

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (8): 1415.doi: 10.7503/cjcu20160173
• Articles Inorganic Chemistry • Previous Articles Next Articles
DU Shichao1, REN Zhiyu2,*( ), WU Jun2, FU Honggang2,*(
), WU Jun2, FU Honggang2,*( )
)
Received:2016-03-23
Online:2016-07-14
Published:2016-07-14
Contact:
REN Zhiyu,FU Honggang
E-mail:zyren@hlju.edu.cn;fuhg@vip.sina.com
Supported by:CLC Number:
TrendMD:
DU Shichao,REN Zhiyu,WU Jun,FU Honggang. Ni-Fe LDH/Reduced Graphene Oxide as Catalyst for Oxygen Evolution Reaction[J]. Chem. J. Chinese Universities, 2016, 37(8): 1415.
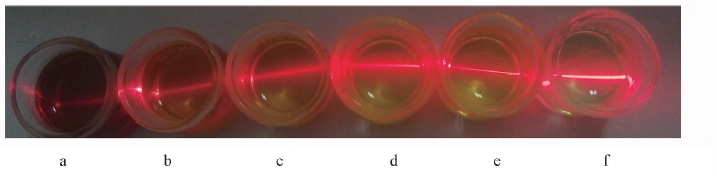
Fig.2 Photos of colloidal solutions of exfoliated nanosheets Tyndall effect was visible when irradiated with a laserbeam. a. Ni-Fe(1∶2) LDH; b. Ni-Fe(1∶1) LDH; c. Ni-Fe(2∶1) LDH; d. Ni-Fe(5∶1) LDH; e. Ni-Fe(10∶1) LDH; f. Ni(OH)2.
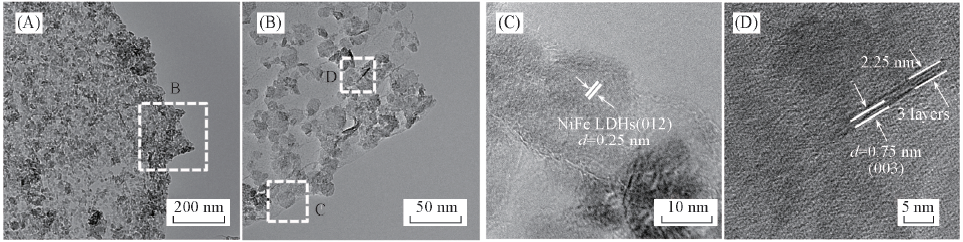
Fig.4 Typical TEM(A, B) and HRTEM(C, D) images of the exfoliated Ni-Fe(5∶1) LDH/rGO composite (B) is the amplification image of the corresponding dotted box in (A); (C) and (D) are the amplification images of the corresponding dotted box in (B).
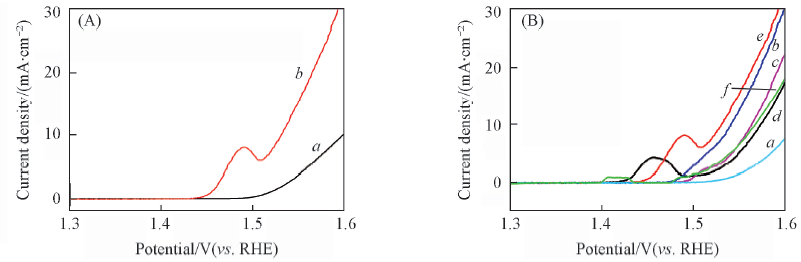
Fig.6 Polarization curves of bulk(a) and exfoliated(b) Ni-Fe(10∶1) LDH(A) and exfoliated Ni-Fe(1∶2) LDH(a), Ni-Fe(1∶1) LDH(b), Ni-Fe(2∶1) LDH(c), Ni-Fe(5∶1) LDH(d), Ni-Fe(10∶1) LDH(e), Ni(OH)2(f)(B) in 1 mol/L KOH
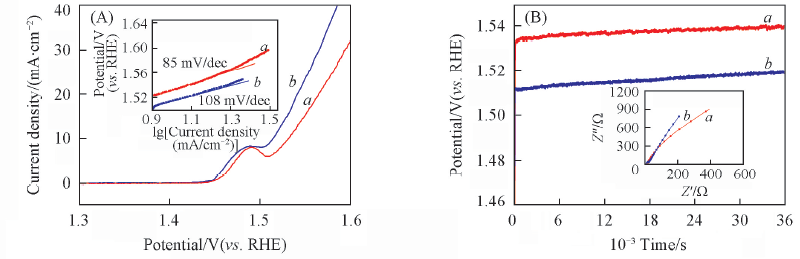
Fig.7 Polarization curves(A) and chronopotentiometry(B) of exfoliated Ni-Fe(10∶1) LDH(a) and NiFe(10∶1) LDH/rGO(b) The inset in (A) shows the Tafel plots; the inset in (B) shows the electrochemical impedance spectra.
| Material | Onset potential/ V(vs. RHE) | Potential at 10 mA·cm-2/ V(vs. RHE) | Slope of Tafel plot/ (mV·dec-1) | Ref. |
|---|---|---|---|---|
| NiFe LDH/CNT | 1.500 | 35 | [ | |
| Exfoliated NiFe LDH | 1.493 | 1.532 | 40 | [ |
| NiCo LDH/CP* | 1.535 | 1.597 | 40 | [ |
| NG-NiCo LDH | 1.580 | 614 | [ | |
| CoNi LDH | 1.590 | [ | ||
| NiCo LDH/Ni foam | 1.520 | 1.650 | 113 | [ |
| Ni2/3Fe1/3-rGO | 1.440 | 1.470 | 40 | [ |
| NiCo2O4 nanosheets | 1.550 | 30 | [ | |
| Ni0.6Co2.4O4/Ni foil | 1.570 | 1.760 | [ | |
| Ni-NG | 1.550 | 188 | [ | |
| Exfoliated Ni-Fe LDH | 1.470 | 1.530 | 108 | This work |
| Exfoliated Ni-Fe LDH/rGO | 1.470 | 1.515 | 85 | This work |
Table 1 Comparison of catalytic performance of exfoliated Ni-Fe LDH/rGO to Fe,Ni-based LDHs and oxides
| Material | Onset potential/ V(vs. RHE) | Potential at 10 mA·cm-2/ V(vs. RHE) | Slope of Tafel plot/ (mV·dec-1) | Ref. |
|---|---|---|---|---|
| NiFe LDH/CNT | 1.500 | 35 | [ | |
| Exfoliated NiFe LDH | 1.493 | 1.532 | 40 | [ |
| NiCo LDH/CP* | 1.535 | 1.597 | 40 | [ |
| NG-NiCo LDH | 1.580 | 614 | [ | |
| CoNi LDH | 1.590 | [ | ||
| NiCo LDH/Ni foam | 1.520 | 1.650 | 113 | [ |
| Ni2/3Fe1/3-rGO | 1.440 | 1.470 | 40 | [ |
| NiCo2O4 nanosheets | 1.550 | 30 | [ | |
| Ni0.6Co2.4O4/Ni foil | 1.570 | 1.760 | [ | |
| Ni-NG | 1.550 | 188 | [ | |
| Exfoliated Ni-Fe LDH | 1.470 | 1.530 | 108 | This work |
| Exfoliated Ni-Fe LDH/rGO | 1.470 | 1.515 | 85 | This work |
| [1] | Lewis N., S. , Nocera D., G. , Proc. Natl. Acad. Sci., 2006, 103( 43), 15729- 15735 |
| [2] |
Jiao, Y. , Zheng, Y. , Jaroniec, M. , Qiao S., Z. , Chem. Soc. Rev., 2015, 44, 2060- 2086
doi: 10.1039/c4cs00470a URL pmid: 25672249 |
| [3] |
Wang, H. , Dai, H. , Chem. Soc. Rev., 2013, 42, 3088- 3113
doi: 10.1021/ja3089923 URL |
| [4] | Kanan M., W. , Nocera D., G. , Science, 2008, 321, 1072- 1075 |
| [5] |
McCrory C. C., L. , Jung, S. , Peters J., C. , Jaramillo T., F. , J. Am. Chem. Soc., 2013, 135( 45), 16977- 16987
doi: 10.1021/ja407115p URL pmid: 24171402 |
| [6] |
Trotochaud, L. , Ranney J., K. , Williams K., N. , Boettcher S., W. , J. Am. Chem. Soc., 2012, 134, 17253- 17261
doi: 10.1021/ja307507a URL pmid: 22991896 |
| [7] |
张艳梅, 张静, 李晓芳, 储刚, 田苗苗, 权春善. 高等学校化学学报, 2016, 37( 3), 573- 580
doi: 10.7503/cjcu20150511 URL |
|
Zhang Y., M. , Zhang, J. , Li X., F. , Chu, G. , Tian M., M. , Quan C., S. , Chem. J. Chinese Universities, 2016, 37( 3), 573- 580 (
doi: 10.7503/cjcu20150511 URL |
|
| [8] |
郭亚军, 张龙, 后洁琼, 马泳波, 秋虎, 张文娟, 杜雪岩. 高等学校化学学报, 2016, 37( 6), 1202- 1207
doi: 10.7503/cjcu20150965 URL |
|
Guo Y., J. , Zhang, L. , Hou J., Q. , Ma Y., B. , Qiu, H. , Zhang W., J. , Du X., Y. , Chem. J. Chinese Universities, 2016, 37( 6), 1202- 1207 (
doi: 10.7503/cjcu20150965 URL |
|
| [9] | Seiger H., N. , Shair R., C. , J. Electrochem. Soc., 1961, 108( 2), 163- 167 |
| [10] |
Corrigan D., A. , J. Electrochem. Soc., 1987, 13( 24), 377- 384
doi: 10.1149/1.2100463 URL |
| [11] |
Bowen C., T. , Davis H., J. , Henshaw B., F. , Int. J. Hydrogen Energ., 1984, 9( 1/2), 59- 66
doi: 10.1016/0360-3199(84)90032-6 URL |
| [12] |
Wang, Q. , O’, Hare D. , Chem. Rev., 2012, 112, 4124- 4155
doi: 10.1021/cr200434v URL pmid: 22452296 |
| [13] |
Song, F. , Hu, X. , Nat. Commun., 2014, 5, 4477- 4486
doi: 10.1038/ncomms5477 URL pmid: 25030209 |
| [14] |
Luo, J. , Im J., H. , Mayer M., T. , Schreier, M. , Nazeeruddin M., K. , Park N., G. , Tilley S., D. , Fan H., J. , Graetzel, M. , Science, 2014, 345( 6024), 1593- 1596
doi: 10.1126/science.1258307 URL pmid: 25258076 |
| [15] | Wang, Y. , Li, F. , Dong, S. , J. Colloid Interface Sci., 2016, 467, 28- 34 |
| [16] |
Butler S., Z. , Shawna M., H. , Linyou, C. , ACS Nano, 2013, 7( 4), 2898- 2926
doi: 10.1021/nn401053g URL pmid: 23590723 |
| [17] |
Liang, H. , Meng, F. , Acevedo M., C. , Li, L. , Forticaux, A. , Xiu, L. , Wang, Z. , Jin, S. , Nano Lett., 2015, 15( 2), 1421- 1427
doi: 10.1021/nl504872s URL pmid: 25633476 |
| [18] | Liu, X. , Wang, X. , Yuan, X. , Donga, W. , Huang, F. , J. Mater. Chem. A, 2016, 4, 167- 172 |
| [19] |
Wang H., L. , Robinson J., T. , Li X., L. , Dai H., J. , J. Am. Chem. Soc., 2009, 131, 9910- 9911
doi: 10.1021/ja904251p URL pmid: 19580268 |
| [20] |
Chen, S. , Duan J., J. , Jaroniec, M. , Qiao S., Z. , Angew. Chem. Int. Ed., 2013, 52( 51), 13567- 13570
doi: 10.1002/anie.201306166 URL pmid: 24346940 |
| [21] | Valdez, R. , Grotjahn D., B. , Smith D., K. , Quintana J., M. , Olivas, A. , Int. J. Electrochem. Sci., 2015, 10, 909- 918 |
| [22] |
Jiang, J. , Zhang A., L. , Li L., L. , Ai L., H. , J. Power Sources, 2015, 278, 445- 451
doi: 10.1016/j.jpowsour.2014.12.085 URL |
| [23] |
Ma, W. , Ma R., Z. , Wang C., X. , Liang J., B. , Liu X., H. , Zhou K., C. , Sasaki, T. , ACS Nano, 2015, 9( 2), 1977- 1984
doi: 10.1021/nn5069836 URL pmid: 25605063 |
| [24] | Bao, J. , Zhang X., D. , Fan, B. , Zhang J., J. , Zhou, M. , Yang W., L. , Hu, X. , Wang, H. , Pan B., C. , Xie, Y. , Angew. Chem. Int. Ed., 2015, 54, 7399- 7404 |
| [25] | Lambert T., N. , Vigil J., A. , White S., E. , Davis D., J. , Limmer S., J. , Burton P., D. , Coker E., N. , Beecheme T., E. , Brumbach M., T. , Chem. Commun., 2015, 51, 9511- 9514 |
| [26] | Chen, S. , Duan J., J. , Ran J., R. , Jaroniecb, M. , Qiao S., Z. , Energy Environ. Sci., 2013, 6, 3693- 3699 |
| [1] | MA Jun, ZHONG Yang, ZHANG Shanshan, HUANG Yijun, ZHANG Lipeng, LI Yaping, SUN Xiaoming, XIA Zhenhai. Design and Theoretical Calculation of Heteroatoms Doped Graphdiyne Towards Efficiently Catalyzing Oxygen Reduction and Evolution Reactions [J]. Chem. J. Chinese Universities, 2021, 42(2): 624. |
| [2] | CHANG Jianhong, XU Guojie, LI Hui, FANG Qianrong. Quinone-based Covalent Organic Frameworks for Efficient Oxygen Evolution Reaction† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1609. |
| [3] | JIN E,SONG Kaixu,CUI Lili. Preparation and Electrocatalytic Performance of Carbon Material Co-doped by Bimetal Phosphide and Heteroatom † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1362. |
| [4] | LIU Lu,WU Hanyue,LI Jing,SHE Lan. Tuning Microstructures of Iron-Nickel Alloy Catalysts for Efficient Oxygen Evolution Reaction † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1083. |
| [5] | GUAN Fanglan,LI Xin,ZHANG Qun,GONG Yan,LIN Ziyu,CHEN Yao,WANG Lejun. Fabrication and Capacitance Performance of Laser-machined RGO/MWCNT/CF In-plane Flexible Micro-supercapacitor † [J]. Chem. J. Chinese Universities, 2020, 41(2): 300. |
| [6] | JIANG Yuanyuan, LI Boyu, LU Yizhong, WU Tongshun, HAN Dongxue. Oxygen Evolution Reaction Electrocatalytic Performance Analysis of Electroless Plated Ni-Bx [J]. Chem. J. Chinese Universities, 2020, 41(12): 2774. |
| [7] | REN Xiangrong,ZHOU Qi. Preparation of Nanoporous Ni and NiO and Their Electrocatalytic Activities for Oxygen Evolution Reaction † [J]. Chem. J. Chinese Universities, 2020, 41(1): 162. |
| [8] | ZHANG Min, CHEN Mengwei, GAO Hong, BI Yanfeng. Synthesis, Structure and Electrochemical Properties of Sulfonylcalix[4]arene Supported Co16 Cluster † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2052. |
| [9] | JIANG Yilan, YUAN Long, WANG Xiyang, HUANG Keke, FENG Shouhua. Effect of Defect Tuning on the Catalytic Behavior of Perovskite Structure Lanthanum Manganite† [J]. Chem. J. Chinese Universities, 2018, 39(3): 416. |
| [10] | LI Weilun, YAO Ying, ZHANG Cunzhong. Applications of Carbon Fiber Ultra-microelectrode and Powder Microelectrode in Exploring Influences of Non-aqueous Solvents and Cathode Materials on ORR and OER† [J]. Chem. J. Chinese Universities, 2017, 38(4): 642. |
| [11] | FENG Xiaolei, QU Zongkai, CHEN Jun, WANG Dengdeng, CHEN Xu, YANG Wensheng. NiFe2O4/NiO Nanocomposites as Electrocatalysts for Oxygen Evolution Reaction† [J]. Chem. J. Chinese Universities, 2017, 38(11): 1999. |
| [12] | LI Yingying, ZHANG Qi, ZHANG Yiheng, WANG Lei. Preparation of NF/rGO/Co3O4/NiO Electrode and Its Application in Supercapacitor† [J]. Chem. J. Chinese Universities, 2017, 38(11): 2031. |
| [13] | HAO Hongying, WANG Xi, SHAO Ziqiang, YANG Rongjie. Transparent Flexible Conductive Thin Films Based on Cellulose Nanofibers by Layer-by-layer Assembly Method and Its Fabricated Electrochromic Flexible Supercapacitors† [J]. Chem. J. Chinese Universities, 2015, 36(9): 1838. |
| [14] | SUN Ren-Xing1, XU Hai-Bo1*, WAN Nian-Fang2, WANG Jia1. Syntheses of IrOx-TiO2 Nano-powers from TiN via Impregnation-thermal Decomposition Method and Its Characterization [J]. Chem. J. Chinese Universities, 2007, 28(5): 904. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||