

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (3): 498.doi: 10.7503/cjcu20180590
• Physical Chemistry • Previous Articles Next Articles
LIU Ben, ZHANG Xingying, CHEN Shaoyun*( ), HU Chenglong
), HU Chenglong
Received:2019-08-23
Online:2019-01-24
Published:2019-01-24
Contact:
CHEN Shaoyun
E-mail:cescsy@jhun.edu.cn
Supported by:CLC Number:
TrendMD:
LIU Ben,ZHANG Xingying,CHEN Shaoyun,HU Chenglong. Preparation and Electrochemical Energy Storage Performance of One Dimensional Orderly Polyaniline Nanowires Array†[J]. Chem. J. Chinese Universities, 2019, 40(3): 498.
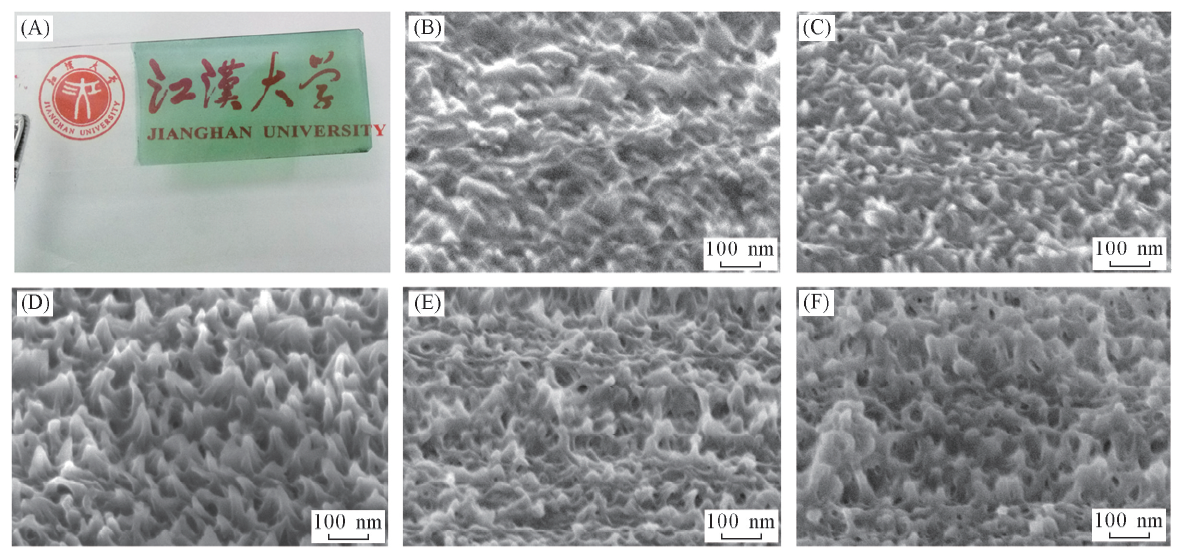
Fig.2 Optical picture of as-prepared PANI(A) and SEM images of the as-prepared PANI upon different concentration of ANI(B—F)cANI/(mol·L-1): (B) 0.02; (C) 0.05; (D) 0.10; (E) 0.20; (F) 0.30.
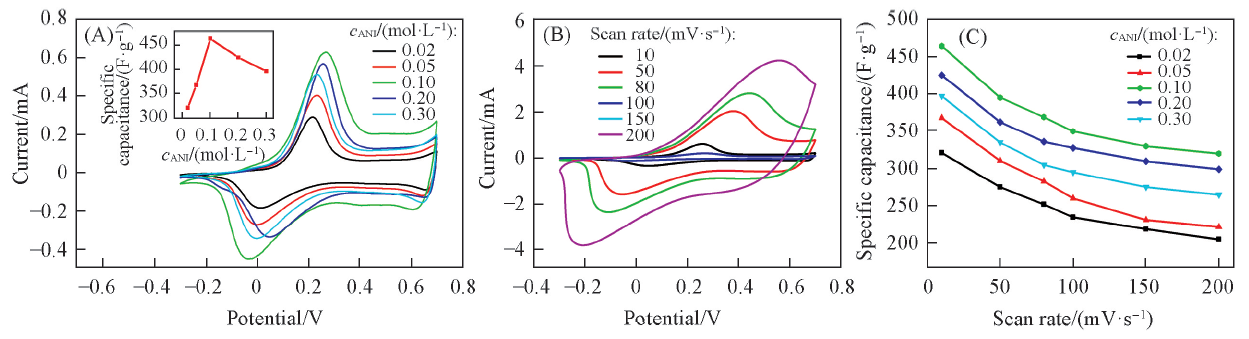
Fig.3 Cyclic voltammograms of the as-prepared PANI upon different concentrations of aniline by galvanostatic current method(0.03 mA/cm2)(A), cyclic voltammograms of PANI at different scan rates(cANI=0.1 mol/L)(B) and plots of specific capacitance as a function of the scan rate(C)The inset of (A) shows the relationship between specific capacitance and concentration of aniline.
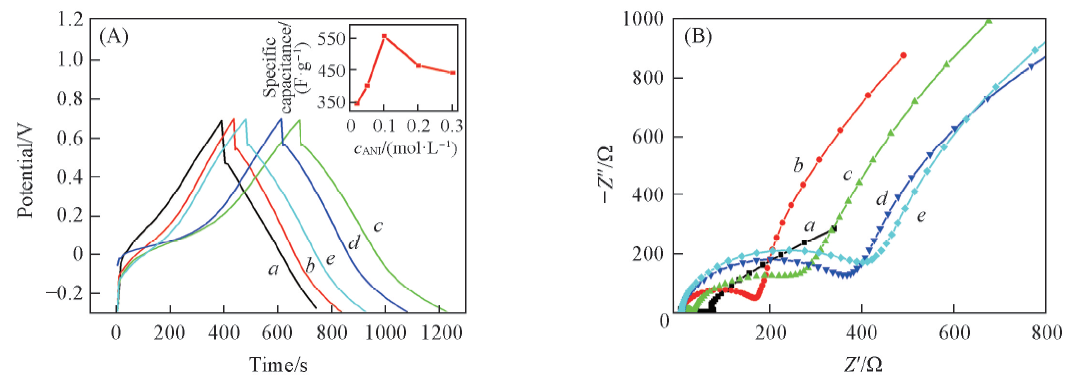
Fig.4 Charge-discharging curves(A) and Nyquist polts(B) of the as-prepared PANI upon different concentration of anilinecANI/(mol·L-1): a. 0.02; b. 0.05; c. 0.10; d. 0.20; e. 0.30. The inset of (A) shows the relationship between specific capacitance and concentration of aniline.
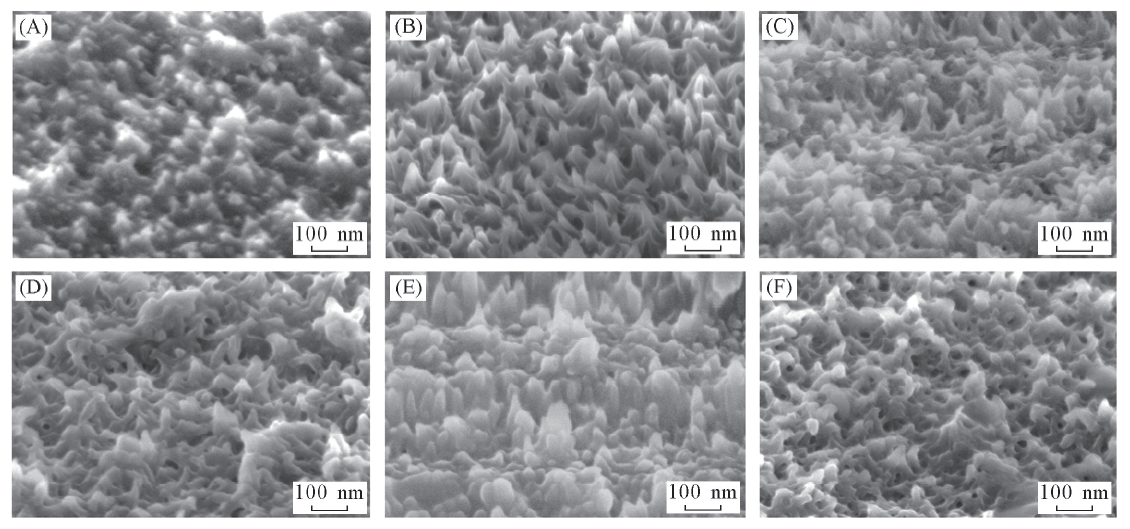
Fig.5 SEM images of the as-prepared PANI at different galvanostatic currents(cANI=0.1 mol/L) Current density/(mA·cm-2): (A) 0.01; (B) 0.03; (C) 0.05; (D) 0.07; (E) 0.09; (F) 0.13.
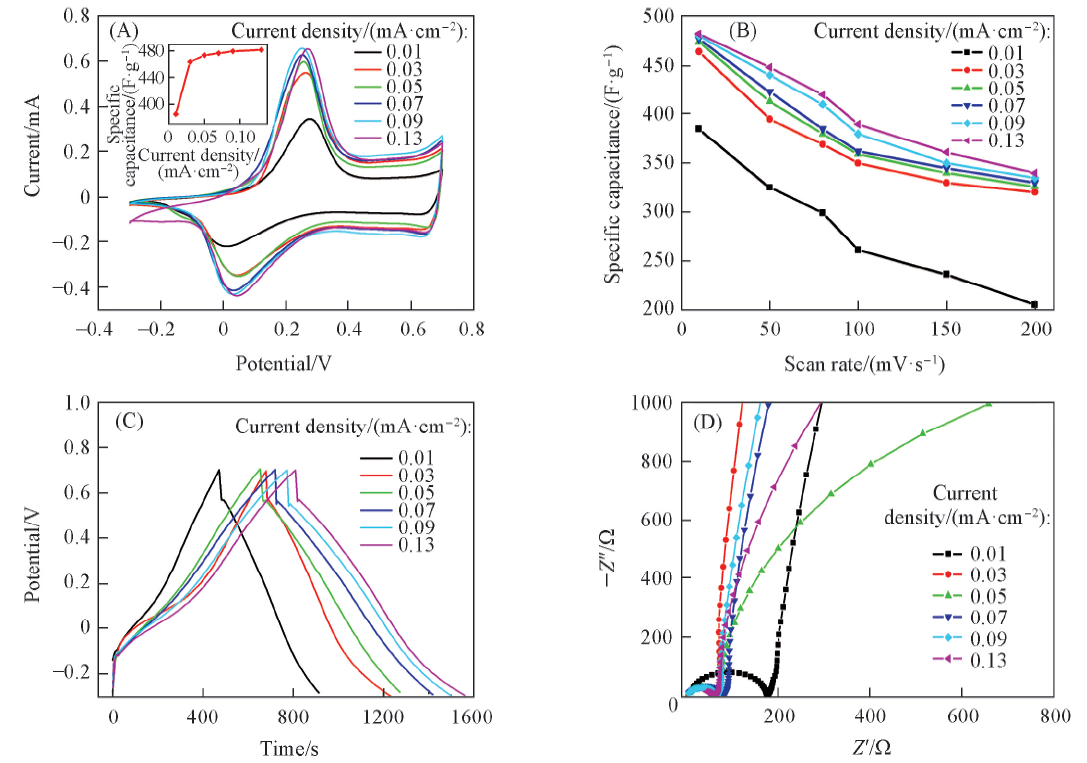
Fig.6 Cyclic voltammograms of the as-prepared PANI nanostructure at different galvanostatic currents at the scan rate of 10 mV/s(A), plots of specific capacitance as a function of scan rate(B) and charge-discharge curves(C) and Nyquist polts(D) of the as-prepared PANI nanostructureThe inset of (A) shows the relationship between specific capacitance and galvanostatic current.
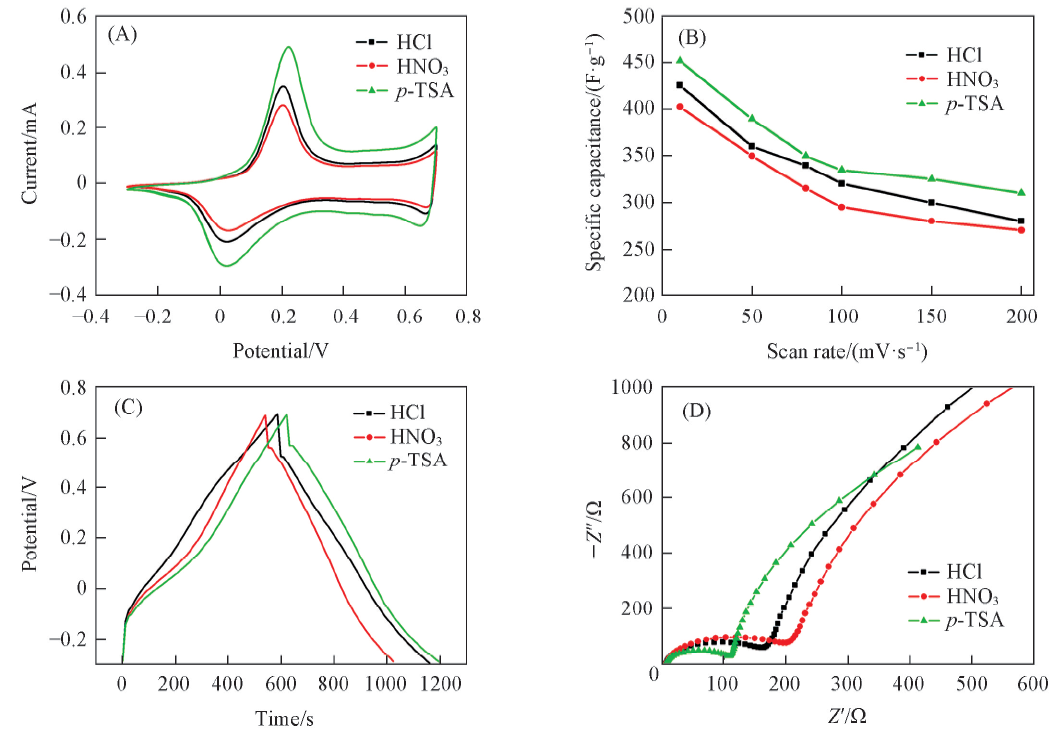
Fig.8 Cyclic voltammograms of the as-prepared PANI nanostructure with different protonic acid at deposition current density of 0.03 mA/cm2 and cANI of 0.1 mol/L(A), plots of specific capacitance as a function of scan rate(B), charge-discharging curves at 1 A/g(C) and Nyquist polts(D) of the as-prepared PANI nanostructures
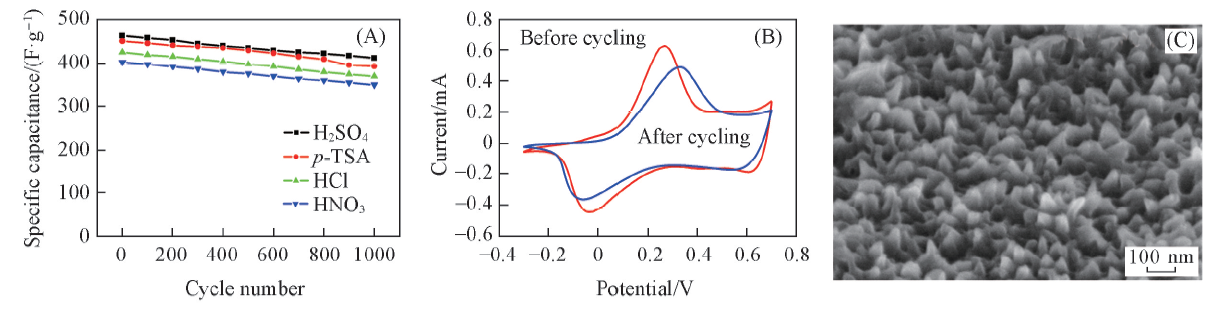
Fig.9 Cycling stability of PANI nanostructure with different protonic acid(A), CV curves of PANI nanostructure before cycle and after 1000 cycles in H2SO4(B) and SEM image of PANI nanostructure after 1000 cycles in H2SO4(C)
| [1] | Zhang L. L., Zhao X. S., Chem. Soc. Rev., 2009, 38(9), 2520—2531 |
| [2] | Snook G. A., Kao P., Best A. S. J., Power Sources, 2011, 196(1), 1—12 |
| [3] | Yu Z., Tetard L., Zhai L., Thomas J., Energy Environ.Sci., 2015, 8(3), 702—730 |
| [4] | Ji H., Zhao X., Qiao Z., Jung J., Zhu Y., Lu Y., Ruoff R. S., Nat. Commun., 2014, 5, 3317 |
| [5] | Lu Q., Chen J. G., Xiao J. Q., Angew. Chem. Int. Ed., 2013, 52(7), 1882—1889 |
| [6] | Liu T., Finn L., Yu M., Wang H., Zhai T., Lu X., Li Y., Nano Lett., 2014, 14(5), 2522—2527 |
| [7] | Cong H. P., Ren X. C., Wang P., Yu S. H., Energy Environ. Sci., 2013, 6(4), 1185—1191 |
| [8] | Wang Y. G., Li H. Q., Xia Y. Y., Adv. Mater., 2006, 18(19), 2619—2623 |
| [9] | Hu C.L.., Chen S. Y., Wang Y., Peng X. H., Zhang W. H., Chen J.,J. Power Sources, 2016, 321, 94—101 |
| [10] | Wang Y., Xu S. Q., Liu W. F., Cheng H., Chen S. Y., Liu X. Q., Liu J. Y., Tai Q. D., Hu C. L., Electrochim. Acta, 2017, 254, 25—35 |
| [11] | Xu J., Wang K., Zu S. Z., Han B. H., Wei Z., ACS Nano, 2010, 4(9), 5019—5026 |
| [12] | Hui N., Chai F., Lin P., Song Z., Sun X., Li Y., Luo X., Electrochim. Acta, 2016, 199, 234—241 |
| [13] | Wang H., Feng Q., Gong F., Li Y., Zhou G., Wang Z. S., J. Mater. Chem. A, 2013, 1(1), 97—104 |
| [14] | Chang H., Yuan Y., Shi N., Guan Y., Anal. Bioanal. Chem., 2007, 79(13), 5111—5115 |
| [15] | Cao Y., Mallouk T. E., Chem. Mater., 2008, 20(16), 5260—5265 |
| [16] | Huang J., Virji S., Weiller B.H.., Kaner R. B.,J. Am. Chem. Soc., 2003, 125(2), 314—315 |
| [17] | Wei Z., Wan M., Adv. Mater., 2002, 14(18), 1314—1317 |
| [18] | Chiou N. R., Lu C., Guan J., Lee L. J., Epstein A. J., Nat. Nanotechnol., 2007, 2(6), 354—357 |
| [19] | Wang K., Huang J., Wei Z., J. Phys. Chem. C, 2010, 114(17), 8062—8067 |
| [20] | Wang Y., Xu S. Q., Cheng H., Liu W. F., Chen F., Liu X. Q., Liu J. Y., Chen S. Y., Hu C. L., Appl. Surf. Sci., 2019, 428, 315—321 |
| [21] | Yang P., Mai W., Nano Energy, 2014, 8, 274—290 |
| [22] | Wang L., Ye Y., Lu X., Wen Z., Li Z., Hou H., Song Y., Sci. Rep., 2013, 3, 3568—3577 |
| [23] | Tu J., Hou J., Wang W., Jiao S., Zhu H., Synth. Met., 2011, 61(13/14), 1255—1258 |
| [24] | Zhang X., Goux W.J.., Manohar S. K.,J. Am. Chem. Soc., 2004, 126(14), 4502—4503 |
| [25] | Li G. R., Feng Z. P., Zhong J. H., Wang Z. L., Tong Y. X., Macromolecules, 2010, 43(5), 2178—2183 |
| [26] | Chen W., Rakhi R. B., Alshareef H. N., J. Mater. Chem. A, 2013, 1(10), 3315—3324 |
| [27] | Lin W., Xu K., Xin M., Peng J., Xing Y. Chen M., RSC Adv., 2014, 4(74), 39508—39518 |
| [28] | Liu T. Y., Finn L., Yu M. H., Wang H. Y., Zhai T., Lu X. H., Tong Y. X., Li Y., Nano Lett., 2014, 14(5), 2522—2527 |
| [1] | HOU Congcong, WANG Huiying, LI Tingting, ZHANG Zhiming, CHANG Chunrui, AN Libao. Preparation and Electrochemical Properties of N-CNTs/NiCo-LDH Composite [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220351. |
| [2] | LIANG Yu, LIU Huan, GONG Lige, WANG Chunxiao, WANG Chunmei, YU Kai, ZHOU Baibin. Synthesis and Supercapacitor Properties of Biimidazole-modified {SiW12O40} Hybrid [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210556. |
| [3] | WEI Yuchen, WU Tingting, YANG Lei, JIN Biyu, LI Hongqiang, HE Xiaojun. Preparation and Supercapacitive Performance of Naphthalene-based Interconnected Porous Carbon Nanocapsules [J]. Chem. J. Chinese Universities, 2021, 42(9): 2852. |
| [4] | YANG Sixian, ZHONG Wenyu, LI Chaoxian, SU Qiuyao, XU Bingjia, HE Guping, SUN Fengqiang. Photochemical Fabrication and Performance of Polyaniline Nanowire/SnO2 Composite Photocatalyst [J]. Chem. J. Chinese Universities, 2021, 42(6): 1942. |
| [5] | SUN Hao, GONG Jie, YANG Yan, WANG Xinqing, CHEN Huidong. Synthesis of Three-dimensional Ordered In2O3 Nanowire Arrays and the Effect of Nanostructure Order on Gas Sensitivity [J]. Chem. J. Chinese Universities, 2021, 42(6): 1730. |
| [6] | ZOU Junyan, ZHANG Yanyan, CHEN Shi, SHAO Huaiyu, TANG Yuxin. Recent Development on Surface-interface Chemistry of All-solid-state Lithium Batteries [J]. Chem. J. Chinese Universities, 2021, 42(4): 1005. |
| [7] | HUANG Dongxue, ZHANG Ying, ZENG Ting, ZHANG Yuanyuan, WAN Qijin, YANG Nianjun. Transition Metal Sulfides Hybridized with Reduced Graphene Oxide for High-Performance Supercapacitors [J]. Chem. J. Chinese Universities, 2021, 42(2): 643. |
| [8] | SHA Huiwen, MA Weiting, ZHOU Xiaojuan, SONG Weixing. One-step Preparation and Applications of Laser Induced Three-dimensional Reticular Graphene [J]. Chem. J. Chinese Universities, 2021, 42(2): 607. |
| [9] | CHEN Minghua, LI Hongwu, FAN He, LI Yu, LIU Weiduo, XIA Xinhui, CHEN Qingguo. Research Progress of Two-dimensional Transition Metal Dichalcogenides in Supercapacitors [J]. Chem. J. Chinese Universities, 2021, 42(2): 539. |
| [10] | LI Xiaoqian, ZHANG Hua, LU Haijian, LIU Chang, LIU Qinglong, MA Xiayu, FANG Yuanping, LIANG Dapeng. Mechanism of Photocatalytic Degradation of Rhodamine B by TiO2 Nanowire Array with Internal Extraction Electrospray Ionization Mass Spectrometry [J]. Chem. J. Chinese Universities, 2020, 41(9): 2003. |
| [11] | ZHANG Weiguo, FAN Songhua, WANG Hongzhi, YAO Suwei. Synthesis of Self-assembled α-Fe2O3/Graphene Hydrogel for Supercapacitors with Promising Electrochemical Properties [J]. Chem. J. Chinese Universities, 2020, 41(8): 1850. |
| [12] | REN Wen, ZHANG Guoli, YAN Han, HU Xinghua, LI Kun, WANG Jingfeng, LI Ruiqi. Preparation of Superhydrophobic Polyaniline/Polytetrafluoroethylenethylene Composite Membrane and Its Separation Ability for Oil-Water Emulsion † [J]. Chem. J. Chinese Universities, 2020, 41(4): 846. |
| [13] | GUAN Fanglan,LI Xin,ZHANG Qun,GONG Yan,LIN Ziyu,CHEN Yao,WANG Lejun. Fabrication and Capacitance Performance of Laser-machined RGO/MWCNT/CF In-plane Flexible Micro-supercapacitor † [J]. Chem. J. Chinese Universities, 2020, 41(2): 300. |
| [14] | LING Xuxia, LONG Zhu, WANG Shihua, LI Zhiqiang, GUO Shuai, ZHANG Dan. Surface Modified Aramid Pulp with Polyaniline and Conductivity of Its Paper-based Materials [J]. Chem. J. Chinese Universities, 2020, 41(11): 2553. |
| [15] | WANG Yihan,YIN Qiang,DU Kai,YIN Qinjian. Polypyrrole/Polyaniline Nanocomposite Nanotubes with Enhanced Thermoelectric Properties † [J]. Chem. J. Chinese Universities, 2020, 41(1): 175. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||