

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (6): 1164.doi: 10.7503/cjcu20180833
• Organic Chemistry • Previous Articles Next Articles
SHI Mai1, JIANG Rui2, CUI Xinxia1, ZHANG Xin2, SHEN Shigang1, DING Liang2( ), PAN Xuefeng1,2,3(
), PAN Xuefeng1,2,3( )
)
Received:2018-12-13
Online:2019-06-10
Published:2019-04-18
Supported by:CLC Number:
TrendMD:
SHI Mai,JIANG Rui,CUI Xinxia,ZHANG Xin,SHEN Shigang,DING Liang,PAN Xuefeng. Preparation, Structure and Pharmaceutical Analysis of Protamine-siRNA Complexes†[J]. Chem. J. Chinese Universities, 2019, 40(6): 1164.
| V(Total)/μL | V(siRNA)/μL | V(Protamine)/μL | V(HEPES)/μL |
|---|---|---|---|
| 50.0 | 2.5 | 2.5 | 45.0 |
| 50.0 | 5.0 | 5.0 | 40.0 |
| 50.0 | 7.5 | 7.5 | 35.0 |
| 50.0 | 10.0 | 10.0 | 30.0 |
| 50.0 | 12.5 | 12.5 | 25.0 |
| 50.0 | 15.0 | 15.0 | 20.0 |
Table 1 Preparation of protamine-siRNA-1 complex with a mass ratio of 20∶1
| V(Total)/μL | V(siRNA)/μL | V(Protamine)/μL | V(HEPES)/μL |
|---|---|---|---|
| 50.0 | 2.5 | 2.5 | 45.0 |
| 50.0 | 5.0 | 5.0 | 40.0 |
| 50.0 | 7.5 | 7.5 | 35.0 |
| 50.0 | 10.0 | 10.0 | 30.0 |
| 50.0 | 12.5 | 12.5 | 25.0 |
| 50.0 | 15.0 | 15.0 | 20.0 |
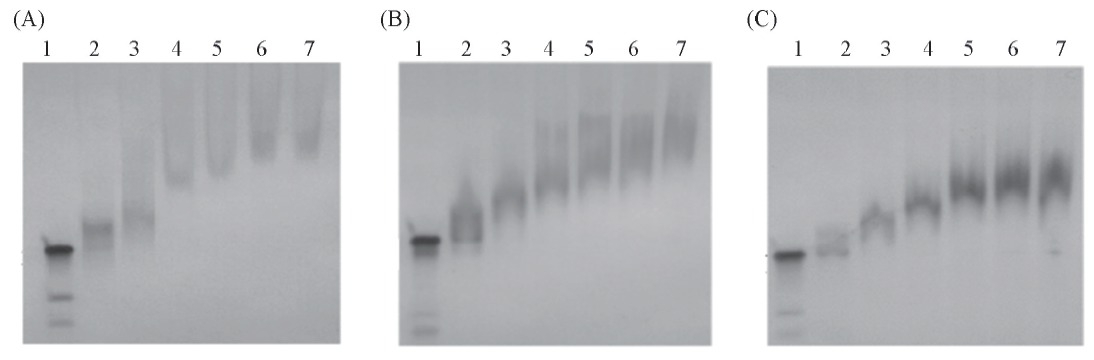
Fig.1 PAGE analysis on the formations of protamine-siRNA complexes Protamine-siRNA-1 complexes in (A) or protamine-siRNA-2 complexes in (B) were formed by protamine and siRNA by mass ratios of 15∶1, 20∶1, 25∶1, 30∶1, 35∶1, 40∶1, respectively. Lane 1: siRNA-1(A) or siRNA-2(B); lanes 2—7: protamine-siRNA complexes formed by mass ratios of protamine to siRNA-1(A) or siRNA-2(B) by 15∶1, 20∶1, 25∶ 1, 30∶ 1, 35∶ 1, 40∶ 1, respectively. Protamine-siRNA-1 complexes formed by a mass ratio of protamine to siRNA-1 by 20∶1(C). Lane 1: siRNA-1, lanes 2—7: protamine-siRNA-1 complexes formed by simultaneously increasing the protamine and siRNA-1 by 2.5, 5.0, 7.5, 10.0, 12.5, 15.0 μL, respectively.
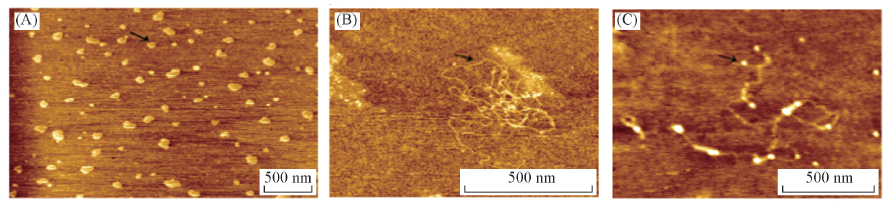
Fig.4 AFM visualizations of protamine-siRNA complexes with the mass ratios of protamine to siRNA 20∶1(A, toroid) and 40∶1(B, filamentous) and protamine proteins(white dots) in the filamentous protamine-siRNA complexes(C)
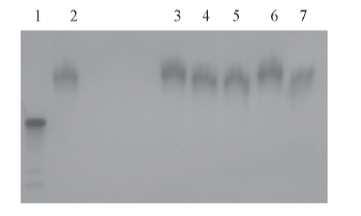
Fig.5 PAGE analysis on the formation of disulfide bonds in the protamine-siRNA complexes Lane 1: siRNA, lanes 2—7: protamine-siRNA complexes of filament morphologies treated using β-mercaptoethanol with a final concentration of 0, 5, 10, 15, 20 and 40 mmol/L, respectively.
| Mass ratio of protamine to siRNA | d(90)/nm | Mass ratio of protamine-siRNA | d(90)/nm |
|---|---|---|---|
| 15∶1 | 217.5±6.7 | 30∶1 | 325.8±7.6 |
| 20∶1 | 220.0±7.2 | 35∶1 | 384.2±6.2 |
| 25∶1 | 275.5±5.0 | 40∶1 | 405.0±4.3 |
Table 2 Particle sizes of the protamine-siRNA complexes
| Mass ratio of protamine to siRNA | d(90)/nm | Mass ratio of protamine-siRNA | d(90)/nm |
|---|---|---|---|
| 15∶1 | 217.5±6.7 | 30∶1 | 325.8±7.6 |
| 20∶1 | 220.0±7.2 | 35∶1 | 384.2±6.2 |
| 25∶1 | 275.5±5.0 | 40∶1 | 405.0±4.3 |
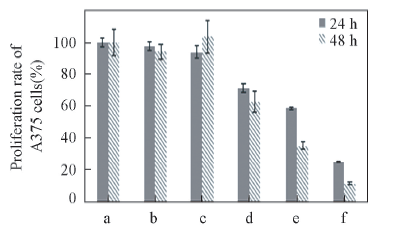
Fig.7 Effects of protamine-siRNA complexes on the proliferation of A375 cells a. Control; b. lipoHigh; c. phospholipid vesicles; d. siRNA; e. spherical complexes; f. filamentous complexes.
| [1] | Fire A., Xu S., Montgomery M. K., Kostas S. A., Drive S. E., Mello C. C., Nature,1998, 391(6669), 806-811 |
| [2] | Pan X.F., Molecular Biology of Genetic Diseases, Chemical Industry Press, Beijing, 2014, 436-447 |
| (潘学峰. 基因疾病的分子生物学, 北京: 化学工业出版社, 2014, 436-447) | |
| [3] | Peedicayil J., Indian J. Med. Res., 2006, 123(1), 17-24 |
| [4] | Jiang N., Pan X. F., Biotechnology Bulletin,2015, 31(4), 105-119 |
| (姜楠,潘学峰. 生物技术通报, 2015, 31(4), 105-119) | |
| [5] | Tatiparti K., Sau S., Kashaw S. K., Iyer A. K., Nanomaterials,2017, 7(4), 77-94 |
| [6] | Rengaswamy V., Zimmer D., Süss R., Rössler J., J. Control Release,2016, 235, 319-327 |
| [7] | Hao H. W., Zhen Y. S., Wang Z. C., Chen F. L., Xie X., Cell Biol. Int., 2013, 37, 860-864 |
| [8] | Hutchison J. M., Rau D. C., DeRouchey J. E., Biophys. J., 2017, 113(9), 1925-1933 |
| [9] | Warrant R. W., Kim S. H., Nature,1978, 271(5641), 130-135 |
| [10] | Balhorn R., Genome. Biol., 2007, 8(9), 227-235 |
| [11] | He H. N., Ye J. X., Liu E. G., Liang Q. L., Liu Q., Yang V. C., J. Control Release,2014, 193, 63-73 |
| [12] | Rui P. M., Ostermeier G. C.,Krawetz S. A,.J. Biol. Chem., 2004, 279(50), 51862-51868 |
| [13] | Cornetta K., Anderson W. F., J. Virol. Methods,1989, 23(2), 187-194 |
| [14] | Pillaiyar T., Manickam M., Namasivayam V., J. Enzym. Inhib. Med. Ch., 2017, 32(1), 403-425 |
| [15] | Hudjashov G., Villems R., Kivisild T., PloS One,2013, 8(9), e74307 |
| [16] | Wang J. H., Pei Y. Y., Xu H. D., Li L. J., Wang Y. Q., Liu G. L., Qu Y., Zhang N., Biomed Rep., 2016, 5(1), 87-92 |
| [17] | Elbashir S. M., Harborth J., Weber K., Tuschl T., Methods,2002, 26(2), 199-213 |
| [18] | Minakshi P., Ranjan K., Kumar P., Prasad G., Adv. Anim. Vet. Sci., 2013, 1(4S), 20-23 |
| [19] | Petrov A. S., Bowman J. C., Harvey S. C., Williams L. D., RNA,2011, 17(2), 291-298 |
| [20] | Misra V. K., Draper D. E., P. Natl. Acad. Sci. USA,2001, 98(22), 12456-12461 |
| [21] | Iii R. J. T., Draper D. E., Nucleic Acids Res., 2017, 45(8), 4733-4742 |
| [22] | Koo H., Park I., Lee Y., Kim H. G., Jung J. H., Lee J. H., Kim Y., Kim J. H., Park J. W., J. Am. Chem. Soc., 2016, 138(36), 11664-11671 |
| [23] | Schön P., Methods, 2016, 103, 25-33 |
| [24] | Tusup M., Pascolo S., Methods in Molecular Biology,2017, 1499, 155-163 |
| [25] | Rio D. C., Jr A. M., Hannon G. J., Nilsen T. W., Cold Spring Harbor Protocols,2010, 2010(6), pdb.prot5443 |
| [26] | Gilmore J., Deguchi K., Takeyasu K., Microscopy and Imaging Science: Practical Approaches to Applied Research and Education, Formatex Research Center, Badajoz, 2017, 300-306 |
| [27] | Bowman J. C., Lenz T. K., Hud N. V., Williams L. D., Curr. Opin. Struct.Biol., 2012, 22(3), 262-272 |
| [1] | Jinhan Sheng, Qizhen Zheng, Ming Wang. Non-viral delivery of CRISPR/Cas9 Genome Editing [J]. Chem. J. Chinese Universities, 2022, 43(Album-4): 20220344. |
| [2] | WU Yushuai, SHANG Yingxu, JIANG Qiao, DING Baoquan. Research Progress of Controllable Self-assembled DNA Origami Structure as Drug Carrier [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220179. |
| [3] | REN Yushuang, GUO Yuanyuan, LIU Xueyi, SONG Jie, ZHANG Chuan. Platinum(Ⅳ) Prodrug-grafted Phosphorothioate DNA and Its Self-assembled Nanostructure for Targeted Drug Delivery [J]. Chem. J. Chinese Universities, 2020, 41(8): 1721. |
| [4] | ZHANG Kaixiang, LIU Junjie, SONG Qiaoli, WANG Danyu, SHI Jinjin, ZHANG Haiyue, LI Jinghong. Multifunctional DNA Nanoflowers for Autophagy Inhibition and Enhanced Antitumor Chemotherapy† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1461. |
| [5] | HOU Chunxi, LI Yijia, WANG Tingting, LIU Shengda, YAN Tengfei, LIU Junqiu. Application of Elastin-like Polypeptides in Supramolecular Assembly † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1163. |
| [6] | ZHAO Yu, CAO Wanqing, LIU Yang. Recent Advances in Polymeric Nano-sized Carrier Systems † [J]. Chem. J. Chinese Universities, 2020, 41(5): 909. |
| [7] | FAN Ye, LI Qian, FANG Yun, XIA Yongmei. Fabrication of Lamellar Liquid Crystals of Conjugated Linoleic Acid as Drug Delivery Systems † [J]. Chem. J. Chinese Universities, 2020, 41(4): 750. |
| [8] | WANG Xinghuo,TANG Jun,YANG Yingwei. Mesoporous Silica Nanoparticles-Based Stimuli-Responsive Drug Delivery Systems Gated by Polymers † [J]. Chem. J. Chinese Universities, 2020, 41(1): 28. |
| [9] | LIU Xiaozhou, WANG Yujie, LIU Yaozu, LI Zonglong, LI Hui, FANG Qianrong, JIN Yongri. A Covalent Organic Framework with High Surface Area for Drug Delivery † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1813. |
| [10] | CHEN Dongdong, SONG Wenzhi, LI Hui, HE Dan, SUN Junqi, YIN Wanzhong. Layer-by-layer Assembled Polymeric Complexes Films for High Loading and Differential Release of Macromolecular and Small Molecular Drugs† [J]. Chem. J. Chinese Universities, 2019, 40(3): 592. |
| [11] | ZHAO Junqiang, YAN Caixia, CHEN Ze, YANG Ning, FENG Xia, ZHAO Yiping, CHEN Li. Synthesis and Self-assembly Properties of Intracellular Redox Bioresponsive Block Copolymers with Hepatoma-targeting Groups† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1592. |
| [12] | YANG Zechuan, LI Fan, HUANG Qingrong, ZHANG Guo, SHI Tongfei. Synthesis and Properties of the Amino Acid Functionalized Curcumin/His-Pectin Colloidal Particles† [J]. Chem. J. Chinese Universities, 2016, 37(2): 381. |
| [13] | LUO Zewei, WANG Yimin, LIU Kunping, WEI Fujing, LI Yu, DUAN Yixiang. Preparation of a Functionalized Graphene and Its Role as Delivery Carrier for Anti-cancer Drug† [J]. Chem. J. Chinese Universities, 2016, 37(10): 1900. |
| [14] | ZHANG Fada, LIU Yi, XU Jingcheng, LI Shengjuan, WANG Xiunan, SUN Yue, ZHAO Xinluo. Molecular Dynamics Study on Binding Strength and Conformation of Dendrimer-based Drug Delivery Systems† [J]. Chem. J. Chinese Universities, 2015, 36(6): 1156. |
| [15] | CHEN Jie, LI Xiaozhou, TIAN Huayu, ZHU Xiaojuan, CHEN Xuesi. Application of Zwitterionic Polymers in the Treatment of Malignant Tumors† [J]. Chem. J. Chinese Universities, 2015, 36(11): 2148. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||