

Chem. J. Chinese Universities ›› 2020, Vol. 41 ›› Issue (1): 162.doi: 10.7503/cjcu20190340
• Physical Chemistry • Previous Articles Next Articles
Received:2019-06-17
Online:2020-01-10
Published:2019-11-21
Contact:
Qi ZHOU
E-mail:zhouxq301@sina.com
Supported by:CLC Number:
TrendMD:
REN Xiangrong,ZHOU Qi. Preparation of Nanoporous Ni and NiO and Their Electrocatalytic Activities for Oxygen Evolution Reaction †[J]. Chem. J. Chinese Universities, 2020, 41(1): 162.
| Alloy | w(%) | ||
|---|---|---|---|
| O | Ni | Al | |
| Ni25Al75 | 7.35 | 88.55 | 4.10 |
| Ni30Al70 | 5.27 | 91.54 | 3.18 |
| Alloy | w(%) | ||
|---|---|---|---|
| O | Ni | Al | |
| Ni25Al75 | 7.35 | 88.55 | 4.10 |
| Ni30Al70 | 5.27 | 91.54 | 3.18 |
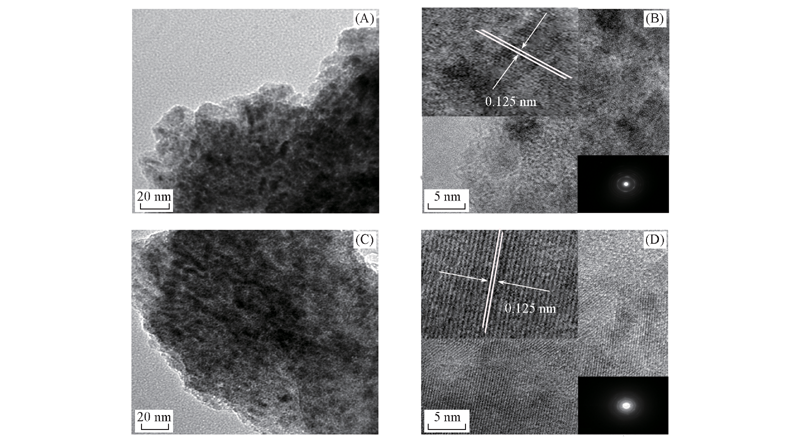
Fig.4 TEM(A, C) and HRTEM(B, D) images of dealloyed Ni25Al75(A, B) and Ni30Al70(C, D) The insets in upper left corner and the lower right corner of (B) and (D) show a local enlarged image and a selected area electron diffraction pattern of the corresponding samples, respectively.
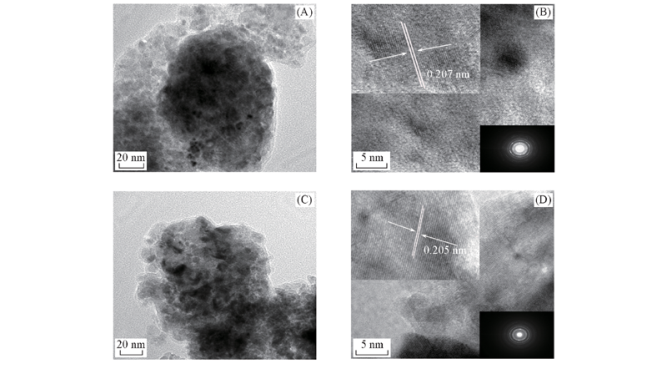
Fig.8 TEM(A, C) and HRTEM(B, D) images of NiO formed from Ni25Al75(A, B) and Ni30Al70(C, D) The insets in the upper left corner and the lower right corner of (B) and (D) show a local enlarged image and a selected area electron diffraction of the corresponding samples, respectively.
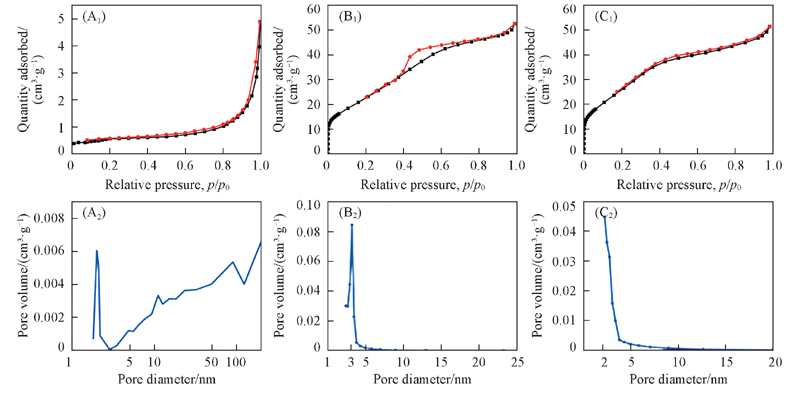
Fig.9 N2 adsorption-desorption isotherms(A1—C1) and the pore size distributions(A2—C2) of Ni and NiO (A) Nanoporous Ni formed from Ni25Al75; (B, C) nanoporous Ni and NiO formed from Ni30Al70, respectively.
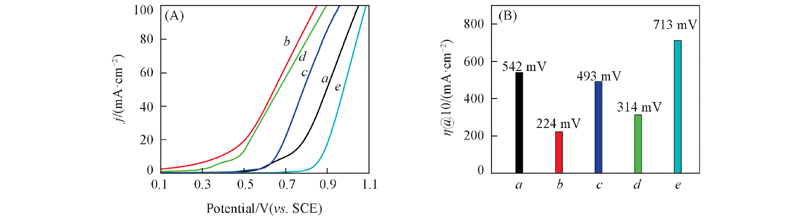
Fig.10 Anodic polarization plots of the Ni, NiO electrode(A) and overpotential histogram of the Ni, NiO electrodes obtained from graph(A) at 10 mA/cm2(B) a. Ni from Ni25Al75; b. Ni from Ni30A70; c. NiO from Ni25Al75; d. NiO from Ni30Al70; e. foam Ni.
| Electrode | b/(mV·dec-1) | a/mV | j0/(mA·cm-2) |
|---|---|---|---|
| Ni from Ni25Al75 | 261.09 | 1106.49 | 0.05781 |
| Ni from Ni30Al70 | 194.06 | 620.72 | 0.63297 |
| NiO from Ni25Al75 | 201.90 | 846.87 | 0.06390 |
| NiO from Ni30Al70 | 232.23 | 769.06 | 0.48794 |
| Electrode | b/(mV·dec-1) | a/mV | j0/(mA·cm-2) |
|---|---|---|---|
| Ni from Ni25Al75 | 261.09 | 1106.49 | 0.05781 |
| Ni from Ni30Al70 | 194.06 | 620.72 | 0.63297 |
| NiO from Ni25Al75 | 201.90 | 846.87 | 0.06390 |
| NiO from Ni30Al70 | 232.23 | 769.06 | 0.48794 |
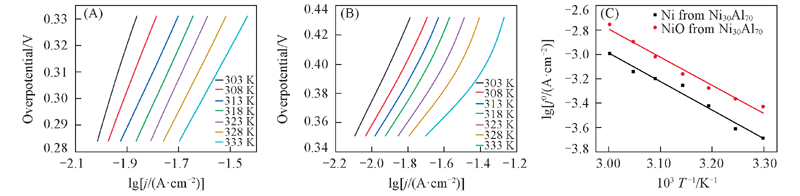
Fig.11 Tafel curves of nanoporous Ni(A) and NiO(B) obtained from Ni30Al70 at different temperatures in 1 mol/L NaOH solution and OER Arrhenius plots on the Ni, NiO electrode formed from Ni30Al70 alloy(C)
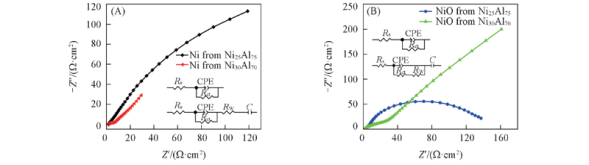
Fig.12 Nyquist plots for the Ni(A), NiO(B) electrodes at equilibrium potential Insets of (A) are equivalent circuit models of nanoporous Ni formed from Ni25Al75(up) and Ni30Al70(down) alloys, respectively; Insets of (B) are equivalent circuit models of nanoporous NiO formed from Ni25Al75(up) and Ni30Al70(down) alloys, respectively.
| Electrode | Rs/(Ω·cm-2) | CPE/F | Rct/(Ω·cm-2) | C/F | Warburg Y0/ (Ω-1·cm-2· |
|---|---|---|---|---|---|
| Ni from Ni25Al75 | 1.724 | 0.005269 | 456.6 | | |
| Ni from Ni30Al70 | 1.587 | 0.004831 | 4.125 | 0.521 | 0.1069 |
| NiO from Ni25Al75 | 1.773 | 0.01208 | 173.1 | | |
| NiO from Ni30Al70 | 1.864 | 0.00539 | 6.754 | 0.371 | 0.01938 |
| Electrode | Rs/(Ω·cm-2) | CPE/F | Rct/(Ω·cm-2) | C/F | Warburg Y0/ (Ω-1·cm-2· |
|---|---|---|---|---|---|
| Ni from Ni25Al75 | 1.724 | 0.005269 | 456.6 | | |
| Ni from Ni30Al70 | 1.587 | 0.004831 | 4.125 | 0.521 | 0.1069 |
| NiO from Ni25Al75 | 1.773 | 0.01208 | 173.1 | | |
| NiO from Ni30Al70 | 1.864 | 0.00539 | 6.754 | 0.371 | 0.01938 |
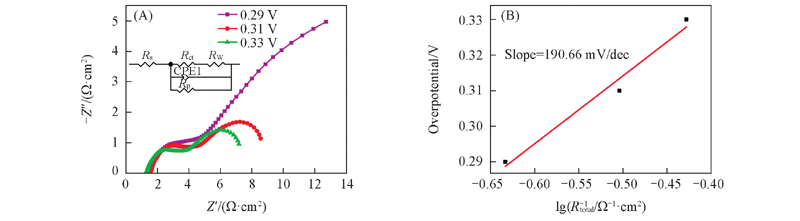
Fig.13 Nyquist plots for the Ni electrode formed from Ni30Al70 alloy at different overpotential(A) and the overpotential vs. lgRtotal-1 plot(B) The inset is equivalent circuit model of nanoporous Ni formed from Ni30Al70 alloy at different overpotential.
| η/V | Rs/(Ω·cm-2) | CPE/F | Rct/(Ω·cm-2) | Rp/(Ω·cm-2) | Warburg Y0/ (Ω-1·cm-2·S0.5) |
|---|---|---|---|---|---|
| 0.29 | 1.551 | 0.01469 | 3.021 | 30.36 | 0.2573 |
| 0.31 | 1.6 | 0.0027 | 1.95 | 8.745 | 0.1455 |
| 0.33 | 1.344 | 0.003215 | 1.639 | 7.347 | 0.1733 |
| η/V | Rs/(Ω·cm-2) | CPE/F | Rct/(Ω·cm-2) | Rp/(Ω·cm-2) | Warburg Y0/ (Ω-1·cm-2·S0.5) |
|---|---|---|---|---|---|
| 0.29 | 1.551 | 0.01469 | 3.021 | 30.36 | 0.2573 |
| 0.31 | 1.6 | 0.0027 | 1.95 | 8.745 | 0.1455 |
| 0.33 | 1.344 | 0.003215 | 1.639 | 7.347 | 0.1733 |
| E/V(vs. SCE) | 1015/(cm2·s-1) | |
|---|---|---|
| Ni | NiO | |
| 0.16 | 3.653 | 0.3442 |
| 0.21 | 4.628 | 0.2108 |
| 0.26 | 6.507 | 0.2685 |
| 0.31 | 15.520 | 0.3689 |
| 0.36 | 69.970 | 0.6541 |
| E/V(vs. SCE) | 1015/(cm2·s-1) | |
|---|---|---|
| Ni | NiO | |
| 0.16 | 3.653 | 0.3442 |
| 0.21 | 4.628 | 0.2108 |
| 0.26 | 6.507 | 0.2685 |
| 0.31 | 15.520 | 0.3689 |
| 0.36 | 69.970 | 0.6541 |
| Electrode | Cdl/μF | S/cm2 | r | (j0/r)/(mA·cm-2) |
|---|---|---|---|---|
| Ni | 642333 | 32117 | 32117 | 1.9708×10-5 |
| NiO | 562440 | 28122 | 28122 | 1.7351×10-5 |
| Electrode | Cdl/μF | S/cm2 | r | (j0/r)/(mA·cm-2) |
|---|---|---|---|---|
| Ni | 642333 | 32117 | 32117 | 1.9708×10-5 |
| NiO | 562440 | 28122 | 28122 | 1.7351×10-5 |
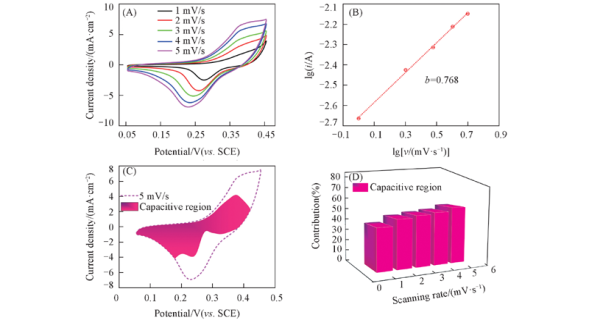
Fig.15 Electrochemical properties and kinetics of nanoporous Ni formed from Ni30Al70 alloy (A) CV curves at 1—5 mV/s; (B) relationship between lgip and lgv; (C) area contribution of capacitance curve at 5 mV/s; (D) contribution diagram of capacitance at different scanning rates.
| [1] |
Rausch B., Symes M. D ., Chisholm G., Cronin L.,. Science, 2014,345(6202), 1326— 1330
doi: 10.1126/science.1257443 URL pmid: 25214625 |
| [2] | Feng X. L., Qu Z. K., Chen J., Wang D. D., Chen X., Yang W. S., Chem. J. Chinese Universities, 2017,38(11), 1999— 2005 |
| ( 冯晓磊, 曲宗凯, 陈俊, 王登登, 陈旭, 杨文胜. 高等学校化学学报, 2017,38(11), 1999— 2005) | |
| [3] | Zhao D. D., Zhang N., Pu L. Z., Shao Q., Huang X. Q., J. Electrochem., 2018,24(5), 52— 62 |
| ( 赵丹丹, 张楠, 卜令正, 邵琪, 黄小青. 电化学, 2018,24(5), 52— 62) | |
| [4] | Liu W., Fabrication of Transition Metal Based Nanosheet Array Electrodes and Their Catalytic Performance Toward Photo-electrochemical Oxygen Evolution Reaction, Zhejiang University, Hangzhou, 2018 |
| (刘伟. 过渡金属基纳米片阵列电极材料的制备及其光/电催化产氧性能研究,.杭州: 浙江大学, 2018) | |
| [5] |
Lee Y., Jin S., May K. J ., Perry E. E., Yang S. H.,. Journal of Physical Chemistry Letters, 2015,3(3), 399— 404
doi: 10.1021/jz2016507 URL pmid: 26285858 |
| [6] |
Prathap M. U. A ., Satpati B., Srivastava R.,. Electrochemical Acta, 2014,130, 368— 380
doi: 10.1016/j.electacta.2014.03.043 URL |
| [7] |
Fominykh K., Feckl J. M ., Sicklinger J., Doblinger M., Bocklein S., Ziegler J., Peter L., Rathousky J., Scheidt E. W., Bein T., Dina F. R.,. Advanced Functional Materials, 2014,24(21), 3123— 3129
doi: 10.1002/adfm.201303600 URL |
| [8] |
Wei G., Xia Z. M ., Cao F. X., Ho J. C., Zheng J., Qu Y. Q.,. Advanced Functional Materials, 2018,28(11), 1706056
doi: 10.1002/adfm.v28.11 URL |
| [9] |
Zou X., Zhang Y ., Chemical Society Reviews, 2015,44(15), 5148— 5180
doi: 10.1039/C4CS00448E URL |
| [10] |
Bain W., Yang Z., Strasser P., Yang R ., Journal of Power Sources, 2014,250(3), 196— 203
doi: 10.1016/j.jpowsour.2013.11.024 URL |
| [11] |
Chen R., Wang H. Y ., Miao J., Yang H., Liu B.,. Nano Energy, 2015,11, 333— 340
doi: 10.1016/j.nanoen.2014.11.021 URL |
| [12] | Zhou Q., Zheng B., Li Z. Y., Wang Y. F., Feng J. W., Chinese Journal of Inorganic Chemistry, 2017,33(8), 1416— 1422 |
| ( 周琦, 郑斌, 李志洋, 王亚飞, 冯基伟. 无机化学学报, 2017,33(8), 1416— 1422) | |
| [13] |
Wang X., Qi Z., Zhao C., Wang W., Zhang Z ., Journal of Physical Chemistry C, 2009,113(30), 13139— 13150
doi: 10.1021/jp902490u URL |
| [14] |
Babar P. T ., Lokhande A. C., Gang M. G., Pawar B. S., Pawar S. M., Kim J. H.,. Journal of Industrial & Engineering Chemistry, 2017,60, 493— 497
doi: 10.1002/bab.1888 URL pmid: 31954377 |
| [15] |
Li J., Luo F., Zhao Q., Li Z., Yuan H., Xiao D ., Journal of Materials Chemistry A, 2014,2(13), 4690— 4697
doi: 10.1039/c3ta14694d URL |
| [16] | Gao X. S., Preparation and Performance of Mesoporous Binary Metal Oxide Nanorods as Oxygen Evolution Catalyst, Taiyuan University of Technology, Taiyuan, 2017 |
| ( 高旭升. 介孔二元金属氧化物纳米棒析氧催化剂的制备及性能研究, 太原: 太原理工大学, 2017) | |
| [17] | Chen G., Study on the Synthesis and Properties of NiO-based Electrode Materials for Supercapacitors, Yunnan University, Kunming, 2016 |
| (陈刚. 基于NiO超级电容器电极材料的制备及其性能研究, 昆明: 云南大学, 2016) | |
| [18] |
Thi T. V ., Rai A. K., Gim J., Kim J.,. Journal of Power Sources, 2015,292, 23— 30
doi: 10.1016/j.jpowsour.2015.05.029 URL |
| [19] |
Zhang J., Cai G., Zhou D., Tang H., Wang X., Gu C., Tu J ., Journal of Materials Chemistry C, 2014,2(34), 7013— 7021
doi: 10.1039/c4tc01033g URL |
| [20] |
Zhu L., Cai Q., Liao F., Sheng M., Wu B., Shao M ., Electrochemistry Communications, 2015,52(15), 29— 33
doi: 10.1016/j.elecom.2015.01.012 URL |
| [21] |
Jeyaprabha C., Sathiyanarayanan S., Venkatachari G ., Applied Surface Science, 2006,253(2), 432— 438
doi: 10.1016/j.apsusc.2005.12.081 URL |
| [22] |
Rakhi R. B ., Chen W., Hedhili M. N., Cha D., Alshareef H. N., ACS Appl. Mater. Interfaces, 2014,6(6), 4196— 4206
doi: 10.1021/am405849n URL pmid: 24580967 |
| [23] |
Min S., Zhao C., Chen G., Zhang Z., Qian X ., Electrochemical Acta, 2014,135(22), 336— 344
doi: 10.1016/j.electacta.2014.05.032 URL |
| [24] |
Zhu L., Lin H., Li Y., Liao F., Lifshitz Y., Sheng M., Shao M ., Nature Communications, 2016,7, 12272
doi: 10.1038/ncomms12272 URL pmid: 27447292 |
| [25] | Wang Y., Sheng M. Q., Weng W. P., Xu J. F., Cao M. Q., Chinese Journal of Materials Research, 2017,31(10), 55— 62 |
| (王玉, 盛敏奇, 翁文凭, 许继芳, 曹孟秋. 材料研究学报, 2017,31(10), 55— 62) | |
| [26] | Shen L., Lv H., Chen S. Q ., Kopold P., Aken P. A., Wu X. J., Maier J., Yu Y.,. Advanced Materials, 2017,29(27), 1602— 1620 |
| [27] | Wang Y. K., Fabrication of Transition Metal Sulfide as Electrode Material and Application for Lithium Ion Capacitor, Lanzhou University of Technology, Lanzhou, 2019 |
| (王雲锴. 过渡金属硫化物电极材料的设计与锂离子电容器应用, 兰州: 兰州理工大学, 2019) | |
| [28] |
Bao J. Z., Wang S. L., Acta Physico-Chimica Sinica, 2011,27(12), 2849— 2856
doi: 10.3866/PKU.WHXB20112849 URL |
|
( 鲍晋珍, 王森林. 物理化学学报, 2011,27(12), 2849— 2856)
doi: 10.3866/PKU.WHXB20112849 URL |
|
| [29] |
Wang L. P., Wang S. L., Duan Q. H., Chinese Journal of Applied Chemistry, 2013,30(6), 690— 697
doi: 10.3724/SP.J.1095.2013.20385 URL |
|
( 王丽品, 王森林, 段钱花. 应用化学, 2013,30(6), 690— 697)
doi: 10.3724/SP.J.1095.2013.20385 URL |
| [1] | MA Jun, ZHONG Yang, ZHANG Shanshan, HUANG Yijun, ZHANG Lipeng, LI Yaping, SUN Xiaoming, XIA Zhenhai. Design and Theoretical Calculation of Heteroatoms Doped Graphdiyne Towards Efficiently Catalyzing Oxygen Reduction and Evolution Reactions [J]. Chem. J. Chinese Universities, 2021, 42(2): 624. |
| [2] | CHANG Jianhong, XU Guojie, LI Hui, FANG Qianrong. Quinone-based Covalent Organic Frameworks for Efficient Oxygen Evolution Reaction† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1609. |
| [3] | JIN E,SONG Kaixu,CUI Lili. Preparation and Electrocatalytic Performance of Carbon Material Co-doped by Bimetal Phosphide and Heteroatom † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1362. |
| [4] | LIU Lu,WU Hanyue,LI Jing,SHE Lan. Tuning Microstructures of Iron-Nickel Alloy Catalysts for Efficient Oxygen Evolution Reaction † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1083. |
| [5] | HAN Zhiying,LI Youji,CHEN Feitai,TANG Senpei,WANG Peng. Preparation of ZnO/Ag2O Nanofibers by Coaxial Electrospinning and Study of Their Photocatalytic Properties † [J]. Chem. J. Chinese Universities, 2020, 41(2): 308. |
| [6] | JIANG Yuanyuan, LI Boyu, LU Yizhong, WU Tongshun, HAN Dongxue. Oxygen Evolution Reaction Electrocatalytic Performance Analysis of Electroless Plated Ni-Bx [J]. Chem. J. Chinese Universities, 2020, 41(12): 2774. |
| [7] | ZHOU Qi, LI Zhiyang, WANG Fan. Effect of Mo on the Skeleton Structure and Hydrogen Evolution Performance of Ni-Mo Alloys Electrode Prepared by De-alloying [J]. Chem. J. Chinese Universities, 2019, 40(8): 1717. |
| [8] | ZHANG Min, CHEN Mengwei, GAO Hong, BI Yanfeng. Synthesis, Structure and Electrochemical Properties of Sulfonylcalix[4]arene Supported Co16 Cluster † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2052. |
| [9] | JIANG Yilan, YUAN Long, WANG Xiyang, HUANG Keke, FENG Shouhua. Effect of Defect Tuning on the Catalytic Behavior of Perovskite Structure Lanthanum Manganite† [J]. Chem. J. Chinese Universities, 2018, 39(3): 416. |
| [10] | CHEN Chen, LI Li, CHEN Jinhua, ZHANG Xiaohua, XU Jie, LI Yibo, WEI Jie. Preparation and Electrocatalytic Performance for Methanol Oxidation of Pt-CeO2/Sodium-4-styrenesulfonate Functionalized Carbon Nanotube Composites† [J]. Chem. J. Chinese Universities, 2018, 39(1): 157. |
| [11] | LI Weilun, YAO Ying, ZHANG Cunzhong. Applications of Carbon Fiber Ultra-microelectrode and Powder Microelectrode in Exploring Influences of Non-aqueous Solvents and Cathode Materials on ORR and OER† [J]. Chem. J. Chinese Universities, 2017, 38(4): 642. |
| [12] | FENG Xiaolei, QU Zongkai, CHEN Jun, WANG Dengdeng, CHEN Xu, YANG Wensheng. NiFe2O4/NiO Nanocomposites as Electrocatalysts for Oxygen Evolution Reaction† [J]. Chem. J. Chinese Universities, 2017, 38(11): 1999. |
| [13] | DU Shichao,REN Zhiyu,WU Jun,FU Honggang. Ni-Fe LDH/Reduced Graphene Oxide as Catalyst for Oxygen Evolution Reaction [J]. Chem. J. Chinese Universities, 2016, 37(8): 1415. |
| [14] | KONG Qing-Mei, JIANG Yu-Zhi, CHEN Chong, ZHOU Yi-Ming, LU Tian-Hong, CHEN Yu*, TANG Ya-Wen*. Preparation of Ir/CNTs Catalyst and Its Electrocatalytic Performance for Ammonia Oxidation [J]. Chem. J. Chinese Universities, 2010, 31(11): 2260. |
| [15] | SUN Ren-Xing1, XU Hai-Bo1*, WAN Nian-Fang2, WANG Jia1. Syntheses of IrOx-TiO2 Nano-powers from TiN via Impregnation-thermal Decomposition Method and Its Characterization [J]. Chem. J. Chinese Universities, 2007, 28(5): 904. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
