

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (7): 1467.doi: 10.7503/cjcu20180194
• Physical Chemistry • Previous Articles Next Articles
LIU Jinghua, DING Tong, TIAN Ye*( ), LI Xingang*(
), LI Xingang*( )
)
Received:2018-03-12
Online:2018-07-10
Published:2018-06-13
Contact:
TIAN Ye,LI Xingang
E-mail:tianye@tju.edu.cn;xingang_li@tju.edu.cn
Supported by:TrendMD:
LIU Jinghua, DING Tong, TIAN Ye, LI Xingang. Enhanced CO Oxidation Performance over Potassium-promoted Pt/TiO2 Catalysts†[J]. Chem. J. Chinese Universities, 2018, 39(7): 1467.
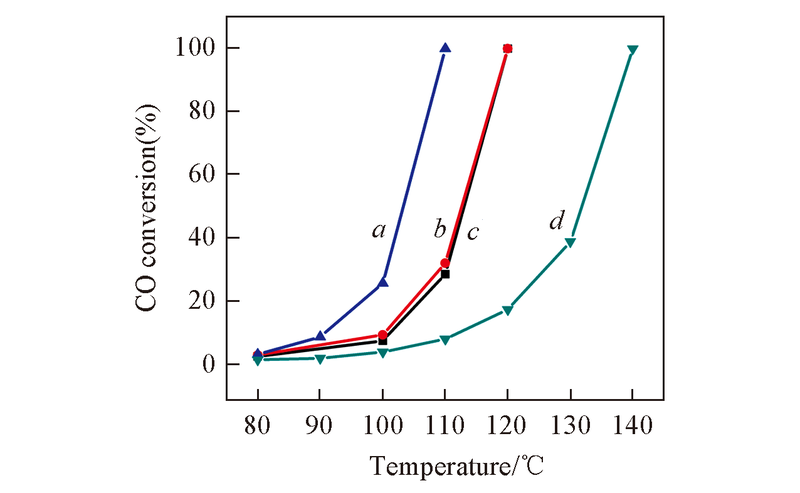
Fig.1 CO conversions of the catalysts at different reaction temperatures^a. 0.3K-Pt/TiO2; b. 0.1K-Pt/TiO2; c. 0K-Pt/TiO2; d. 0.5K-Pt/TiO2. Reaction conditions: mcat =40 mg; feeding gas compositions: 0.9%CO+24%O2+N2 balance; flow rate: 150 mL/min.
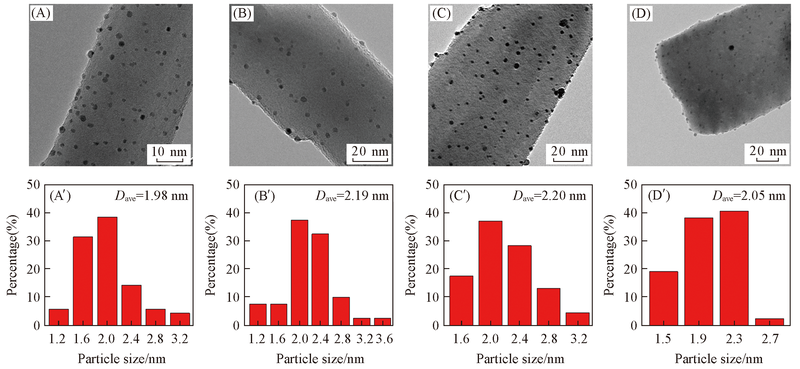
Fig.4 HRTEM images(A—D) and size distributions(A'—D') of Pt of catalysts 0K-Pt/TiO2(A, A'), 0.1K-Pt/TiO2(B, B'), 0.3K-Pt/TiO2(C, C') and 0.5K-Pt/TiO2(D, D')
| Catalyst | OOH/(OOH+OL) | OSCC(110 ℃)/ (μmol[O]·g-1) | Catalyst | OOH/(OOH+OL) | OSCC(110 ℃)/ (μmol[O]·g-1) |
|---|---|---|---|---|---|
| 0K-P | 0.11 | — | 0.3K-Pt/TiO2 | 0.34 | 961.6 |
| 0K-Pt/TiO2 | 0.27 | 912.1 | 0.5K-Pt/TiO2 | 0.25 | 805.2 |
| 0.1K-Pt/TiO2 | 0.29 | 929.2 |
Table 1 Ratios of the different surface oxygen species and the OSCC values of the catalysts
| Catalyst | OOH/(OOH+OL) | OSCC(110 ℃)/ (μmol[O]·g-1) | Catalyst | OOH/(OOH+OL) | OSCC(110 ℃)/ (μmol[O]·g-1) |
|---|---|---|---|---|---|
| 0K-P | 0.11 | — | 0.3K-Pt/TiO2 | 0.34 | 961.6 |
| 0K-Pt/TiO2 | 0.27 | 912.1 | 0.5K-Pt/TiO2 | 0.25 | 805.2 |
| 0.1K-Pt/TiO2 | 0.29 | 929.2 |
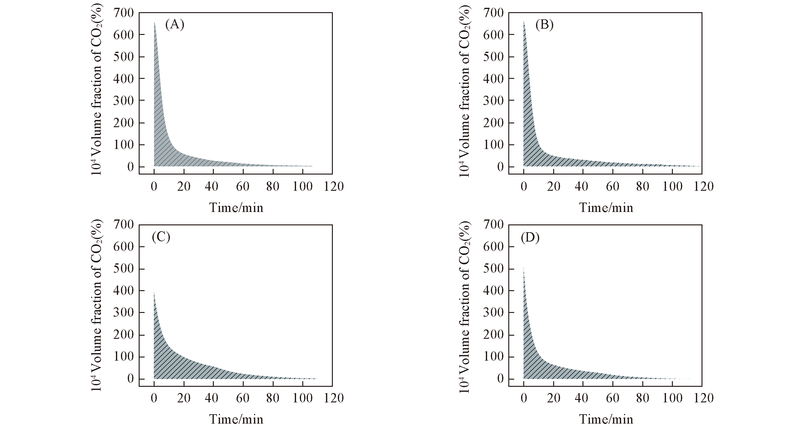
Fig.7 Curves of CO2 concentrations as a function of time during isothermal CO oxidation at 110 ℃^ (A) 0K-Pt/TiO2; (B) 0.1K-Pt/TiO2; (C) 0.3K-Pt/TiO2; (D) 0.5K-Pt/TiO2.
| Catalyst | Dispersion of Pta(%) | Loadingb(mass fraction, %) | |
|---|---|---|---|
| Pt | K | ||
| 0K-Pt/TiO2 | 33.6 | 1.0 | 0 |
| 0.1K-Pt/TiO2 | 33.9 | 1.0 | 0.1 |
| 0.3K-Pt/TiO2 | 40.1 | 1.0 | 0.3 |
| 0.5K-Pt/TiO2 | 25.7 | 1.0 | 0.5 |
Table 2 Dispersion of Pt and the loadings of Pt and K in the catalysts
| Catalyst | Dispersion of Pta(%) | Loadingb(mass fraction, %) | |
|---|---|---|---|
| Pt | K | ||
| 0K-Pt/TiO2 | 33.6 | 1.0 | 0 |
| 0.1K-Pt/TiO2 | 33.9 | 1.0 | 0.1 |
| 0.3K-Pt/TiO2 | 40.1 | 1.0 | 0.3 |
| 0.5K-Pt/TiO2 | 25.7 | 1.0 | 0.5 |
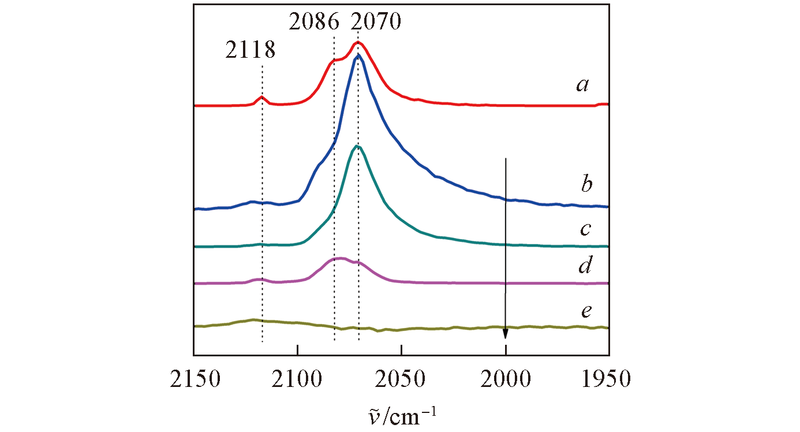
Fig.8 In situ DRIFTS spectra of the catalysts^a. Exposure of 0K-Pt-TiO2 in 1%CO/N2 flow for 10 min, and then in N2 flow for 10 min at 25 ℃; b. exposure of 0.3 K-Pt-TiO2 in 1%CO/N2 flow for 10 min, and then in N2 flow for 10 min at 25 ℃; exposure of 0.3K-Pt/TiO2 in 24%O2/N2 flow for 10 min at 25 ℃(c), 60 ℃(d), 70 ℃(e).
| [1] | Li J., Chai S. J., Bai X. Q., Li X. G., Chem. Ind. & Eng., 2017, 34(6), 1—5 |
| (李静,柴澍靖,白雪芹,李新刚.化学工业与工程, 2017, 34(6), 1—5) | |
| [2] | Chen X., Zhang J. F., Huang Y., Tong Z. Q., Huang M., Chem. Ind. & Eng. Pro., 2009, 28(8), 1355—1359 |
| (陈霞,张俊丰,黄妍,童志权,黄明.化工进展, 2009,28(8), 1355—1359) | |
| [3] | Green I. X., Tang W., Neurock M., Yates J. T., Science, 2011, 333(6043), 736—739 |
| [4] | Xie X., Li Y., Liu Z. Q., Haruta M., Shen W., Nature, 2009, 458(7239), 746—749 |
| [5] | Xu J., Deng Y. Q., Luo Y., Mao W., Yang X. J., Han Y. F., J.Catal., 2013, 300, 225—234 |
| [6] | Wang C., Guo L. H., Li X., Ma K., Ding T., Wang X. L., Cheng Q., Tian Y., Chem. J. Chinese Universities, 2017, 38(12), 2296—2305 |
| (王成, 郭丽红, 李新刚, 马奎, 丁彤, 王新雷, 程庆鹏, 田野.高等学校化学学报,2017, 38(12), 2296—2305) | |
| [7] | Chen M. S., Cai Y., Yan Z., Gath K. K., Axnanda S., Goodman D. W., Surf. Sci., 2007, 601(23), 5326—5331 |
| [8] | Ouyang X., Scott S. L., J. Catal., 2010, 273(2), 83—91 |
| [9] | Ye Q., Zhao J. S., Li D. H., Zhao J., Cheng S. Y., Kang T. F., Acta Phys.-Chim.Sin., 2011, 27(1), 169—176 |
| (叶青,赵建生,李冬辉,赵俊,程水源,康天放.物理化学学报, 2011, 27(1), 169—176) | |
| [10] | Chen M. S., Acta Phys.-Chim.Sin., 2017, 33(12), 2424—2437 |
| (陈明树.物理化学学报, 2017, 33(12), 2424—2437) | |
| [11] | Chen C., Li L., Chen J. H., Zhang X. H., Xu J., Li Y. B., Wei J., Chem. J. Chinese Universities, 2018, 39(1), 157—165 |
| (陈晨, 李丽, 陈金华, 张小华, 许杰, 李益波, 韦杰.高等学校化学学报,2018, 39(1), 157—165) | |
| [12] | Qadir K., Kim S. H., Kim S. M., Ha H., Park J.Y., J. Phys. Chem. C, 2012, 116, 24054—24059 |
| [13] | Liu L. Q., Zhou F., Wang L. G., Qi X. J., Shi F., Deng Y. Q., J.Catal., 2010, 274, 1—10 |
| [14] | Qiao B., Wang A., Yang X., Allard L. F., Jiang Z., Cui Y., Liu J., Li J., Zhang T., Nature Chem., 2011, 3, 634—641 |
| [15] | Yi T., Yuan F., Zheng B., Yu H., Xie Y., Chem. Res. Chinese Universities, 2017, 34(6), 631—637 |
| [16] | Lee C. H., Chen Y. W., Ind. Eng. Chem. Res., 1997, 36(5), 1498—1506 |
| [17] | Wu H. C., Liu L. C., Yang S. M., Appl. Catal.A, 2001, 211(2), 159—165 |
| [18] | Zhang R., Miller J. T., Baertsch C. D., J.Catal., 2012, 294, 69—78 |
| [19] | Cao C. M., Li X. G., Zha Y. Q., Hu T. D., Meng M., Nanoscale, 2016, 8(11), 5857—55864 |
| [20] | Takeguchi T., Manabe S., Kikuchi R., Eguchi K., Kanazawa T., Matsumoto S., Ueda W., Appl. Catal. A, 2005, 293, 91—96 |
| [21] | Cai J. M., Wang Y. T., Zhu Y. M., Wu M. Q., Zhang H., Li X. G., Jiang Z., Meng M., ACS Appl. Mater. Interfaces, 2015, 7(45), 24987—24992 |
| [22] | Chen G., Mi C. T., Lü H., Hao C. P., Huang Y., Song Y. K., Chem. J. Chinese Universities, 2016, 37(1), 126—133 |
| (陈刚, 米灿根, 吕洪, 郝传璞, 黄宇, 宋宇琨.高等学校化学学报,2016, 37(1), 126—133) | |
| [23] | Zhang J., Xu Q., Feng Z., Li M., Li C., Angew. Chem. Int. Ed., 2008, 47(9), 1766—1769 |
| [24] | Yang D., Liu H., Zheng Z., Yuan Y., Zhao J. C., Waclawik E. R., Ke X., Zhu H. J., Am. Chem. Soc., 2009, 131, 17885—17893 |
| [25] | Zhu J. L., Xia X. F., Zhu S. S., Liu X., Li H. X., Chem. J. Chinese Universities, 2016, 37(10), 1833—1839 |
| (朱洁莲, 夏晓峰, 朱珊珊, 刘湘, 李和兴.高等学校化学学报,2016, 37(10), 1833—1839) | |
| [26] | Guan H., Lin J., Qiao B., Yang X., Li L., Miao S., Liu J., Wang A., Wang X., Zhang T., Angew. Chem. Int.Ed., 2016, 55(8), 2820—2824 |
| [27] | Zhu Y. M., Liu D. S., Meng M., Chem.Commun., 2014, 50(45), 6049—6051 |
| [28] | Naldoni A., Allieta M., Santangelo S., Marelli M., Fabbri F., Cappelli S., Bianchi C. L., Psaro R., Santo V. D., J. Am. Chem. Soc., 2012, 134(18), 7600—7603 |
| [29] | Li C., Sivaranjani K., Ji M. K., Catal. Today, 2016, 265, 45—51 |
| [30] | Huang H., Leung D. Y. C., ACS Catal., 2011, 1(4), 348—354 |
| [31] | Chua Y. P. G., Gunasooriya G. T. K. K., Saeys M., Seebauer E., J.Catal., 2014, 311(3), 306—313 |
| [32] | Mergler Y., Aalst A., Delft J., Nieuwenhuys B., Appl. Catal. B, 1996, 10, 245—261 |
| [33] | Satsuma A., Osaki K., Yanagihara M., Ohyama J., Shimizu K., Appl. Catal. B, 2013, 132, 511—518 |
| [34] | Liu H., Jia A., Wang Y., Luo M. F., Chin. J.Catal., 2015, 36(11), 1976 |
| [35] | Kim G. J., Dong W. K., Hong S. C., J. Phys. Chem. C, 2016, 120(32), 17996—18004 |
| [36] | Jiang Z., Yang Y., Shangguan W., Jiang Z., J. Phys. Chem. C, 2012, 116(36), 19396—19404 |
| [37] | Panagiotopouloua P., Christodoulakisa A., Kondaridesa D. I., Boghosiana S., J.Catal., 2006, 24(2), 114—125 |
| [38] | Alexeev O. S., Chin S., Engelhard M., Ortiz-Soto L., Amiridis M., J. Phys. Chem. B, 2006, 109(49), 23430—23443 |
| [1] | JIANG Shan, SHEN Qianqian, LI Qi, JIA Husheng, XUE Jinbo. Pd-loaded Defective TiO2 Nanotube Arrays for Enhanced Photocatalytic Hydrogen Production Performance [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220206. |
| [2] | GAO Zhongnan, GUO Lihong, ZHAO Dongyue, LI Xingang. Effect of A Site-deficiency on the Structure and Catalytic Oxidation Activity of the La-Sr-Co-O Perovskite [J]. Chem. J. Chinese Universities, 2021, 42(9): 2869. |
| [3] | WU Qiliang, MEI Jinghao, LI Zheng, FAN Haidong, ZHANG Yanwei. Photo-thermal Coupling Water Splitting over Fe-doped TiO2 with Various Nanostructures [J]. Chem. J. Chinese Universities, 2021, 42(6): 1837. |
| [4] | ZHANG Guoqiang, SUN Yuchen, SHI Yabo, ZHENG Huayan, LI Zhong, SHANGGUAN Ju, LIU Shoujun, SHI Pengzheng. Surface Properties of Ce1-xMnxO2 Catalyst on the Catalytic Activities for Direct Synthesis of DMC from CO2 and Methanol [J]. Chem. J. Chinese Universities, 2020, 41(9): 2061. |
| [5] | JIN Xin, FENG Xilan, LIU Dapeng, SU Yutong, ZHANG Zheng, ZHANG Yu. Auto-redox Strategy for the Synthesis of Co3O4/CeO2 Nanocomposites and Their Structural Optimization Towards Catalytic CO Oxidation [J]. Chem. J. Chinese Universities, 2020, 41(4): 652. |
| [6] | WANG Rui, HUANG Xinsong, LIU Tian⁃Fu, CAO Rong. Metal-organic Frameworks for CO Oxidation [J]. Chem. J. Chinese Universities, 2020, 41(10): 2174. |
| [7] | REN Wei, TIAN Ye, XING Lingli, YANG Yuexi, DING Tong, LI Xingang. K Promoted Nanosheets-like Hydrotalcite-derived CoAlO Metal Oxides for Catalytic Soot Combustion [J]. Chem. J. Chinese Universities, 2019, 40(8): 1670. |
| [8] | WU Binquan, WANG Sheng, HUANG Liang, QIN Feng, HUANG Zhen, XU Hualong, SHEN Wei. Preparation of Ce Modified Mg-Al Mixed Metal Oxides by Aqueous Reconstruction for Vapor Self-condensation of Acetone† [J]. Chem. J. Chinese Universities, 2016, 37(4): 745. |
| [9] | KONG Lingzhi, WANG Qian, XU Li, YAN Yongsheng, LI Huaming, YANG Xiangguang. Influence of CuO on Ce-Zr-O2 Dispersion on Catalytic Properties in CO Oxidation† [J]. Chem. J. Chinese Universities, 2015, 36(7): 1372. |
| [10] | LIU Qingyu, HE Shenggui. Oxidation of Carbon Monoxide on Atomic Clusters† [J]. Chem. J. Chinese Universities, 2014, 35(4): 665. |
| [11] | GUO Ming, PU Zhi-Ying, BI Qing-Yuan, LU Ji-Qing, LUO Meng-Fei*. Raman Spectroscopic Study of Oxygen Vacancies in Ce1-xTbxO2-δ Mixed Oxides [J]. Chem. J. Chinese Universities, 2009, 30(8): 1645. |
| [12] | BI Yu-Shui1*, LÜ Gong-Xuan2. Controlled Preparation of Nano-Au/NaZSM-5 Catalyst and Its Catalytic Performance [J]. Chem. J. Chinese Universities, 2009, 30(1): 129. |
| [13] | GUO Xian-Zhi*, HUANG Jing, WANG Yan-Mei, WANG Shu-Rong, ZHANG Bao-Long, WU Shi-Hua. Preparation, Characterization and CO Oxidation Catalytic Properties of CuO/TiO2 Catalysts Supported on Porous Microspheres Composed of TiO2 Nanocrystals [J]. Chem. J. Chinese Universities, 2008, 29(6): 1220. |
| [14] | ZHANG Guo-Fang1,3, XUE Yan-Feng2, XU Jiao-Xing1, QIU Xiao-Qing1, LI Guang-She1, LI Li-Ping1*. Hydrothermal Synthesis and Characterization of Nanometer Ce1-xCoxO2-δ Solid Solutions [J]. Chem. J. Chinese Universities, 2007, 28(4): 603. |
| [15] | MA Jing-Meng, LU Ji-Qing, WANG Yue-Juan, BAO Ming-Min, LUO Meng-Fei*. Preparation of Highly-specific Surface Area MnOx-CeO2 Catalysts by a Template Precipitation Method and Its Performance for Low Temperature CO Oxidation [J]. Chem. J. Chinese Universities, 2007, 28(11): 2112. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||