO2 Prepared by Oxalate Thermal Decomposition Method for Preferential Oxidation of CO† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2296. |
| [10] | LIU Yaoyao, DING Tong, ZHAO Dongyue, GAO Zhongnan, GUO Lihong, TIAN Ye, LI Xingang. Effect of Potassium Loading on the NOx Storage and Reduction Performance of the CuO/K2CO3/MgAl2O4 Catalyst at High Temperature† [J]. Chem. J. Chinese Universities, 2017, 38(11): 2006. |
| [11] | HE Jianping, ZHANG Lei, CHEN Lin, YANG Zhanxu, TONG Yufei. Effect of CeO2 on Cu/Zn-Al Catalysts Derived from Hydrotalcite Precursor for Methanol Steam Reforming† [J]. Chem. J. Chinese Universities, 2017, 38(10): 1822. |
| [12] | ZENG Liangpeng, HUANG Fan, ZHU Xing, ZHENG Min, LI Kongzhai. Chemical Looping Conversion of Methane over CeO2-based and Co3O4-based Co3O4-CeO2 Oxygen Carriers:Controlling of Product Selectivity† [J]. Chem. J. Chinese Universities, 2017, 38(1): 115. |
| [13] | LIU Rong, ZHA Fei, YANG Aimei, CHANG Yue. Synthesis of Light Olefins from CO2 Hydrogenation Catalyzed over Rare Earths Modified CuO-ZnO-ZrO2/SAPO-34† [J]. Chem. J. Chinese Universities, 2016, 37(5): 964. |
| [14] | WANG Wenliang, GENG Jing, LI Lufei, CHANG Jianmin. Catalytic Properties of Fast Pyrolysis Char Loaded with Cu-Zn on Alkali Lignin Pyrolysis for Monophenols† [J]. Chem. J. Chinese Universities, 2016, 37(4): 736. |
| [15] | LIN Xiao, LI Youji, LI Ming, TANG Ningmei, LI Ziqin, HAN Zhiying. Preparation and Photoelectrocatalytic Performance of CuO/V2O5/FTO Composite Nanofibers Electrode† [J]. Chem. J. Chinese Universities, 2016, 37(12): 2246. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||