

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (7): 1592.doi: 10.7503/cjcu20170714
• Polymer Chemistry • Previous Articles Next Articles
ZHAO Junqiang*( ), YAN Caixia, CHEN Ze, YANG Ning, FENG Xia, ZHAO Yiping, CHEN Li
), YAN Caixia, CHEN Ze, YANG Ning, FENG Xia, ZHAO Yiping, CHEN Li
Received:2017-11-09
Online:2018-07-10
Published:2018-06-01
Contact:
ZHAO Junqiang
E-mail:junqiangzhao@163.com
Supported by:CLC Number:
TrendMD:
ZHAO Junqiang, YAN Caixia, CHEN Ze, YANG Ning, FENG Xia, ZHAO Yiping, CHEN Li. Synthesis and Self-assembly Properties of Intracellular Redox Bioresponsive Block Copolymers with Hepatoma-targeting Groups†[J]. Chem. J. Chinese Universities, 2018, 39(7): 1592.
| Sample | Composition(/unit)(1H NMR) | Mn (1H NMR) | Mw/Mn(GPC) | Mn(PMAIpGP)/Mn(PPDSMA) (1H NMR) | |
|---|---|---|---|---|---|
| MAIpGP | PDSMA | ||||
| PMpPP(Ⅰ) | 25.0 | 29.0 | 16013 | 1.09 | 0.63 |
| PMpPP(Ⅱ) | 25.0 | 39.0 | 18566 | 1.32 | 0.50 |
Table 1 Chemical structure and compositions of PMpPP(Ⅰ) and PMgPP(Ⅱ)
| Sample | Composition(/unit)(1H NMR) | Mn (1H NMR) | Mw/Mn(GPC) | Mn(PMAIpGP)/Mn(PPDSMA) (1H NMR) | |
|---|---|---|---|---|---|
| MAIpGP | PDSMA | ||||
| PMpPP(Ⅰ) | 25.0 | 29.0 | 16013 | 1.09 | 0.63 |
| PMpPP(Ⅱ) | 25.0 | 39.0 | 18566 | 1.32 | 0.50 |
| Sample | NC NPs | CC NPs | ||
|---|---|---|---|---|
| Size/nm | PDI | Size/nm | PDI | |
| PMgPPI | 23.9±0.8 | 0.14 | 22.7±1.5 | 0.16 |
| PMgPPII | 26.0±1.6 | 0.15 | 23.7±0.2 | 0.18 |
Table 2 Characteristics of blank PMgPP-NC NPs and PMgPP-CC NPs
| Sample | NC NPs | CC NPs | ||
|---|---|---|---|---|
| Size/nm | PDI | Size/nm | PDI | |
| PMgPPI | 23.9±0.8 | 0.14 | 22.7±1.5 | 0.16 |
| PMgPPII | 26.0±1.6 | 0.15 | 23.7±0.2 | 0.18 |
| Sample | NC/DOX NPs | CC/DOX NPs | ||||||
|---|---|---|---|---|---|---|---|---|
| Size/nm | PDI | DLC(%) | DLE(%) | Size/nm | PDI | DLC(%) | DLE(%) | |
| PMgPP(Ⅰ) | 21.5 ± 4.8 | 0.16 | 11.9 | 79.3 | 20.1 ± 4.8 | 0.19 | 11.7 | 78.0 |
| PMgPP(Ⅱ) | 25.0 ± 3.2 | 0.19 | 12.0 | 80.0 | 24.8 ± 3.9 | 0.18 | 12.5 | 83.3 |
Table 3 Characteristics of PMgPP-NC/DOX NPs and PMgPP-CC/DOX NPs*
| Sample | NC/DOX NPs | CC/DOX NPs | ||||||
|---|---|---|---|---|---|---|---|---|
| Size/nm | PDI | DLC(%) | DLE(%) | Size/nm | PDI | DLC(%) | DLE(%) | |
| PMgPP(Ⅰ) | 21.5 ± 4.8 | 0.16 | 11.9 | 79.3 | 20.1 ± 4.8 | 0.19 | 11.7 | 78.0 |
| PMgPP(Ⅱ) | 25.0 ± 3.2 | 0.19 | 12.0 | 80.0 | 24.8 ± 3.9 | 0.18 | 12.5 | 83.3 |
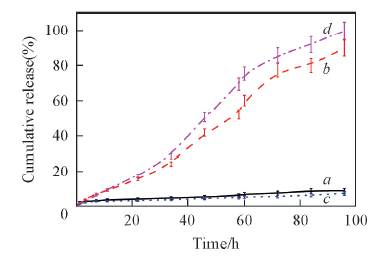
Fig.6 GSH-triggered DOX release profiles from PMgPP(Ⅱ)-NC/DOX NPs(a, b) and PMgPP(Ⅱ)-CC/DOX NPs(c, d)^Concentration of GSH(mmol·L-1): a, c. 0; b, d. 10.
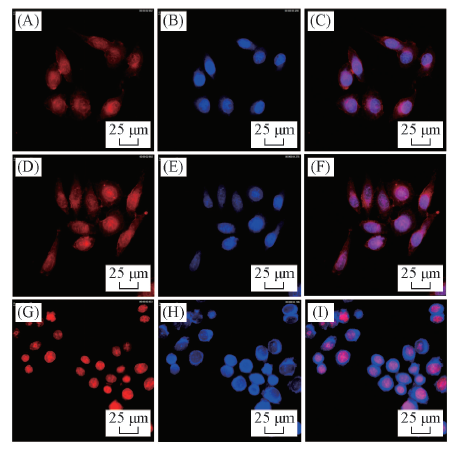
Fig.7 CLSM images of HepG-2 cells after incubated with PMgPP(Ⅱ)-NC/DOX NPs(A—C), PMgPP(Ⅱ)-CC/DOX NPs(D—F) and free DOX(G—I) at equivalent DOX concentration of 10.0 μg/mL for 8 h^Images from left to right show DOX fluorescence in cells(red) and cell nuclei stained by DAPI(blue) and overlays of two images.
| [1] | Dai Y. L., Xu C., Sun X. L., Chen X. Y., Chem. Soc. Rev., 2017, 46(12), 3830—3852 |
| [2] | Li R., Xie Y., J. Controlled Release, 2017, 251, 49—67 |
| [3] | Tibbitt M. W., Dahlman J. E., Langer R., J. Am. Chem. Soc., 2016, 138(3), 704—717 |
| [4] | Shi J. J., Kantoff P. W., Wooster R., Farokhzad O. C., Nature Reviews Cancer, 2017, 17, 20—37 |
| [5] | Blum A. P., Kammeyer J. K., Rush A. M., Callmann C. E., Hahn M. E., Gianneschi N. C., J. Am. Chem. Soc., 2015, 137(6), 2140—2154 |
| [6] | Karimi M., Ghasemi A., Zangabad P. S., Rahighi R., Moosavi Basri S. M., Mirshekari H., Amiri M., Chem. Soc. Rev., 2016, 45(5), 1457—1501 |
| [7] | Zhang X. J., Dong H., Fu S. L., Zhong Z. L., Zhuo R., Macromol. Rapid Commun., 2016, 37, 993—997 |
| [8] | Li D. W., Bu Y. Z., Zhang L. N., Wang X., Yang Y. Y., Zhuang Y. P., Yang F., Shen H., Wu D. C., Biomacromolecules, 2016, 17(1), 291—300 |
| [9] | Talelli M., Barz M., Rijcken C. J., Kiessling F., Hennink W. E., Lammers T., Nano Today, 2015, 10(1), 93—117 |
| [10] | Xiao W. W., Suby N., Xiao K., Lin T. Y., Awwad N. A., Lam K. S., Li Y. P., J. Controlled Release, 2017, 264, 169—179 |
| [11] | Huang M. M., Zhao K. J., Wang L., Lin S. Q., Li J. J., Chen J. B., Zhao C. G., Ge Z. S., ACS Appl. Mater. Interfaces, 2016, 8, 11226—11236 |
| [12] | Zhang Q. Q., He J. L., Zhang M. Z., Ni P. H., J. Mater. Chem. B, 2015, 3(24), 4922—4932 |
| [13] | Kong F. P., Liang Z. Y., Luan D. G., Liu X. J., Xu K. H., Tang B., Anal. Chem., 2016, 88(12), 6450—6456 |
| [14] | Zhang P., Zhang H. Y., He W. X., Zhao D. J., Song A. X., Luan Y. X., Biomacromolecules, 2016, 17, 1621—1632 |
| [15] | Deng B., Ma P., Xie Y., Nanoscale, 2015, 7(30), 12773—12795 |
| [16] | Han H. S., Choi K. Y., Ko H., Jeon J., Saravanakumar G., Suh Y. D., Lee D. S., Park J. H., J. Controlled Release, 2015, 200, 158—166 |
| [17] | Bertrand N., Wu J., Xu X. Y., Kamaly N., Farokhzad O. C., Adv. Drug Delivery Rev., 2014, 66, 2—25 |
| [18] | Hu X. L., Liu G. H., Li Y., Wang X. R., Liu S. Y., J. Am. Chem. Soc., 2015, 137(1), 362—368 |
| [19] | Fu L. Y., Sun C. Y., Yan L. F., ACS Appl. Mater. Interfaces, 2015, 7(3), 2104—2115 |
| [20] | Wang Z., Luo T., Sheng R. L., Li H., Sun J. J., Cao A., Biomacromolecules, 2016, 17(1), 98—110 |
| [21] | Wang Y., Hong C. Y., Pan C. Y., Biomacromolecules, 2013, 14(5), 1444—1451 |
| [22] | Moad G., Chong Y. K., Postma A., Rizzardo E., Thang S. H., Polymer, 2005, 46(19), 8458—8468 |
| [23] | Chen W., Meng F. H., Cheng R., Deng C., Feijen J., Zhong Z. Y., J. Controlled Release, 2015, 210, 125—133 |
| [1] | Jinhan Sheng, Qizhen Zheng, Ming Wang. Non-viral delivery of CRISPR/Cas9 Genome Editing [J]. Chem. J. Chinese Universities, 2022, 43(Album-4): 20220344. |
| [2] | WU Yushuai, SHANG Yingxu, JIANG Qiao, DING Baoquan. Research Progress of Controllable Self-assembled DNA Origami Structure as Drug Carrier [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220179. |
| [3] | REN Yushuang, GUO Yuanyuan, LIU Xueyi, SONG Jie, ZHANG Chuan. Platinum(Ⅳ) Prodrug-grafted Phosphorothioate DNA and Its Self-assembled Nanostructure for Targeted Drug Delivery [J]. Chem. J. Chinese Universities, 2020, 41(8): 1721. |
| [4] | ZHANG Kaixiang, LIU Junjie, SONG Qiaoli, WANG Danyu, SHI Jinjin, ZHANG Haiyue, LI Jinghong. Multifunctional DNA Nanoflowers for Autophagy Inhibition and Enhanced Antitumor Chemotherapy† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1461. |
| [5] | HOU Chunxi, LI Yijia, WANG Tingting, LIU Shengda, YAN Tengfei, LIU Junqiu. Application of Elastin-like Polypeptides in Supramolecular Assembly † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1163. |
| [6] | ZHAO Yu, CAO Wanqing, LIU Yang. Recent Advances in Polymeric Nano-sized Carrier Systems † [J]. Chem. J. Chinese Universities, 2020, 41(5): 909. |
| [7] | FAN Ye, LI Qian, FANG Yun, XIA Yongmei. Fabrication of Lamellar Liquid Crystals of Conjugated Linoleic Acid as Drug Delivery Systems † [J]. Chem. J. Chinese Universities, 2020, 41(4): 750. |
| [8] | WANG Xinghuo,TANG Jun,YANG Yingwei. Mesoporous Silica Nanoparticles-Based Stimuli-Responsive Drug Delivery Systems Gated by Polymers † [J]. Chem. J. Chinese Universities, 2020, 41(1): 28. |
| [9] | LIU Xiaozhou, WANG Yujie, LIU Yaozu, LI Zonglong, LI Hui, FANG Qianrong, JIN Yongri. A Covalent Organic Framework with High Surface Area for Drug Delivery † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1813. |
| [10] | SHI Mai,JIANG Rui,CUI Xinxia,ZHANG Xin,SHEN Shigang,DING Liang,PAN Xuefeng. Preparation, Structure and Pharmaceutical Analysis of Protamine-siRNA Complexes† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1164. |
| [11] | CHEN Dongdong, SONG Wenzhi, LI Hui, HE Dan, SUN Junqi, YIN Wanzhong. Layer-by-layer Assembled Polymeric Complexes Films for High Loading and Differential Release of Macromolecular and Small Molecular Drugs† [J]. Chem. J. Chinese Universities, 2019, 40(3): 592. |
| [12] | YUAN Jie, LIU Qingchuan, XU Guangcan, LIANG Guangyi, XU Bixue. Synthesis and Anti-HBV Activity Evaluation of the Galactopyranosyl Derivatives of MTS Based on Click Reaction† [J]. Chem. J. Chinese Universities, 2016, 37(7): 1307. |
| [13] | YANG Zechuan, LI Fan, HUANG Qingrong, ZHANG Guo, SHI Tongfei. Synthesis and Properties of the Amino Acid Functionalized Curcumin/His-Pectin Colloidal Particles† [J]. Chem. J. Chinese Universities, 2016, 37(2): 381. |
| [14] | LUO Zewei, WANG Yimin, LIU Kunping, WEI Fujing, LI Yu, DUAN Yixiang. Preparation of a Functionalized Graphene and Its Role as Delivery Carrier for Anti-cancer Drug† [J]. Chem. J. Chinese Universities, 2016, 37(10): 1900. |
| [15] | ZHANG Fada, LIU Yi, XU Jingcheng, LI Shengjuan, WANG Xiunan, SUN Yue, ZHAO Xinluo. Molecular Dynamics Study on Binding Strength and Conformation of Dendrimer-based Drug Delivery Systems† [J]. Chem. J. Chinese Universities, 2015, 36(6): 1156. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||