

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (4): 642.doi: 10.7503/cjcu20160727
• Physical Chemistry • Previous Articles Next Articles
LI Weilun1, YAO Ying1,2,3,*( ), ZHANG Cunzhong1,2,3,*(
), ZHANG Cunzhong1,2,3,*( )
)
Received:2016-10-19
Online:2017-04-10
Published:2017-03-23
Contact:
YAO Ying,ZHANG Cunzhong
E-mail:yaoying@bit.edu.cn;czzhangchem@bit.edu.cn
CLC Number:
TrendMD:
LI Weilun, YAO Ying, ZHANG Cunzhong. Applications of Carbon Fiber Ultra-microelectrode and Powder Microelectrode in Exploring Influences of Non-aqueous Solvents and Cathode Materials on ORR and OER†[J]. Chem. J. Chinese Universities, 2017, 38(4): 642.
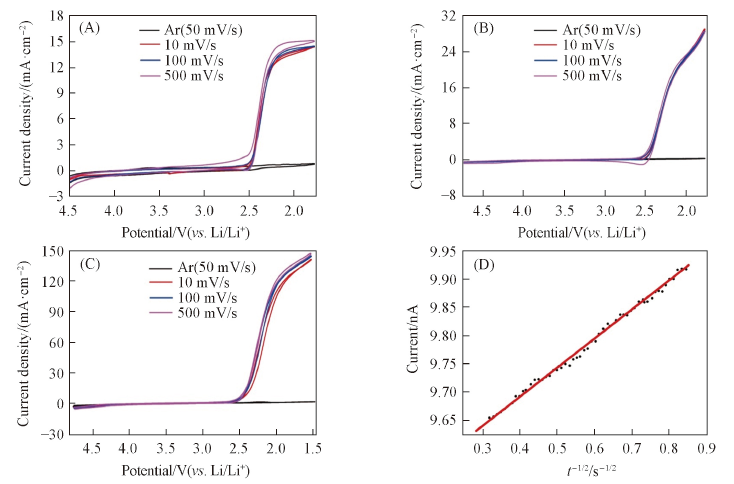
Fig.1 Current-voltage behavior observed on CFUME in O2-saturated 0.1 mol/L TBACF3SO3/DMSO(A), 0.1 mol/L TBACF3SO3/MeCN(B), 0.1 mol/L TBACF3SO3/TEGDME(C) and Cottrell plots obtained for the current time transients recorded in O2 saturated 0.1 mol/L TBACF3SO3/DMSO(D)
| Solvent | 105Diffusion coefficient /(cm2·s-1) | Solubility of oxygen/(mmol·L-1) |
|---|---|---|
| DMSO | 2.65 | 2.38 |
| MECN | 6.51 | 8.54 |
| TEGDME | 3.27 | 4.15 |
Table 1 Oxygen diffusion coefficient and solubility in electrolytes
| Solvent | 105Diffusion coefficient /(cm2·s-1) | Solubility of oxygen/(mmol·L-1) |
|---|---|---|
| DMSO | 2.65 | 2.38 |
| MECN | 6.51 | 8.54 |
| TEGDME | 3.27 | 4.15 |
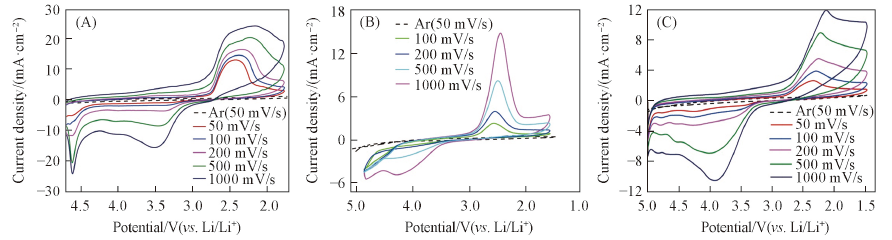
Fig.2 Scan rate dependent cyclic voltammograms obtained on CFUME in O2-saturated 0.1 mol/L LiCF3SO3/DMSO(A), 0.1 mol/L LiCF3SO3/MeCN(B) and 0.1 mol/L LiCF3SO3/TEGDME(C)
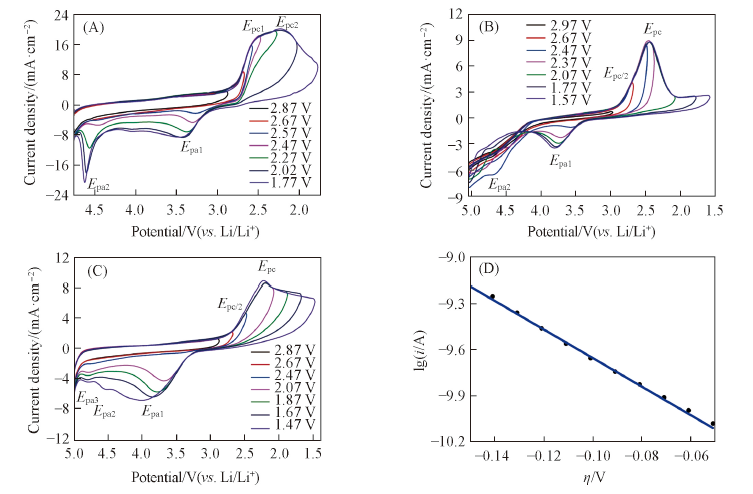
Fig.3 Cyclic voltammograms for the ORR and OER on CFUME at various potential windows in O2-saturated 0.1 mol/L LiCF3SO3/DMSO(A), 0.1 mol/L LiCF3SO3/MeCN(B), 0.1 mol/L LiCF3SO3/TEGDME(C) and cathodic Tafel plot obtained in O2-saturated 0.1 mol/L LiCF3SO3/DMSO during ORR(D)(A)—(C) Scan rate: 500 mV/s; (D) scan rate: 10 mV/s.
| Electrode | Electrolyte | Tafel slope/(mV·dec-1) | 105 Exchange current density(A·cm-2) | 105 Standard rate constant/(cm·s-1) |
|---|---|---|---|---|
| CFUME | 0.1 mol/L Li+/DMSO | 115 | 17.4 | 75.8 |
| CFUME | 0.1 mol/L Li+/MeCN | 111 | 15.2 | 19.4 |
| CFUME | 0.1 mol/L Li+/TEGDME | 121 | 1.75 | 4.37 |
| Super P PME | 1 mol/L Li+/TEGDME | 117 | 1.45 | 3.62 |
| CAB PME | 1 mol/L Li+/TEGDME | 119 | 1.25 | 3.12 |
Table 2 Kinetics parameters of ORR obtained from OCP for different electrolytes and electrodes(scan rate: 10 mV/s)
| Electrode | Electrolyte | Tafel slope/(mV·dec-1) | 105 Exchange current density(A·cm-2) | 105 Standard rate constant/(cm·s-1) |
|---|---|---|---|---|
| CFUME | 0.1 mol/L Li+/DMSO | 115 | 17.4 | 75.8 |
| CFUME | 0.1 mol/L Li+/MeCN | 111 | 15.2 | 19.4 |
| CFUME | 0.1 mol/L Li+/TEGDME | 121 | 1.75 | 4.37 |
| Super P PME | 1 mol/L Li+/TEGDME | 117 | 1.45 | 3.62 |
| CAB PME | 1 mol/L Li+/TEGDME | 119 | 1.25 | 3.12 |
| Electrode | Electrolyte | Epc1 /V | Epa1/V | Epa2/V | |Epc1-Epa1|/V |
|---|---|---|---|---|---|
| CFUME | 0.1 mol/L Li+/DMSO | 2.53 | 3.43 | 4.62 | 0.90 |
| CFUME | 0.1 mol/L Li+/MeCN | 2.45 | 3.79 | 4.67 | 1.34 |
| CFUME | 0.1 mol/L Li+/TEGDME | 2.23 | 3.83 | 4.55 | 1.60 |
| Super P PME | 1 mol/L Li+/TEGDME | 2.50 | 3.63 | 4.63 | 1.13 |
| CAB PME | 1 mol/L Li+/TEGDME | 2.45 | 3.82 | 4.43 | 1.37 |
Table 3 Properties of peak potentials during ORR and OER for different electrolytes and electrodes(scan rate: 500 mV/s)
| Electrode | Electrolyte | Epc1 /V | Epa1/V | Epa2/V | |Epc1-Epa1|/V |
|---|---|---|---|---|---|
| CFUME | 0.1 mol/L Li+/DMSO | 2.53 | 3.43 | 4.62 | 0.90 |
| CFUME | 0.1 mol/L Li+/MeCN | 2.45 | 3.79 | 4.67 | 1.34 |
| CFUME | 0.1 mol/L Li+/TEGDME | 2.23 | 3.83 | 4.55 | 1.60 |
| Super P PME | 1 mol/L Li+/TEGDME | 2.50 | 3.63 | 4.63 | 1.13 |
| CAB PME | 1 mol/L Li+/TEGDME | 2.45 | 3.82 | 4.43 | 1.37 |
| Electrode | Electrolyte | Jpc1 /(mA·cm-2) | Jpa1/(mA·cm-2) | Jpa1/Jpc1(%) |
|---|---|---|---|---|
| CFUME | 0.1 mol/L Li+/DMSO | 13.56 | 5.14 | 37.91 |
| CFUME | 0.1 mol/L Li+/MeCN | 7.21 | 2.77 | 38.42 |
| CFUME | 0.1 mol/L Li+/TEGDME | 6.43 | 5.45 | 84.50 |
| Super P PME | 1 mol/L Li+/TEGDME | 5.20 | 1.22 | 23.45 |
| CAB PME | 1 mol/L Li+/TEGDME | 4.24 | 1.02 | 24.05 |
Table 4 Properties of peak currents during ORR and OER for different electrolytes and electrodes(scan rate: 500 mV/s)
| Electrode | Electrolyte | Jpc1 /(mA·cm-2) | Jpa1/(mA·cm-2) | Jpa1/Jpc1(%) |
|---|---|---|---|---|
| CFUME | 0.1 mol/L Li+/DMSO | 13.56 | 5.14 | 37.91 |
| CFUME | 0.1 mol/L Li+/MeCN | 7.21 | 2.77 | 38.42 |
| CFUME | 0.1 mol/L Li+/TEGDME | 6.43 | 5.45 | 84.50 |
| Super P PME | 1 mol/L Li+/TEGDME | 5.20 | 1.22 | 23.45 |
| CAB PME | 1 mol/L Li+/TEGDME | 4.24 | 1.02 | 24.05 |
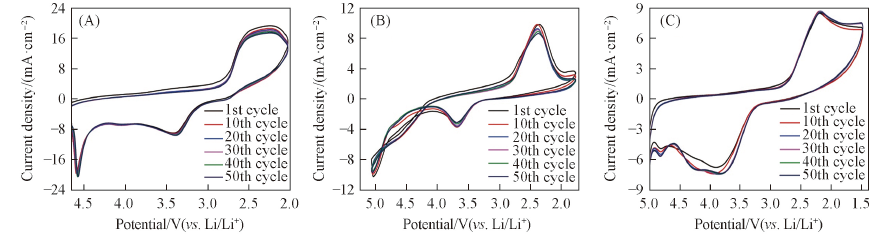
Fig.4 Cyclability of the ORR/OER on CFUME at the scan rate of 500 mV/s in O2-saturated 0.1 mol/L LiCF3SO3/DMSO(A), 0.1 mol/L LiCF3SO3/MeCN(B) and 0.1 mol/L LiCF3SO3/TEGDME(C)
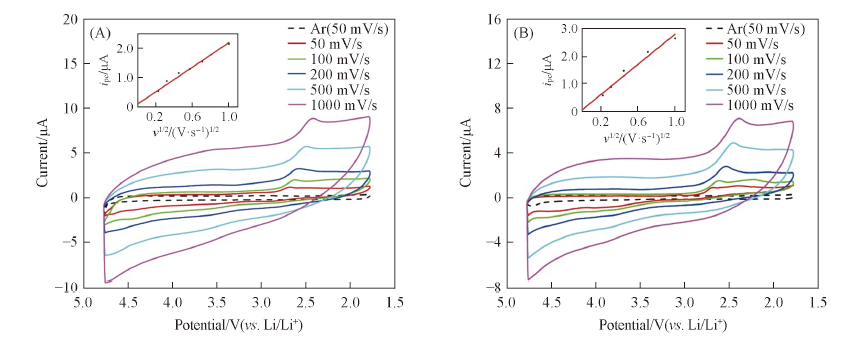
Fig.5 Scan rate dependent cyclic voltammograms obtained in O2-saturated 1 mol/L LiCF3SO3/TEGDME on Super P PME(A) and CAB PME(B)Insets of (A) and (B): peak current vs. square root of the scan rate.
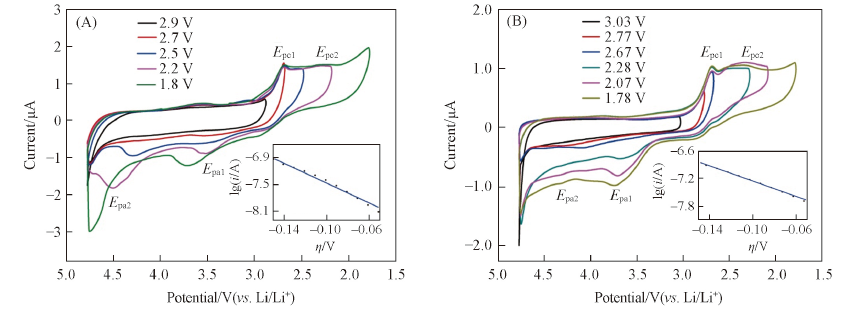
Fig.6 Cyclic voltammograms for the ORR and OER in O2-saturated 1 mol/L LiCF3SO3/TEGDME at various potential windows on Super P PME(A) and CAB PME(B)Scan rate: 50 mV/s. Inset: cathodic Tafel plot; scan rate: 10 mV/s.
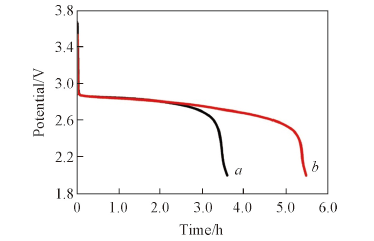
Fig.8 Galvanostatic discharge curves in O2-saturated 1 mol/L LiCF3SO3/TEGDME on different powder microelectrodesCurrent density: 0.2 mA/cm2. a. Super P PME; b. CAB PME.
| [1] | Ma Z., Yuan X. X., Li L., Ma Z. F., Wilkinson D. P., Zhang L., Zhang J. J., Energy Environ. Sci., 2015, 8(8), 2144—2198 |
| [2] | Cheng F., Liang J., Tao Z., Chen J., Adv. Mater., 2011, 23(15), 1695—1715 |
| [3] | Bhatt M. D., Geaney H., Nolan M., O'Dwyer C., Phys. Chem. Chem. Phys., 2014, 16(24), 12093—12130 |
| [4] | Zhang L., Liu Q. H., Duan X. B., Hua X. H., Zhu D., Chen Y. G., Chem. J. Chinese Universities, 2016, 37(4), 682—687 |
| (张蕾, 刘清华, 段晓波, 华小虎, 朱丁, 陈云贵. 高等学校化学学报, 2016,37(4), 682—687) | |
| [5] | Akhtar N., Akhtar W., Int. J. Energy Res., 2015, 39(3), 303—316 |
| [6] | Ibrahim H., Ilinca A., Perron J., Renew. Sust. Energ. Rev., 2008, 12(5), 1221—1250 |
| [7] | Chen H., Cong T. N., Yang W., Tan C., Li Y., Ding Y., Prog. Nat. Sci., 2009, 19(3), 291—312 |
| [8] | Bruce P. G., Freunberger S. A., Hardwick L. J., Tarascon J.M., Nat. Mater., 2012, 11(1), 19—29 |
| [9] | Kraytsberg A., Ein-Eli Y., J. Power Sources, 2011, 196(3), 886—893 |
| [10] | Shao Y. Y., Ding F., Xiao J., Zhang J., Xu W., Park S., Zhang J. G., Wang Y., Liu J., Adv. Funct. Mater., 2013, 23(8), 987—1004 |
| [11] | Lu J., Li L., Park J. B., Sun Y. K., Wu F., Amine K., Chem. Rev., 2014, 114(11), 5611—5640 |
| [12] | Zhang Z., Lu J., Assary R. S., Du P., Wang H. H., Sun Y. K., Qin Y., Lau K. C., Greeley J., Redfern P. C., J. Phys. Chem. C, 2011, 115(51), 25535—25542 |
| [13] | Xu W., Xu K., Viswanathan V. V., Towne S. A., Hardy J. S., Xiao J., Hu D. H., Wang D. Y., Zhang J. G., J. Power Sources, 2011, 196(22), 9631—9639 |
| [14] | Freunberger S. A., Chen Y. H., Peng Z. Q., Griffin J. M., Hardwick L. J., Barde F., Novak P., Bruce P. G., J. Am. Chem. Soc., 2011, 133(20), 8040—8047 |
| [15] | McCloskey B. D., Bethune D. S., Shelby R. M., Girishkumar G., Luntz A. C., J. Phys. Chem. Lett., 2011, 2(10), 1161—1166 |
| [16] | Bryantsev V. S., Giordani V., Walker W., Blanco M., Zecevic S., Sasaki K., Uddin J., Addison D., Chase G. V., J. Phys. Chem. A, 2011, 115(44), 12399—12409 |
| [17] | Bryantsev V. S., Blanco M., J. Phys. Chem. Lett., 2011, 2(5), 379—383 |
| [18] | McCloskey B. D., Bethune D. S., Shelby R. M., Mori T., Scheffler R., Speidel A., Sherwood M., Luntz A. C., J. Phys. Chem. Lett., 2012, 3(20), 3043—3047 |
| [19] | Duan D. H., You X., Ren W. J., Wei H. K., Liu H. H., Liu S. B., Int. J. Hydrog. Energy, 2015, 40(34), 10847—10855 |
| [20] | Jung H. G., Hassoun J., Park J. B., Sun Y. K., Scrosati B., Nat. Chem., 2012, 4(7), 579—585 |
| [21] | Jung H. G., Kim H. S., Park J. B., Oh I. H., Hassoun J., Yoon C. S., Scrosati B., Sun Y. K., Nano Lett., 2012, 12(8), 4333—4335 |
| [22] | Oldham K. B., Cardwell T. J., Santos J. H., Bond A. M., J. Electroanal. Chem., 1997, 430(1), 25—37 |
| [23] | Jin Y., Luo G. A., Chem. J. Chinese Universities, 2003, 24(7), 1180—1184 |
| (金亚, 罗国安. 高等学校化学学报, 2003,24(7), 1180—1184) | |
| [24] | Gonçalves A. M., Mathieu C., Herlem M., Etcheberry A., J. Electroanal. Chem., 1999, 477(2), 140—145 |
| [25] | Augustin M., Yezerska O., Fenske D., Bardenhagen I., Westphal A., Knipper M., Plaggenborg T., Kolny-Olesiak J., Parisi J., Electrochim. Acta, 2015, 158, 383—389 |
| [26] | Ming J., Park J. B., Kim H. S., Yoon C. S., Elia G. A., Scrosati B., Sun Y. K., Hassoun J., Solid State Ion, 2015, 278, 133—137 |
| [27] | Cheon S. E., Kwon C. W., Kim D. B., Hong S. J., Kim H. T., Kim S. W., Electrochim. Acta, 2000, 46, 599—605 |
| [28] | Shu J., Shui M., Huang F. T., Xu D., Ren Y. L., Hou L., Cui J., Xu J. J., J. Phys. Chem. C, 2011, 115(14): 6954—6960 |
| [29] | Zoski C. G., Electroanal., 2002, 14(15), 1041—1051 |
| [30] | Laoire C. O., Mukerjee S., Abraham K. M., Plichta E. J., Hendrickson M. A., J. Phys. Chem. C, 2010, 114(19), 9178—9186 |
| [31] | Abraham K. M., J. Electrochem. Soc., 2015, 162(2), A3021—A3031 |
| [32] | Gunasekara I., Mukerjee S., Plichta E. J., Hendrickson M. A., Abraham K. M., J. Electrochem. Soc., 2014, 161(3), A381—A392 |
| [33] | Burke C. M., Pande V., Khetan A., Viswanathan V., McCloskey B. D., Proc. Natl. Acad. Sci. USA,2015, 112(30), 9293—9298 |
| [1] | JIANG Yilan, YUAN Long, WANG Xiyang, HUANG Keke, FENG Shouhua. Effect of Defect Tuning on the Catalytic Behavior of Perovskite Structure Lanthanum Manganite† [J]. Chem. J. Chinese Universities, 2018, 39(3): 416. |
| [2] | LIU Pei, CHENG Qingqing, CHEN Chi, ZOU Liangliang, ZOU Zhiqing, YANG Hui. Preparation and Oxygen Reduction Reaction Catalytic Performance of Fe, N Co-doped Carbon Nanofibers with Encapsulated Iron Nitride† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2492. |
| [3] | KANG Huan, LI Shang, LIU Chang, GUO Wei, PAN Mu. Synthesis of Ordered Mesoporous Fe-N-C-PANI Catalyst via Self-assembly and Its Oxygen Reduction Reaction Activity in Acid Medium [J]. Chem. J. Chinese Universities, 2017, 38(8): 1423. |
| [4] | QIU Guo-Hong, YANG Dan, FENG Xiong-Han, ZHANG Xu-Liang, CHEN Xiu-Hua, ....... Study on the Preparation and Electrochemical Property of Cu2+ Ion Doped Todorokite [J]. Chem. J. Chinese Universities, 2009, 30(8): 1481. |
| [5] | JIN Bao-Duo, GUO Jian-Wei, XIE Xiao-Feng*, WANG Shu-Bo, WANG Jin-Hai. Effect of Operating Condition on Cathodic EIS Parameters in a DMFC [J]. Chem. J. Chinese Universities, 2008, 29(11): 2258. |
| [6] | YANG Tian-Ming, HU Xiao-An, LU Jian-Jie, DENG Min . Development and Application of Polyvinyl Chloride Modified Powder Microelectrode [J]. Chem. J. Chinese Universities, 1998, 19(11): 1743. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||