

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (11): 2520.doi: 10.7503/cjcu20180321
• Physical Chemistry • Previous Articles Next Articles
AI Shujuan1, ZONG Chengxing1, WU Wei1, FENG Jingjing1, JIN Can1, FU Fengzhi1, LIU Jing1,*( ), SUN Donglan2,*(
), SUN Donglan2,*( ), ZHENG Qin3, GUO Yeping3
), ZHENG Qin3, GUO Yeping3
Received:2018-04-25
Online:2018-11-10
Published:2018-06-26
Contact:
LIU Jing,SUN Donglan
E-mail:jingliu@tust.edu.cn;sundonglan@tust.edu.cn
Supported by:CLC Number:
TrendMD:
AI Shujuan, ZONG Chengxing, WU Wei, FENG Jingjing, JIN Can, FU Fengzhi, LIU Jing, SUN Donglan, ZHENG Qin, GUO Yeping. Effect of Derivatives of Glycerol Sulfite as Electrolyte Additive on Electrochemical Performance of Lithium Ion Battery†[J]. Chem. J. Chinese Universities, 2018, 39(11): 2520.
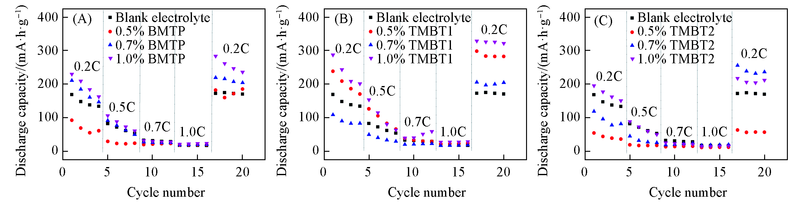
Fig.2 Rate capability of lithium ion batteries with blank electrolyte and different concentration of electrolyte additivesElectrolyte additive: (A) BMTP; (B) TMBT1; (C) TMBT2.
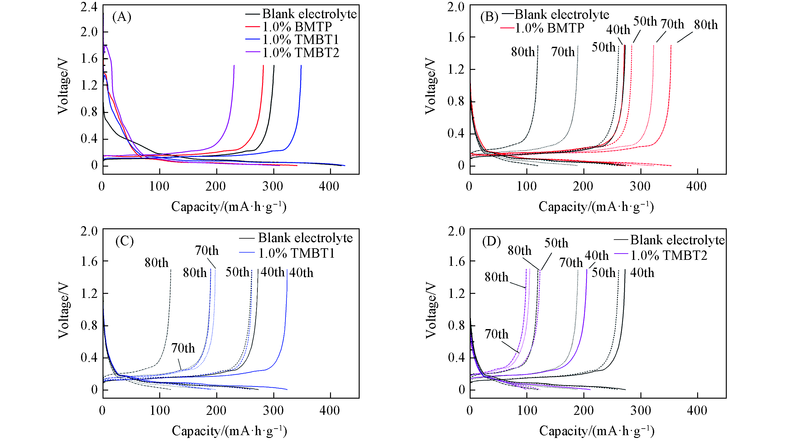
Fig.3 Charge and discharge curves of lithium ion batteries with blank electrolyte and different additives(A) First-cycle with different additives; (B) different cycles with 1.0% BMTP; (C) different cycles with 1.0% TMBT1;(D) different cycles with 1.0% TMBT2.
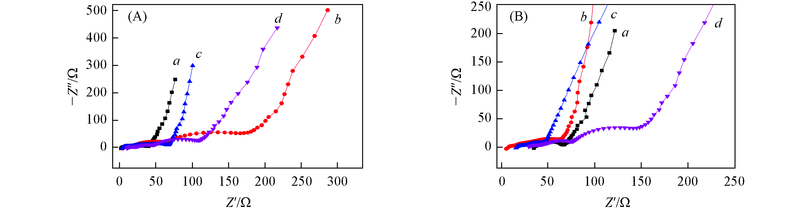
Fig.6 Nyquist plot of lithium ion batteries with blank electrolyte and different electrolyte additives(A) After 2 cycles; (B) after 80 cycles. a. Blank electrolyte; b. 1.0% BMTP; c. 1.0% TMBT1; d. 1.0% TMBT2.
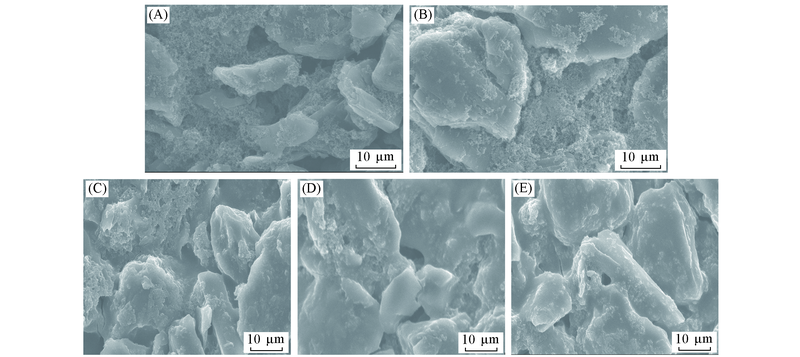
Fig.7 SEM images of graphite electrodes with blank electrolyte and electrolyte with different additives for lithium ion battery(A) Electrode before cycle; (B) electrode with blank electrolyte after 80 cycles; (C) electrode with electrolyte+1.0% BMTP after 80 cycles; (D) electrode with electrolyte+1.0% TMBT1 after 80 cycles; (E) electrode with electrolyte+1.0% TMBT2 after 80 cycles.
| [1] | Lukic S. M., Cao J., Bansal R. C., Rodriguez F., Emadi A., IEEE Transactions on Industrial Electronics, 2008, 55(6), 2258—2267 |
| [2] | Xiong S. Z., Xie K., Hong X. B., Chem. J. Chinese Universities, 2011, 32(11), 2645—2649 |
| (熊仕昭, 谢凯, 洪晓斌. 高等学校化学学报, 2011, 32(11), 2645—2649) | |
| [3] | Scrosati B., Garche J., Journal of Power Sources, 2010, 195(9), 2419—2430 |
| [4] | Wu K., Zhang Y., Zeng Y. Q., Yang J., Progress in Chemistry, 2011, 23(2), 401—409 |
| (吴凯, 张耀, 曾毓群, 杨军. 化学进展, 2011, 23(2), 401—409) | |
| [5] | Song Y. H., Yang Y. X., Hu Z. C., Power System Technology, 2011, 35(4), 1—7 |
| (宋永华, 阳岳希, 胡泽春. 电网技术, 2011, 35(4), 1—7) | |
| [6] | Hu M., Pang X. L., Zhou Z., Journal of Power Sources, 2013, 237(3), 229—242 |
| [7] | Liu G. Q., Wen L., Liu Y. M., Journal of Solid State Electrochemistry, 2010, 14(12), 2191—2202 |
| [8] | Xu M. Q., Xing L. D., Li W. S., A Non-aqueous Electrolyte and Preparation Method and Application for High-voltage Lithium-ion Battery, CN 101702447A,2010-05-05 |
| (徐梦清, 邢丽丹, 李伟善. 用于高电压锂离子电池的非水电解液及其制备方法与应用: CN 101702447A, 2010-05-05) | |
| [9] | Wang D., Li X. H., Wang Z. X., Guo H. J., Xu Y., Fan Y. L., Ru J. J., Electrochimica Acta, 2016, 188, 48—56 |
| [10] | Lee B. R., Noh H. J., Myung S. T., Amine K., Sun Y. K., Journal of the Electrochemical Society, 2011, 158(2), A180—A186 |
| [11] | Lu L. G., Han X. B., Li J. Q., Hua J. F., Ouyang M. G., Journal of Power Sources, 2013, 226(3), 272—288 |
| [12] | Oh S. W., Park S. H., Kim J. H., Bae Y. C., Sun Y. K., Journal of Power Sources, 2006, 157(1), 464—470 |
| [13] | Wang D., Li X. H., Wang Z. X., Gou H. J., Xu Y., Fan Y. L., Electrochimica Acta, 2016, 196, 101—109 |
| [14] | Yan G. C., Li X. H., Wang Z. X., Guo H. J., Wang J. X., Peng W. J., Electrochimca Acta, 2015, 166, 190—196 |
| [15] | Zuo X. X., Fan C. J., Liu J. S., Xiao X., Wu J. H., Nan J. M., Journal of Power Sources, 2013, 229(3), 308—312 |
| [16] | Wrodnigg G. H., Besenhard J. O., Winter M., Journal of The Electrochemical Society, 1999, 146(2), 470—472 |
| [17] | Zhang B. B., Zhou Y., Li X., Ren X. F., Nian H. E., Chinese Journal of Power Sources, 2014, 6, 1167—1173 |
| (张斌斌, 周圆, 李翔, 任秀峰, 年洪恩. 电源技术, 2014, 6, 1167—1173) | |
| [18] | Zhao B. H., Fang L., Chinese Battery Industry, 2013, 18(1), 78—81 |
| (赵本好, 方丽. 电池工业, 2013, 18(1), 78—81) | |
| [19] | Li A. J., Du P., Chen Z. J., Zhao R. R., Huang W. D., Zou L. Y., Huang D. H., Chen H. Y., Ionics, 2015, 21(9), 2431—2438 |
| [20] | Zuo X. X., Li W. S., Liu J. S., Xu M. Q., Chinese Battery Industry, 2006, 11(2), 97—99 |
| (左晓希, 李伟善, 刘建生, 许梦清. 电池工业, 2006, 11(2), 97—99) | |
| [21] | Yu B. T., Qiu W. H., Li F. S., Cheng L., Journal of Power Sources, 2006, 158(2), 1373—1378 |
| [22] | Wrodnigg G. H., Wrodnigg T. M., Besenhard J. O., Winter M., Electrochemistry Communications, 1999, 1(3—4), 148—150 |
| [23] | Guo M. Y., Zong C. X., Ai S. J., Fu F. Z., Wang Q., Liu J., Sun D. L., Guo Y. L., Guo Y. P., Chem. J. Chinese Universities, 2017, 38(10), 1857—1863 |
| (郭梦雅, 宗成星, 艾淑娟, 付凤至, 王琪, 刘靖, 孙冬兰, 郭艳玲, 郭也平. 高等学校化学学报, 2017, 38(10), 1857—1863) | |
| [24] | Dong T., Zhang L., Chen S. M., Lu X. M., Zhang S. J., Ionics., 2015, 21(8), 2109—2117 |
| [25] | Zong C. X., Guo M. Y., Ai S. J., Wu W., Jin C., Liu J., Sun D. L., Journal of Tianjin University of Science and Technology, 2018, 33(6), 73—78 |
| (宗成星, 郭梦雅, 艾淑娟, 吴为, 金灿, 刘靖, 孙冬兰. 天津科技大学学报, 2018, 33(6), 73—78) | |
| [26] | Jing J., Mao L. P., Cui X. L., Li S. Y., Li L. X., New Chemical Materials, 2015, 5, 11—13 |
| (净洁, 毛丽萍, 崔孝玲, 李世友, 李玲霞. 化工新型材料, 2015, 5, 11—13) | |
| [27] | Zhuang Q. C., Wu S., Chemistry Bulletin, 2003, 66(11), 743—747 |
| (庄全超, 武山. 化学通报, 2003, 66(11), 43—747) | |
| [28] | Lemaire M., Bolte J., Tetrahedron: Asymmetry, 1999, 10(24), 4755—4762 |
| [29] | Naji A., Ghanbaja J., Willmann P., Billaud D., Electrochimica Acta, 2000, 45(12), 1893—1899 |
| [30] | Shu Z. X., Mcmillan R., Murray J. J., Davidson I., Journal of the Electrochemical Society, 1995, 142(9), L161—L162 |
| [31] | Li L., Wu F., Chen R. J., Wu S. X., Chem. J. Chinese Universities, 2007, 28(2), 293—296 |
| (李丽, 吴锋, 陈人杰, 吴生先. 高等学校化学学报, 2007, 28(2), 293—296) | |
| [32] | Zhang Q., Synthesis of Fluorine-containing Ester Additive and Application in Lithium Ion Batteries, University of Jinan, Jinan, 2016 |
| (张倩. 含氟酯类添加剂的合成及其在锂离子电池中的应用研究, 济南: 济南大学, 2016) | |
| [33] | Zhen H. H., Lithium-ion Battery Electrolyte, Chemical Industry Press, Beijing, 2007 |
| (郑洪河. 锂离子电池电解质. 北京: 化学工业出版社, 2007) | |
| [34] | Li F. Q., Lan Y. Q., Zhang Z. A., Gao H. Q., Yang J., Acta Physico-Chimica Sinica, 2008, 24(7), 1302—1306 |
| (李凡群, 赖延清, 张治安, 高宏权, 杨娟. 物理化学学报, 2008, 24(7), 1302—1306) | |
| [35] | Huang K. L., Lv Z. Z., Liu S. Q., Battery, 2001, 31(3), 142—145 |
| (黄可龙, 吕正中, 刘素琴. 电池, 2001, 31(3), 142—145) | |
| [36] | Yang J., Jie J. Y., Wang J. L., Chemical Power Test Principle and Technology, Chemical Industry Press, Beijing, 2006 |
| (杨军, 解晶莹, 王久林. 化学电源测试原理与技术, 北京: 化学工业出版社, 2006) | |
| [37] | Verma P., Maire P., Novák P., Electrochimica Acta, 2010, 55(22), 6332—6341 |
| [38] | Ryou M. H., Lee D. J., Lee J. N., Lee Y. M., Park J. K., Choi J. W., Advanced Energy Materials, 2012, 2(6), 610 |
| [39] | Chen S. B., Wu X. M., Chen S., Ding Q. C., He H. L., Applied Chemical Industry, 2016, 45(1), 18—25 |
| (陈守彬, 吴显明, 陈上, 丁其晨, 何海亮. 应用化工, 2016, 45(1), 18—25) | |
| [40] | Zhang Y. Y., Zhou Y., Deng X. Y., Du X. Y., Chemistry Bulletin, 2007, 70(12), 929—935 |
| (张昕岳, 周园, 邓小宇, 杜秀月. 化学通报, 2007, 70(12), 929—935) | |
| [41] | Wang E. T., Liu G. Q., Ren Y. Z., Chemical Research, 2007, 18(1), 93—97 |
| (王恩通, 刘根庆, 任引哲. 化学研究, 2007, 18(1), 93—97) | |
| [42] | Yang C. H., Study on AC Impedance Characteristics of LiFePO4 as Cathode Materials for Lithium Ion Batteries, Shandong University of Science and Technology, Qingdao, 2015 |
| (杨传浩. 锂离子电池正极材料LiFePO4交流阻抗的研究, 青岛: 山东科技大学, 2015) | |
| [43] | Xu J., Yao W. H., Yao Y. W., Wang Z. C., Yang Y., Acta Physico-Chimica Sinica, 2009, 25(2), 201—206 |
| (许杰, 姚万浩, 姚宜稳, 王周成, 杨勇. 物理化学学报, 2009, 25(2), 201—206) |
| [1] | LI Huiyang, ZHU Siying, LI Sha, ZHANG Qiaobao, ZHAO Jinbao, ZHANG Li. Influencing Factors and Promotion Strategies of the First-cycle Coulombic Efficiency of Silicon Suboxide Anodes in Lithium-ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(8): 2342. |
| [2] | LIU Tiefeng, ZHANG Ben, SHENG Ouwei, NAI Jianwei, WANG Yao, LIU Yujing, TAO Xinyong. Research Progress of the Binders for the Silicon Anode [J]. Chem. J. Chinese Universities, 2021, 42(5): 1446. |
| [3] | WANG Renheng, XIAO Zhe, LI Yan, SUN Yiling, FAN Shuting, ZHENG Junchao, QIAN Zhengfang, HE Zhenjiang. Synthesis of Li2FeP2O7 Cathode Material at Different Temperatures and Its Electrochemical Performance for Lithium Ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(4): 1299. |
| [4] | HAN Muyao, ZHAO Lina, SUN Jie. Advances in Silicon and Silicon-based Anode Materials [J]. Chem. J. Chinese Universities, 2021, 42(12): 3547. |
| [5] | ZHANG Chenyang,WEN Yuehua,ZHAO Pengcheng,CHENG Jie,QIU Jingyi,SUN Yanzhi. Effect of Organic Carbon Source on Performance of LiTi2(PO4)3/C Composite Electrodes in Aqueous Solutions † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1352. |
| [6] | JI Tianyi, LIU Xiaoxu, ZHAO Jiupeng, LI Yao. Synthesis and Lithium-storage Characteristics of Three-dimensional Cross-linked Graphene Nanofibers † [J]. Chem. J. Chinese Universities, 2020, 41(4): 821. |
| [7] | FANG Liang,DING Xiaoli,SONG Yun,LIU Dongming,LI Yongtao,ZHANG Qingan. Effect of Morphological Tuning on Electrochemical Performance of Perovskite LaCoO3 Anodes† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1456. |
| [8] | ZHAO Xinyue,WANG Jinglun,YAN Xiaodan,ZHANG Lingzhi. Effect of Nitrile Group Functionalized Organosilicon as Electrolyte Additive on Low-temperature Performance of LiFePO4 Battery† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1258. |
| [9] | Yuzhen DUAN,Jinyu ZHU,Junming GUO,Mingwu XIANG,Xiaofang LIU,Hongli BAI,Changwei SU. Synthesis and Electrochemical Properties of Spinel Lithium Manganese Cathode Material LiNi0.01Co0.03Mn1.96O4 † [J]. Chem. J. Chinese Universities, 2019, 40(12): 2574. |
| [10] | LI Xiangnan,YU Mingming,FAN Yong,WANG Qiuxian,ZHANG Huishuang,YANG Shuting. Study on Electrochemical Performances of N-doped P/C Composite as Anode Material of Lithium Ion Batteries † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2360. |
| [11] | WU Wei, LIU Yuchun, ZHU Guancun, AN Jiayu, DOU Guangpeng, WANG Yuyan, LIU Jing, SUN Donglan, GUO Yeping. Application of Polyethylene Separator Modified by Methyl Acrylic Polymer in Lithium Ion Battery † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2332. |
| [12] | LI Yi, LI Chun, YU Kaifeng. Preparation, Characterization and Electrochemical Properties of Mesoporous Biomass Carbon Derived from Corn Stalk† [J]. Chem. J. Chinese Universities, 2018, 39(4): 607. |
| [13] | SUN Bing,JIANG Shang,WANG Runwei,NI Ling,QIU Shilun,ZHANG Zongtao. Preparation and Application of High Performance Lithium Titanate/reduced Graphene Oxide Nanocomposites for Lithium Batteries† [J]. Chem. J. Chinese Universities, 2018, 39(12): 2767. |
| [14] | XU Dan, XIAO Shanshan, WU Pan, PAN Ying, CHEN Lihua, SU Baolian, XUE Ming, QIU Shilun. Synthesis of Organodiphosphonate MOFs-derived Ni2P/C Composite† [J]. Chem. J. Chinese Universities, 2018, 39(1): 19. |
| [15] | WANG Kun, HUANG Mengyi, ZHANG Xiaosong, HUANG Junjie, DENG Xiang, LIU Changlu. Preparation and Electrochemical Performance of LiNi1/3Co1/3Mn1/3O2@C Composite† [J]. Chem. J. Chinese Universities, 2018, 39(1): 141. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||