

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (4): 665.doi: 10.7503/cjcu20131066
• Review • Previous Articles Next Articles
LIU Qingyu1,2, HE Shenggui1,*( )
)
Received:2013-11-04
Online:2014-04-10
Published:2013-12-30
Contact:
HE Shenggui
E-mail:shengguihe@iccas.ac.cn
Supported by:CLC Number:
TrendMD:
LIU Qingyu, HE Shenggui. Oxidation of Carbon Monoxide on Atomic Clusters†[J]. Chem. J. Chinese Universities, 2014, 35(4): 665.
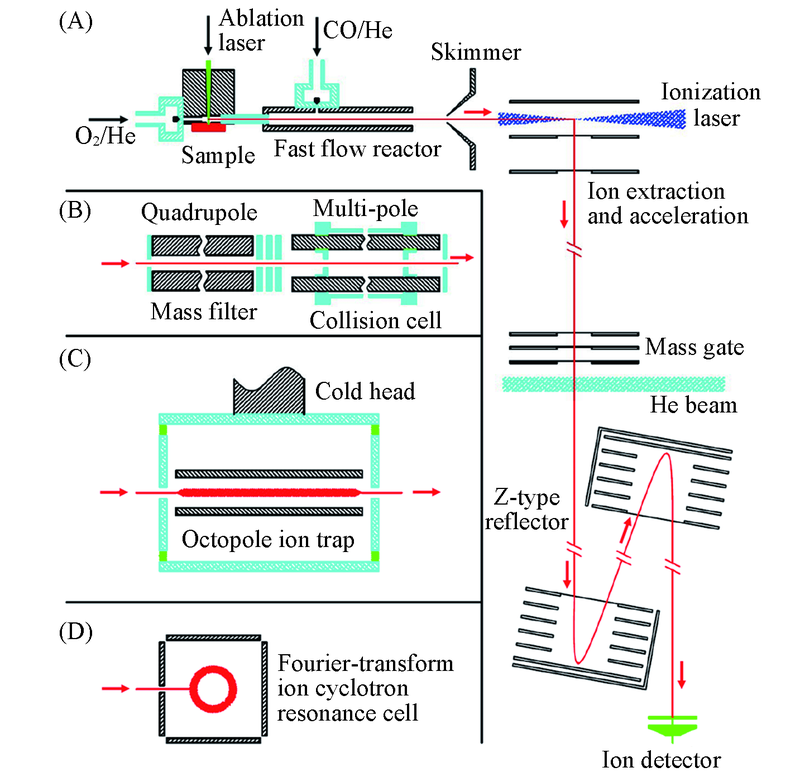
Fig.1 Experimental setups used for studying the reactions between CO and atomic clustersPart (A) shows that a fast flow reactor is coupled with a laser ablation cluster source and a time-of-flight mass spectrometer in which a laser ionizes the neutral clusters, a mass gate selects cluster ions of interest, and a helium beam collides with the selected ions(adapted from refs.[33,42,45]). The reactor in (A) can be replaced by a quadrupole mass filter and a multi-pole(hexapole or octopole) collision cell(B, adapted from refs.[32,46]). The collision cell in (B) can be further replaced by an octopole ion trap(C, adapted from ref.[47]) and a Fourier-transform ion cyclotron resonance cell(D, adapted from refs.[49]). For (B) and (C), the ions coming out of the cell or trap are detected by a quadrupole[46] or a time-of-flight mass spectrometer[33].
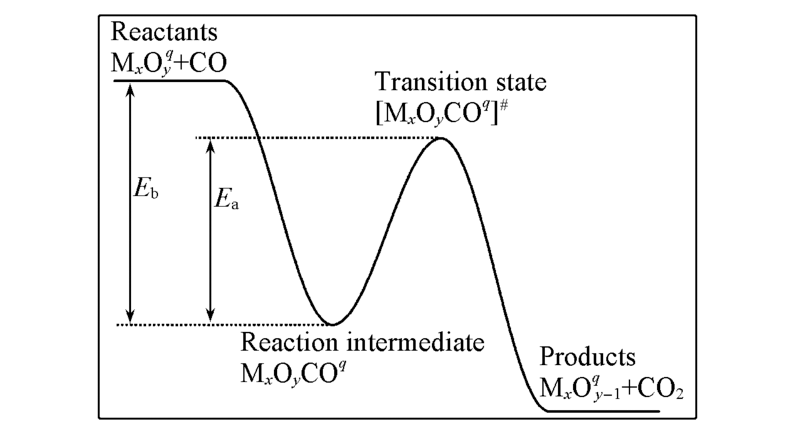
Fig.2 Typical potential energy profile for CO oxidation on the MxOyq clusterThe reaction barrier and the binding energy between CO and MxOyq are indicated by variables Ea and Eb, respectively.
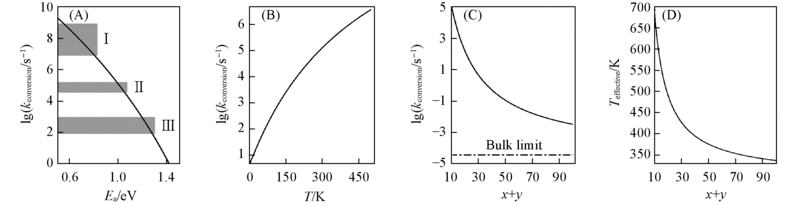
Fig.3 Calculated rates(A—C) and effective temperatures(D) for conversions of MxOyCOq into MxOy-1q+CO2(see Fig.2) The conversion rates with respect to the reaction barrier, the cluster vibrational temperature, and the number of atoms in the cluster are given in (A), (B) and (C), respectively. Eq.(2) in the text is used and the ν, Eb, and Ek are fixed at 3×1012 s-1, 1.5 eV, and 0.14 eV(a center-of-mass velocity of 1 km/s and a reduced mass of 28 amu), respectively. Other parameters fixed: T=298 K and x+y=10 for (A); Ea=1.0 eV and x+y=10 for (B); and Ea=1.0 eV and T=298 K for (C), in which the value denoted as bulk limit is calculated with Eq.(3) in the text. In (A), the ranges labeled as Ⅰ, Ⅱ, and Ⅲ represent the rates of energy dissipation(collision or infrared radiation) in the fast flow reactor[Fig.1(A)], in the collision cell and ion trap[Fig.1(B) and 1(C)], and in the FT-ICR cell[Fig.1(D)]. (D) shows the effective temperate(Teffective) at which the conversion rate of bulk limit(Eq.(3) with T=Teffective) equals to that of the cluster system in (C).
| Species | Cluster | Product | k1 | Reactor | Remark | Year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main | Al2 | Al2 | [1.4×10-11] | B | 2008[ | |||||||||||
| group | Al2 | Al2 | [ca.10-14] | B | 2008[ | |||||||||||
| Al2 | Al2 | [4.6×10-13] | B | 2008[ | ||||||||||||
| CaO+ | Ca+ | 2.5×10-10 | A | 2005[ | ||||||||||||
| CaO+ | Ca+ | (2.8±1.5)×10-10 | A | 2008[ | ||||||||||||
| GeO+ | Ge+ | 2.1×10-10 | A | 2005[ | ||||||||||||
| SrO+ | Sr+ | 1.0×10-10 | A | 2005[ | ||||||||||||
| BaO+ | Ba+ | 2.3×10-11 | A | 2005[ | ||||||||||||
| d-Block | Sc2 | Sc2 | 2.0×10-10 | A | Theory | 2012[ | ||||||||||
| Ti2 | Ti2 | [4.5×10-12] | B | Theory | 2011[ | |||||||||||
| (TiO2)nO- | (TiO2 | ca.10-11 | A | n=3—25, theory(n=3—8) | 2013[ | |||||||||||
| V4 | V4 | 4.4×10-11 | B | Theory | 2013[ | |||||||||||
| VO3 | VO2 | A | Theory | 2013[ | ||||||||||||
| Mn2O4 | Mn2O3 | 6.5×10-13 | A | Theory | 2013[ | |||||||||||
| Mn3O7 | Mn3O6 | 1.9×10-13 | A | Theory | 2013[ | |||||||||||
| FeO+ | Fe+ | 9×10-10 | D | 1981[ | ||||||||||||
| FeO+ | Fe+ | 1.8×10-10 | A | Theory | 2005[ | |||||||||||
| FeO(N2O | Fe+ | ca.10-10 | A | n=0—3 | 1995[ | |||||||||||
| Fe | FeO+, FeCO+ | [5.3×10-12] | B | Theory | 2007[ | |||||||||||
| Fe | FeO+, FeOCO+, Fe+ | B | 2007[ | |||||||||||||
| Fe | Fe | [1.4×10-12] | B | 2007[ | ||||||||||||
| Fe | FeO2CO+, Fe(CO | [5.5×10-12] | B | Loss of molecular O2, theory | 2007[ | |||||||||||
| Fe | Fe | B | 2007[ | |||||||||||||
| Fe2O+ | F | B | Theory | 2007[ | ||||||||||||
| Fe2 | Fe2O+, F | [8.8×10-13] | B | Theory | 2007[ | |||||||||||
| Fe2 | Fe2 | [ca.10-13] | B | 2007[ | ||||||||||||
| Fe2 | Fe2O2CO+, Fe2 | B | Theory | 2007[ | ||||||||||||
| Fe2 | Fe2 | B | Theory | 2007[ | ||||||||||||
| Fe2 | ||||||||||||||||
| FeO2 | FeO | 5.3×10-12 | A | Theory | 2008[ | |||||||||||
| FeO3 | FeO2 | 1.3×10-12 | A | Theory | 2008[ | |||||||||||
| Fe | FeO- | B | 2007[ | |||||||||||||
| Fe | FeO- | 1.9×10-12 | B | Theory | 2010[ | |||||||||||
| Fe | Fe | [ca.10-14] | B | 2007[ | ||||||||||||
| Fe | Fe | B | 2007[ | |||||||||||||
| Fe | Fe | [2.9×10-13] | B | Loss of molecular O2 and | 2007[ | |||||||||||
| CO oxidation | ||||||||||||||||
| Species | Cluster | Product | k1 | Reactor | Remark | Year | ||||||||||
| d-Block | Fe2 | Fe2 | B | 2007[ | ||||||||||||
| Fe2 | Fe2 | 1.8×10-12 | B | 2010[ | ||||||||||||
| Fe2 | Fe2 | B | 2007[ | |||||||||||||
| Fe2 | Fe2 | B | 2007[ | |||||||||||||
| Fe2 | Fe2 | [4.5×10-13] | B | Loss of molecular O2 and | 2007[ | |||||||||||
| CO oxidation | ||||||||||||||||
| CoO+ | Co+ | B | 2008[ | |||||||||||||
| Co | Co+, CoCO+ | [9.3×10-12] | B | Loss of molecular O2,theory | 2008[ | |||||||||||
| Co | CoO+, CoOCO+, Co+ | [7.2×10-12] | B | Loss of molecular O2 | 2008[ | |||||||||||
| Co | Co | B | Loss of molecular O2 | 2008[ | ||||||||||||
| Co2 | CoO2CO+ , Co(CO | [1.3×10-12] | B | Loss of molecular O2, | 2008[ | |||||||||||
| cluster fragmentation | ||||||||||||||||
| Co2 | Co2 | [4.7×10-12] | B | Loss of molecular O2 | 2008[ | |||||||||||
| CoO2CO+, Co(CO | ||||||||||||||||
| Co2 | Co2 | B | Loss of molecular O2 | 2008[ | ||||||||||||
| ConOm | Con | 10-13—10-12 | A | n=3—9, m=3—13, | 2010[ | |||||||||||
| theory(Co3O4) | ||||||||||||||||
| Co | CoO- | B | 2008[ | |||||||||||||
| Co | CoO- | 0.3×10-12 | B | 2010[ | ||||||||||||
| Co | Co | B | 2008[ | |||||||||||||
| Co2 | Co2 | B | 2008[ | |||||||||||||
| Co2 | Co2 | 1.6×10-12 | B | 2010[ | ||||||||||||
| Co2 | Co2 | [4.8×10-14] | B | 2008[ | ||||||||||||
| Co3 | Co3 | [1.4×10-13] | B | 2008[ | ||||||||||||
| Co3 | Co3 | [1.0×10-13] | B | 2008[ | ||||||||||||
| Co2 | ||||||||||||||||
| Ni6 | Ni6 | 2.2×10-13 | A | 2013[ | ||||||||||||
| Ni8 | Ni8 | 1.2×10-13 | A | 2013[ | ||||||||||||
| Ni | NiO- | (9.2±0.5)×10-14 | B | 2009[ | ||||||||||||
| Ni | NiO- | 0.4×10-12 | B | 2010[ | ||||||||||||
| Ni | Ni | B | 2009[ | |||||||||||||
| Ni2 | Ni2 | (1.9±0.1)×10-13 | B | 2009[ | ||||||||||||
| Ni2 | Ni2 | 1.0×10-12 | B | 2010[ | ||||||||||||
| Ni3 | Ni3 | (9.6±0.8)×10-14 | B | 2009[ | ||||||||||||
| Ni4 | Ni4 | (3.0±0.2)×10-13 | B | 2009[ | ||||||||||||
| Cu | CuO- | 0.7×10-12 | B | 2010[ | ||||||||||||
| Cu2 | Cu2 | 1.5×10-12 | B | 2010[ | ||||||||||||
| Cu5 | Cu5O- | B | 2013[ | |||||||||||||
| Cu9 | Cu9O- | B | 2013[ | |||||||||||||
| Zr2 | Zr2 | 1.8×10-10 | A | 2010[ | ||||||||||||
| (ZrO2 | Zrn | 2.4×10-12, 1.6×10-12, | B | n=1—5, k1 for | 2008[ | |||||||||||
| 1.2×10-12, 6.3×10-13 | n=2—5,theory | |||||||||||||||
| (ZrO2)nO- | (ZrO2 | 2.1×10-12, 2.2×10-13, | B | n=1—4, theory | 2009[ | |||||||||||
| 3.4×10-13, 1.8×10-13 | ||||||||||||||||
| (ZrO2)nO- | (ZrO2)nC | ca.10-11 | A | n=3—25, Oxidative adsorption | 2013[ | |||||||||||
| Mo | Mo(CO | A | n=1—3 | 2006[ | ||||||||||||
| C6 | 2007[ | |||||||||||||||
| RhnOm | Rhn | ca.10-10 | A | n=10—28, m=1—5, | 2012[ | |||||||||||
| k1 for m=4, 5 | ||||||||||||||||
| Pd | PdO+, OPdCO+, PdCO+ | B | Loss of molecular O2 and | 2011[ | ||||||||||||
| CO oxidation, theory | ||||||||||||||||
| Pd | Pd | B | Loss of molecular O2 and | 2011[ | ||||||||||||
| OPdCO+ | CO oxidation, theory | |||||||||||||||
| PdnO+ | Pdn(CO | C | n=2—7 | 2012[ | ||||||||||||
| Pdn | Pdn(CO | C | n=4—6 | 2012[ | ||||||||||||
| Species | Cluster | Product | k1 | Reactor | Remark | Year | ||||||||||
| d-Block | Pd6 | Pd6 | C | Theory | 2012[ | |||||||||||
| Pd6 | Pd6O5CO+ | C | Eliminate CO2 and form | 2012[ | ||||||||||||
| Pd6 | ||||||||||||||||
| Ag3 | Ag3On(OCO | C | n=1—3, with N2O | 2011[ | ||||||||||||
| Agn | A | C | n=7, 9, 11 | 2004[ | ||||||||||||
| Ag5 | Ag4 | C | Cluster fragmentation | 2005[ | ||||||||||||
| Ag3C | ||||||||||||||||
| AgnNx | Ag3NO-, AgN2 | C | n=2—5, y>x, cluster | 2007[ | ||||||||||||
| fragmentation | ||||||||||||||||
| La2 | La2O3+e- | 1.9×10-10 | A | Electron detachment, theory | 2012[ | |||||||||||
| Wn | Wn | 3.0×10-12, 3.6×10-13, | B | n=1—3 | 2008[ | |||||||||||
| 5.9×10-13 | ||||||||||||||||
| Re | Re | 3.3×10-10 | D | 2001[ | ||||||||||||
| Re | Re | 2.4×10-10 | D | 2001[ | ||||||||||||
| Os | Os | ≥4.0×10-13 | A | n=1—4, k1 for n=1 | 2005[ | |||||||||||
| Ir | Ir | 3.5×10-10 | A | n=1—3, k1 for n=1 | 2005[ | |||||||||||
| Pt | PtO+, Pt+ | 6.6×10-10 | D | 2001[ | ||||||||||||
| Pt | Pt | 2.3×10-10 | A | n=1—3, k1 for n=1 | 2005[ | |||||||||||
| Pt7 | Pt7 | ca.10-9 | D | n=1—2 | 2004[ | |||||||||||
| Pt4 | Pt4 | 10-11—10-9 | D | n=1—3, 4(slow) | 2007[ | |||||||||||
| Ptn | Ptn | B | n=3—6, m=1—2 | 1998[ | ||||||||||||
| AuO+ | AuCO+ | B | Theory | 2008[ | ||||||||||||
| AuO+ | AuCO+ | 7.93×10-12 | B | 2008[ | ||||||||||||
| Au2O+ | Au2CO+ | B | Theory | 2008[ | ||||||||||||
| Au2O+ | Au2CO+ | 2.42×10-12 | B | 2008[ | ||||||||||||
| Au3O+ | Au3CO+ | 4.98×10-12 | B | 2008[ | ||||||||||||
| Au3 | Au3(CO2 | C | 2011[ | |||||||||||||
| Aun | AunOx(CO | A | n=1—2, m=1—5, | 2004[ | ||||||||||||
| x=0—3, y=0—2 | ||||||||||||||||
| AuO- | Au- | ca.10-11 | A | Theory | 2004[ | |||||||||||
| Au | AuO- | A | Very slow, theory | 2004[ | ||||||||||||
| Au | Au- | ca.10-12 | A | Slow, loss of molecular O2, | 2004[ | |||||||||||
| theory | ||||||||||||||||
| Au2O- | A | ca.10-13 | A | Theory | 2006[ | |||||||||||
| Au2 | A | B | 2003[ | |||||||||||||
| Au2 | A | C | 2005[ | |||||||||||||
| Au2 | A | A | Theory | 2006[ | ||||||||||||
| Au2 | Au2 | ca.10-13 | A | Theory | 2006[ | |||||||||||
| Au2 | Au2 | ca.10-13 | A | Theory | 2006[ | |||||||||||
| Au6 | Au6O-, A | A | CO and O2 co-adsorption | 2002[ | ||||||||||||
| Aun | AuxOy(CO | A | n ≥ 4, Oxidation, replacement | 2006[ | ||||||||||||
| and association | ||||||||||||||||
| f-Block | Cen | Cen | 1.9×10-12 | A | n=2—6, k1 for Ce2 | 2010[ | ||||||||||
| theory(n =2—5) | ||||||||||||||||
| Cen | Cen | 8×10-11 | A | n=4—21, k1 for Ce4 | 2011[ | |||||||||||
| theory(n =1—6) | ||||||||||||||||
| EuO+ | Eu+ | ≥3.3×10-12 | A | 2005[ | ||||||||||||
| YbO+ | Yb+ | 1.2×10-11 | A | 2005[ | ||||||||||||
| Hetero- | AlV | AlV | 4.1×10-10 | D | Theory | 2011[ | ||||||||||
| nuclear | VCoO4 | VCoO3 | ca.10-11 | A | Theory | 2012[ | ||||||||||
| YAl | YAl | D | Theory | 2013[ | ||||||||||||
| AuTi3 | AuTi3O6CO+ | A | Molecular association | 2011[ | ||||||||||||
Table 1 Experimentally studied reactions between the oxide clusters MxOyq(q=0, ±1) and CO*
| Species | Cluster | Product | k1 | Reactor | Remark | Year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main | Al2 | Al2 | [1.4×10-11] | B | 2008[ | |||||||||||
| group | Al2 | Al2 | [ca.10-14] | B | 2008[ | |||||||||||
| Al2 | Al2 | [4.6×10-13] | B | 2008[ | ||||||||||||
| CaO+ | Ca+ | 2.5×10-10 | A | 2005[ | ||||||||||||
| CaO+ | Ca+ | (2.8±1.5)×10-10 | A | 2008[ | ||||||||||||
| GeO+ | Ge+ | 2.1×10-10 | A | 2005[ | ||||||||||||
| SrO+ | Sr+ | 1.0×10-10 | A | 2005[ | ||||||||||||
| BaO+ | Ba+ | 2.3×10-11 | A | 2005[ | ||||||||||||
| d-Block | Sc2 | Sc2 | 2.0×10-10 | A | Theory | 2012[ | ||||||||||
| Ti2 | Ti2 | [4.5×10-12] | B | Theory | 2011[ | |||||||||||
| (TiO2)nO- | (TiO2 | ca.10-11 | A | n=3—25, theory(n=3—8) | 2013[ | |||||||||||
| V4 | V4 | 4.4×10-11 | B | Theory | 2013[ | |||||||||||
| VO3 | VO2 | A | Theory | 2013[ | ||||||||||||
| Mn2O4 | Mn2O3 | 6.5×10-13 | A | Theory | 2013[ | |||||||||||
| Mn3O7 | Mn3O6 | 1.9×10-13 | A | Theory | 2013[ | |||||||||||
| FeO+ | Fe+ | 9×10-10 | D | 1981[ | ||||||||||||
| FeO+ | Fe+ | 1.8×10-10 | A | Theory | 2005[ | |||||||||||
| FeO(N2O | Fe+ | ca.10-10 | A | n=0—3 | 1995[ | |||||||||||
| Fe | FeO+, FeCO+ | [5.3×10-12] | B | Theory | 2007[ | |||||||||||
| Fe | FeO+, FeOCO+, Fe+ | B | 2007[ | |||||||||||||
| Fe | Fe | [1.4×10-12] | B | 2007[ | ||||||||||||
| Fe | FeO2CO+, Fe(CO | [5.5×10-12] | B | Loss of molecular O2, theory | 2007[ | |||||||||||
| Fe | Fe | B | 2007[ | |||||||||||||
| Fe2O+ | F | B | Theory | 2007[ | ||||||||||||
| Fe2 | Fe2O+, F | [8.8×10-13] | B | Theory | 2007[ | |||||||||||
| Fe2 | Fe2 | [ca.10-13] | B | 2007[ | ||||||||||||
| Fe2 | Fe2O2CO+, Fe2 | B | Theory | 2007[ | ||||||||||||
| Fe2 | Fe2 | B | Theory | 2007[ | ||||||||||||
| Fe2 | ||||||||||||||||
| FeO2 | FeO | 5.3×10-12 | A | Theory | 2008[ | |||||||||||
| FeO3 | FeO2 | 1.3×10-12 | A | Theory | 2008[ | |||||||||||
| Fe | FeO- | B | 2007[ | |||||||||||||
| Fe | FeO- | 1.9×10-12 | B | Theory | 2010[ | |||||||||||
| Fe | Fe | [ca.10-14] | B | 2007[ | ||||||||||||
| Fe | Fe | B | 2007[ | |||||||||||||
| Fe | Fe | [2.9×10-13] | B | Loss of molecular O2 and | 2007[ | |||||||||||
| CO oxidation | ||||||||||||||||
| Species | Cluster | Product | k1 | Reactor | Remark | Year | ||||||||||
| d-Block | Fe2 | Fe2 | B | 2007[ | ||||||||||||
| Fe2 | Fe2 | 1.8×10-12 | B | 2010[ | ||||||||||||
| Fe2 | Fe2 | B | 2007[ | |||||||||||||
| Fe2 | Fe2 | B | 2007[ | |||||||||||||
| Fe2 | Fe2 | [4.5×10-13] | B | Loss of molecular O2 and | 2007[ | |||||||||||
| CO oxidation | ||||||||||||||||
| CoO+ | Co+ | B | 2008[ | |||||||||||||
| Co | Co+, CoCO+ | [9.3×10-12] | B | Loss of molecular O2,theory | 2008[ | |||||||||||
| Co | CoO+, CoOCO+, Co+ | [7.2×10-12] | B | Loss of molecular O2 | 2008[ | |||||||||||
| Co | Co | B | Loss of molecular O2 | 2008[ | ||||||||||||
| Co2 | CoO2CO+ , Co(CO | [1.3×10-12] | B | Loss of molecular O2, | 2008[ | |||||||||||
| cluster fragmentation | ||||||||||||||||
| Co2 | Co2 | [4.7×10-12] | B | Loss of molecular O2 | 2008[ | |||||||||||
| CoO2CO+, Co(CO | ||||||||||||||||
| Co2 | Co2 | B | Loss of molecular O2 | 2008[ | ||||||||||||
| ConOm | Con | 10-13—10-12 | A | n=3—9, m=3—13, | 2010[ | |||||||||||
| theory(Co3O4) | ||||||||||||||||
| Co | CoO- | B | 2008[ | |||||||||||||
| Co | CoO- | 0.3×10-12 | B | 2010[ | ||||||||||||
| Co | Co | B | 2008[ | |||||||||||||
| Co2 | Co2 | B | 2008[ | |||||||||||||
| Co2 | Co2 | 1.6×10-12 | B | 2010[ | ||||||||||||
| Co2 | Co2 | [4.8×10-14] | B | 2008[ | ||||||||||||
| Co3 | Co3 | [1.4×10-13] | B | 2008[ | ||||||||||||
| Co3 | Co3 | [1.0×10-13] | B | 2008[ | ||||||||||||
| Co2 | ||||||||||||||||
| Ni6 | Ni6 | 2.2×10-13 | A | 2013[ | ||||||||||||
| Ni8 | Ni8 | 1.2×10-13 | A | 2013[ | ||||||||||||
| Ni | NiO- | (9.2±0.5)×10-14 | B | 2009[ | ||||||||||||
| Ni | NiO- | 0.4×10-12 | B | 2010[ | ||||||||||||
| Ni | Ni | B | 2009[ | |||||||||||||
| Ni2 | Ni2 | (1.9±0.1)×10-13 | B | 2009[ | ||||||||||||
| Ni2 | Ni2 | 1.0×10-12 | B | 2010[ | ||||||||||||
| Ni3 | Ni3 | (9.6±0.8)×10-14 | B | 2009[ | ||||||||||||
| Ni4 | Ni4 | (3.0±0.2)×10-13 | B | 2009[ | ||||||||||||
| Cu | CuO- | 0.7×10-12 | B | 2010[ | ||||||||||||
| Cu2 | Cu2 | 1.5×10-12 | B | 2010[ | ||||||||||||
| Cu5 | Cu5O- | B | 2013[ | |||||||||||||
| Cu9 | Cu9O- | B | 2013[ | |||||||||||||
| Zr2 | Zr2 | 1.8×10-10 | A | 2010[ | ||||||||||||
| (ZrO2 | Zrn | 2.4×10-12, 1.6×10-12, | B | n=1—5, k1 for | 2008[ | |||||||||||
| 1.2×10-12, 6.3×10-13 | n=2—5,theory | |||||||||||||||
| (ZrO2)nO- | (ZrO2 | 2.1×10-12, 2.2×10-13, | B | n=1—4, theory | 2009[ | |||||||||||
| 3.4×10-13, 1.8×10-13 | ||||||||||||||||
| (ZrO2)nO- | (ZrO2)nC | ca.10-11 | A | n=3—25, Oxidative adsorption | 2013[ | |||||||||||
| Mo | Mo(CO | A | n=1—3 | 2006[ | ||||||||||||
| C6 | 2007[ | |||||||||||||||
| RhnOm | Rhn | ca.10-10 | A | n=10—28, m=1—5, | 2012[ | |||||||||||
| k1 for m=4, 5 | ||||||||||||||||
| Pd | PdO+, OPdCO+, PdCO+ | B | Loss of molecular O2 and | 2011[ | ||||||||||||
| CO oxidation, theory | ||||||||||||||||
| Pd | Pd | B | Loss of molecular O2 and | 2011[ | ||||||||||||
| OPdCO+ | CO oxidation, theory | |||||||||||||||
| PdnO+ | Pdn(CO | C | n=2—7 | 2012[ | ||||||||||||
| Pdn | Pdn(CO | C | n=4—6 | 2012[ | ||||||||||||
| Species | Cluster | Product | k1 | Reactor | Remark | Year | ||||||||||
| d-Block | Pd6 | Pd6 | C | Theory | 2012[ | |||||||||||
| Pd6 | Pd6O5CO+ | C | Eliminate CO2 and form | 2012[ | ||||||||||||
| Pd6 | ||||||||||||||||
| Ag3 | Ag3On(OCO | C | n=1—3, with N2O | 2011[ | ||||||||||||
| Agn | A | C | n=7, 9, 11 | 2004[ | ||||||||||||
| Ag5 | Ag4 | C | Cluster fragmentation | 2005[ | ||||||||||||
| Ag3C | ||||||||||||||||
| AgnNx | Ag3NO-, AgN2 | C | n=2—5, y>x, cluster | 2007[ | ||||||||||||
| fragmentation | ||||||||||||||||
| La2 | La2O3+e- | 1.9×10-10 | A | Electron detachment, theory | 2012[ | |||||||||||
| Wn | Wn | 3.0×10-12, 3.6×10-13, | B | n=1—3 | 2008[ | |||||||||||
| 5.9×10-13 | ||||||||||||||||
| Re | Re | 3.3×10-10 | D | 2001[ | ||||||||||||
| Re | Re | 2.4×10-10 | D | 2001[ | ||||||||||||
| Os | Os | ≥4.0×10-13 | A | n=1—4, k1 for n=1 | 2005[ | |||||||||||
| Ir | Ir | 3.5×10-10 | A | n=1—3, k1 for n=1 | 2005[ | |||||||||||
| Pt | PtO+, Pt+ | 6.6×10-10 | D | 2001[ | ||||||||||||
| Pt | Pt | 2.3×10-10 | A | n=1—3, k1 for n=1 | 2005[ | |||||||||||
| Pt7 | Pt7 | ca.10-9 | D | n=1—2 | 2004[ | |||||||||||
| Pt4 | Pt4 | 10-11—10-9 | D | n=1—3, 4(slow) | 2007[ | |||||||||||
| Ptn | Ptn | B | n=3—6, m=1—2 | 1998[ | ||||||||||||
| AuO+ | AuCO+ | B | Theory | 2008[ | ||||||||||||
| AuO+ | AuCO+ | 7.93×10-12 | B | 2008[ | ||||||||||||
| Au2O+ | Au2CO+ | B | Theory | 2008[ | ||||||||||||
| Au2O+ | Au2CO+ | 2.42×10-12 | B | 2008[ | ||||||||||||
| Au3O+ | Au3CO+ | 4.98×10-12 | B | 2008[ | ||||||||||||
| Au3 | Au3(CO2 | C | 2011[ | |||||||||||||
| Aun | AunOx(CO | A | n=1—2, m=1—5, | 2004[ | ||||||||||||
| x=0—3, y=0—2 | ||||||||||||||||
| AuO- | Au- | ca.10-11 | A | Theory | 2004[ | |||||||||||
| Au | AuO- | A | Very slow, theory | 2004[ | ||||||||||||
| Au | Au- | ca.10-12 | A | Slow, loss of molecular O2, | 2004[ | |||||||||||
| theory | ||||||||||||||||
| Au2O- | A | ca.10-13 | A | Theory | 2006[ | |||||||||||
| Au2 | A | B | 2003[ | |||||||||||||
| Au2 | A | C | 2005[ | |||||||||||||
| Au2 | A | A | Theory | 2006[ | ||||||||||||
| Au2 | Au2 | ca.10-13 | A | Theory | 2006[ | |||||||||||
| Au2 | Au2 | ca.10-13 | A | Theory | 2006[ | |||||||||||
| Au6 | Au6O-, A | A | CO and O2 co-adsorption | 2002[ | ||||||||||||
| Aun | AuxOy(CO | A | n ≥ 4, Oxidation, replacement | 2006[ | ||||||||||||
| and association | ||||||||||||||||
| f-Block | Cen | Cen | 1.9×10-12 | A | n=2—6, k1 for Ce2 | 2010[ | ||||||||||
| theory(n =2—5) | ||||||||||||||||
| Cen | Cen | 8×10-11 | A | n=4—21, k1 for Ce4 | 2011[ | |||||||||||
| theory(n =1—6) | ||||||||||||||||
| EuO+ | Eu+ | ≥3.3×10-12 | A | 2005[ | ||||||||||||
| YbO+ | Yb+ | 1.2×10-11 | A | 2005[ | ||||||||||||
| Hetero- | AlV | AlV | 4.1×10-10 | D | Theory | 2011[ | ||||||||||
| nuclear | VCoO4 | VCoO3 | ca.10-11 | A | Theory | 2012[ | ||||||||||
| YAl | YAl | D | Theory | 2013[ | ||||||||||||
| AuTi3 | AuTi3O6CO+ | A | Molecular association | 2011[ | ||||||||||||
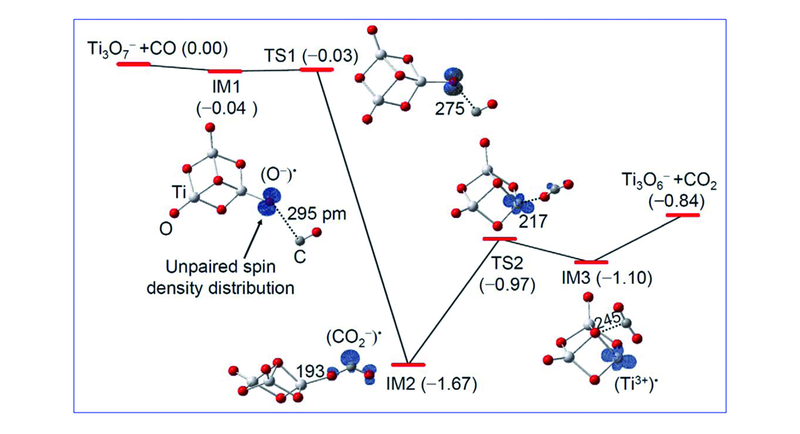
Fig.4 DFT calculated reaction pathway for Ti3O7-+CO→Ti3O6-+CO2[33]The zero-point vibration corrected energies(DH0K/eV) of the reaction intermediates(IM1—IM3), transition states(TS1 and TS2), and products(Ti3O6-+CO2) with respect to the separated reactants(Ti3O7-+CO) are given. Bond lengths in pm are shown.
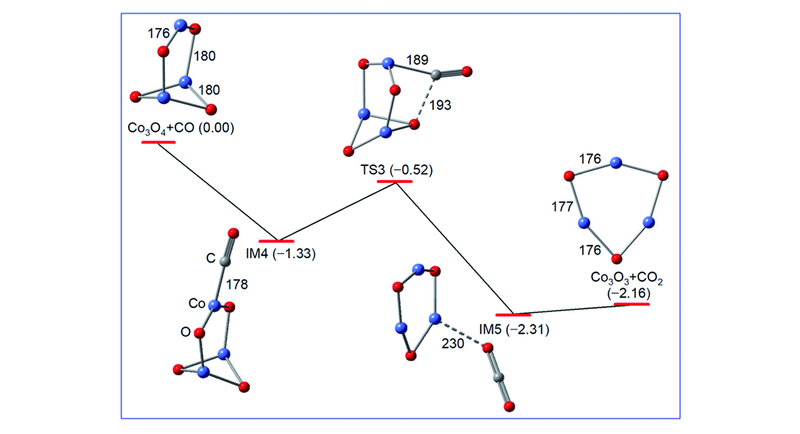
Fig.5 DFT calculated reaction pathway for Co3O4+CO→Co3O3+CO2[65]The zero-point vibration corrected energies(DH0 K/eV) of the reaction intermediates(IM4 and IM5), transition state(TS3), and products(Co3O3+CO2) with respect to the separated reactants(Co3O4+CO) are given. Bond lengths in pm are shown.
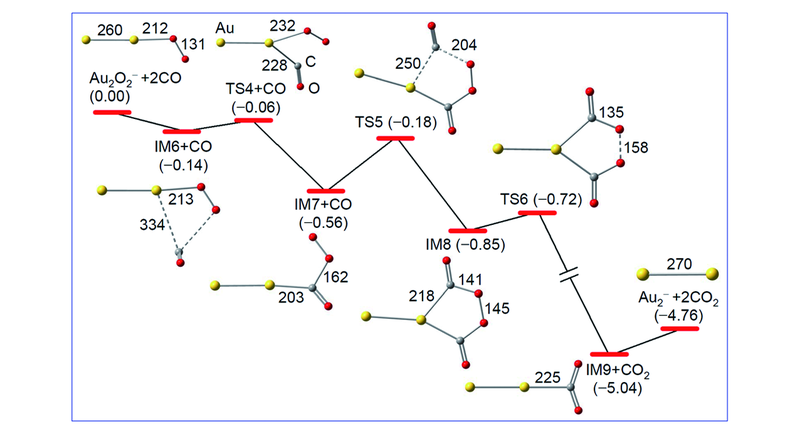
Fig.6 DFT calculated reaction pathway for Au2O2-+2CO→Au2-+2CO2[84] The zero-point vibration corrected energies(DH0 K/eV) of the reaction intermediates(IM6—IM9), transition states(TS4—TS6), and products(Au2-+2CO2) with respect to the separated reactants(Au2O2-+2CO) are given. Bond lengths in pm are shown.
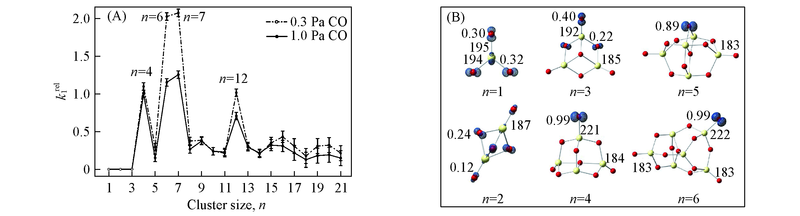
Fig.7 Experimentally determined relative pseudo-first order rate constants(k1) for(CeO2)nO-+CO→ (CeO2)n-+CO2(n=4—21)(A) and DFT calculated structures and profiles of unpaired spin density distributions for(CeO2)nO-(n=1—6) clusters(B)[28] The experiment identified no evidence of reaction between(CeO2)nO-(n=1—3) and CO, so the rate constants are simply set to be zero. The absolute values of k1(Ce4O9-+CO) under the conditions of 0.3 and 1.0 Pa CO in the fast flow reactor are 8.6×10-11 and 7.8×10-11 cm3·molecule-1·s-1, respectively. Abundance of un-reactive cluster isomers can lead to the result that the rate constants under the condition of 0.3 Pa are generally larger than those under 1.0 Pa. The spin density values over oxygen atoms in μB and some Ce—O bond lengths in pm are shown.
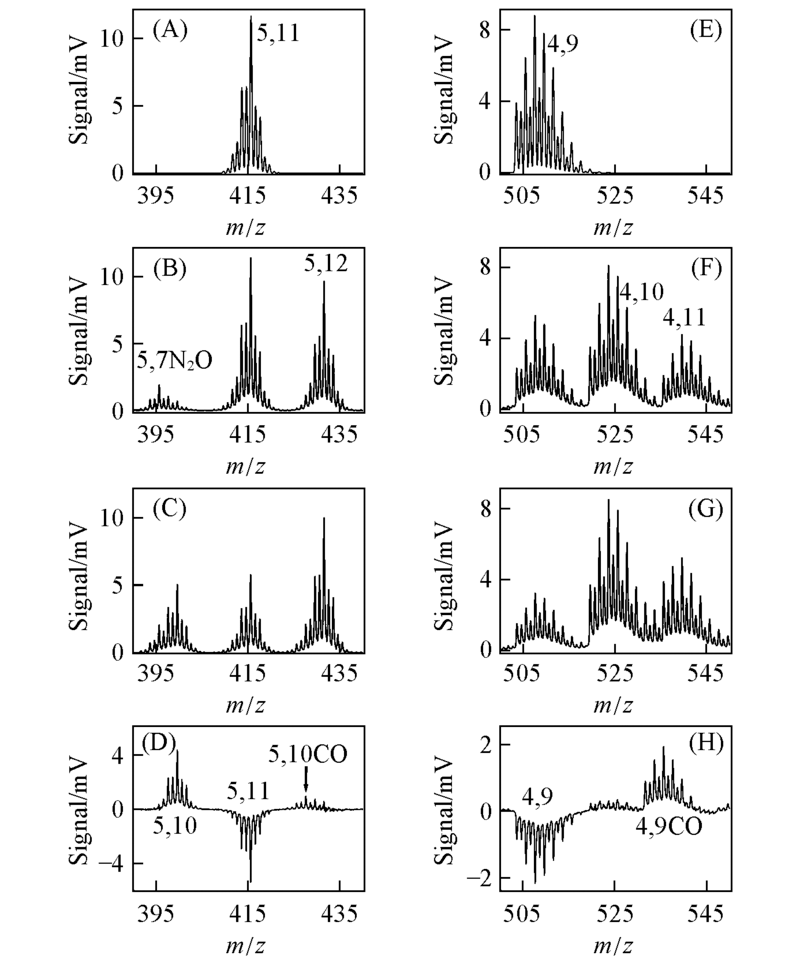
Fig.8 TOF mass spectra for reactions of Ti5Oy-(A—D) and Zr4Oy-(E—H) with CO in the fast flow reactor[33]The numbers x, y denote MxO-y in which M=Ti or Zr. The simulated Ti5O11- and Zr4O9- isotopomers are given in (A) and (E). The reference spectra with N2 in the reactor are given in (B) and(F). The spectra with 1.4 and 0.6 Pa CO in the reactor are given in (C) and (G), respectively. The difference spectra[(D)=(C)-(B) and (H)=(G)-(F)] are also shown.
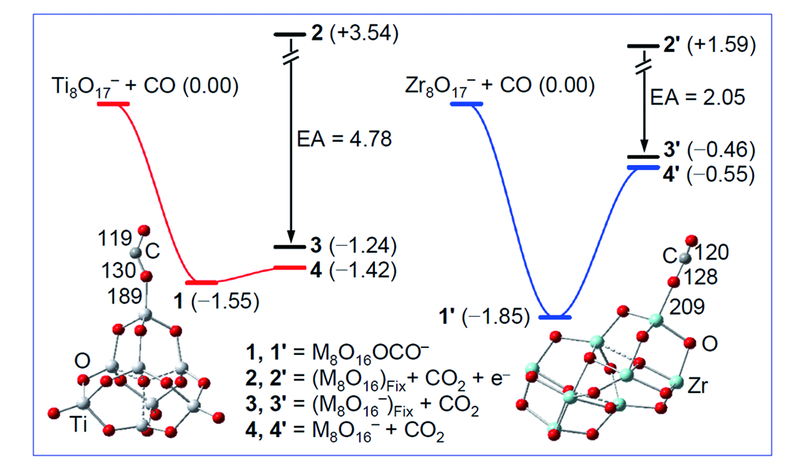
Fig.9 Simplified potential energy profiles for oxidative CO absorption and CO2 desorption in the reactions of CO with M8O17-(M=Ti, Zr) calculated by the DFT[33]The zero-point vibration corrected energies(DH0 K/eV) of the reaction intermediates(1 and 1') and products(4 and 4') with respect to the separated reactants are given. The energies of 2/3 and 2'/3' are calculated with the geometric parameters of M8O16 being fixed at the values of the M8O16 moiety in 1 and 1', respectively(EA denotes electron affinity in eV). Bond lengths in pm are shown.
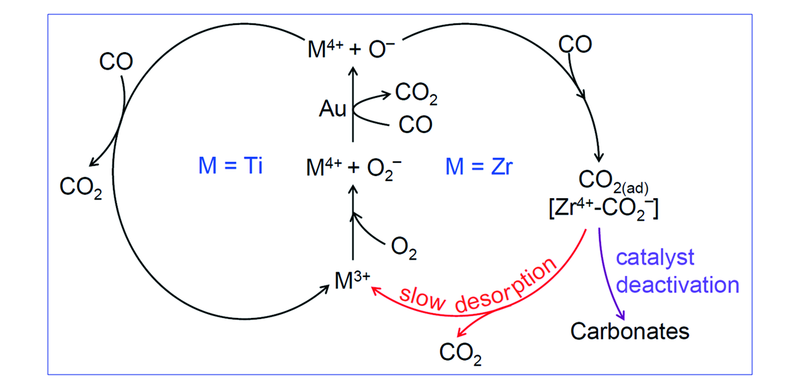
Fig.10 Proposed catalytic cycles involving O2- and O- radicals for low-temperature CO oxidation over titania and zirconia supported gold[33] Gold may participate in the O—O bond activation because it was identified that O2- is unable to react with CO in the absence of gold at low-temperature[103,136,137].
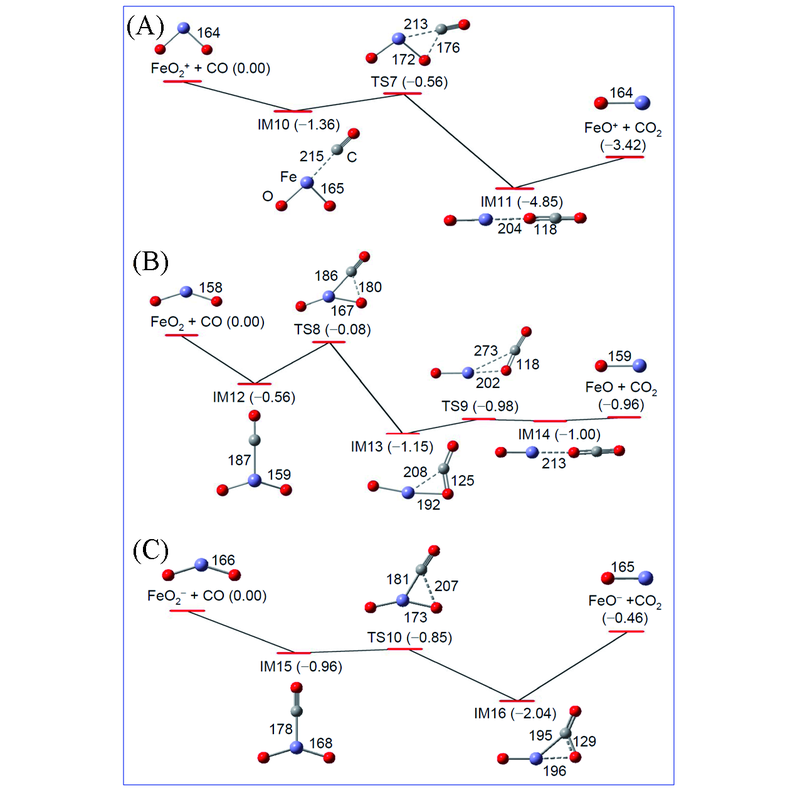
Fig.11 B3LYP/6-311+G* calculated reaction pathways for FeO2q+CO→FeOq+CO2 in which q=+1(A), 0(B) and -1(C)The zero-point vibration corrected energies(ΔH0 K/eV) of the reaction intermediates(IM10—IM16), transition states(TS7—TS10), and products(FeOq+CO2) with respect to the separated reactants(FeO2q+CO) are given. Bond lengths in pm are shown. The structures and mechanisms of FeO2+, FeO2, and FeO2- reaction systems are adapted from refs.[61], [43], and [63], respectively.
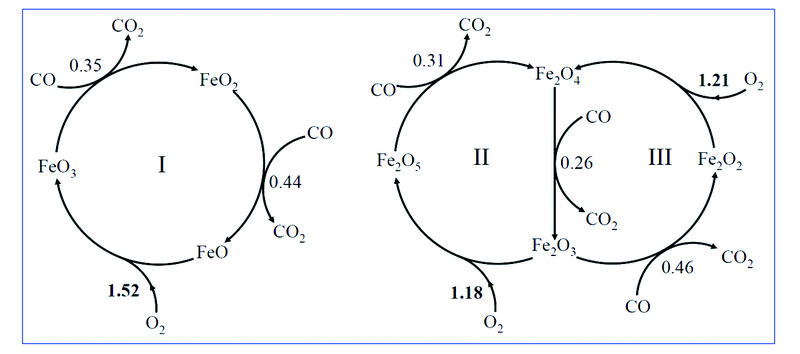
Fig.12 Three model catalytic cycles Ⅰ—Ⅲ for CO oxidation by O2 over FeO1—3 and Fe2O2—5 clusters[43] The free energy barrier(eV) of the rate-limiting step in each elementary reaction is given.
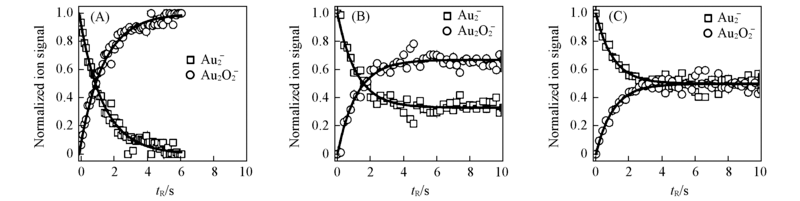
Fig.13 Concentration of all product ions observed during the reactions of mass selected Au2- with CO and O2 inside the octopole ion trap as a function of the reaction time(tR) for different CO partial pressures[87] (A) p(CO)=0 and p(O2)=0.12 Pa, (B) p(CO)=p(O2)=0.12 Pa, (C) p(CO)=2p(O2)=0.24 Pa. The reaction temperature and bath gas(He) pressure are 300 K and 1.2 Pa, respectively.
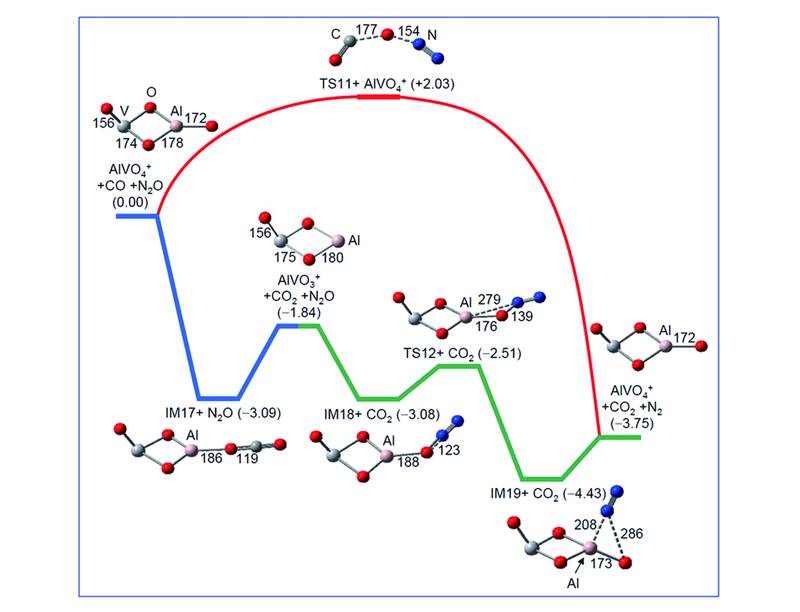
Fig.14 DFT calculated potential energy profile for direct and catalytic oxidations of CO by N2O[91]Catalysts=AlVO3+ and AlVO4+. The energies of the intermediates(IM17—IM19), transition states(TS11 and TS12), and products with respect to the separated reactants are expressed in eV. Bond lengths in pm are shown.
| Catalyst | Oxidant | Reactor | Remark | Year |
|---|---|---|---|---|
| Al2 | N2O | B | 2008[ | |
| Al2 | N2O | B | 2008[ | |
| Ca+, CaO+ | N2O | A | 2005[ | |
| Ti2 | N2O | B | Theory | 2011[ |
| VO2, VO3 | N2O | A | Theory | 2013[ |
| Mn2O4, Mn2O5 | NO2 | A | Theory | 2013[ |
| Fe+, FeO+ | N2O | D | 1981[ | |
| Fe+, FeO+ | N2O | A | Theory | 2005[ |
| Ge+, GeO+ | N2O | A | 2005[ | |
| Sr+, SrO+ | N2O | A | 2005[ | |
| Zrn | N2O | B | n=1—4, theory | 2008[ |
| RhnOm | N2O | A | n=10—28, m=0—5 | 2011[ |
| Pd6 | O2 | C | Theory | 2012[ |
| A | O2 | C | n=7, 9, 11 | 2004[ |
| Ba+, BaO+ | N2O | A | 2005[ | |
| Eu+, EuO+ | N2O | A | 2005[ | |
| Yb+, YbO+ | N2O | A | 2005[ | |
| Os | N2O | A | 2005[ | |
| Ir | N2O | A | 2005[ | |
| Pt | N2O | A | 2005[ | |
| Pt+, Pt | N2O | D | 2001[ | |
| Pt7 | N2O | D | 2004[ | |
| Pt4 | N2O | D | 2007[ | |
| Ptn | O2/N2O | B | n=3—6, m=0—2 | 1998[ |
| A | O2 | C | 2003[ | |
| Au3(CO)2—5 | O2 | A | Theory | 2011[ |
| A | O2 | A | 2002[ | |
| AlV | N2O | D | Theory | 2011[ |
| YAl | N2O | D | Theory | 2013[ |
Table 2 Experimentally studied catalytic oxidations of CO on atomic cluster catalysts
| Catalyst | Oxidant | Reactor | Remark | Year |
|---|---|---|---|---|
| Al2 | N2O | B | 2008[ | |
| Al2 | N2O | B | 2008[ | |
| Ca+, CaO+ | N2O | A | 2005[ | |
| Ti2 | N2O | B | Theory | 2011[ |
| VO2, VO3 | N2O | A | Theory | 2013[ |
| Mn2O4, Mn2O5 | NO2 | A | Theory | 2013[ |
| Fe+, FeO+ | N2O | D | 1981[ | |
| Fe+, FeO+ | N2O | A | Theory | 2005[ |
| Ge+, GeO+ | N2O | A | 2005[ | |
| Sr+, SrO+ | N2O | A | 2005[ | |
| Zrn | N2O | B | n=1—4, theory | 2008[ |
| RhnOm | N2O | A | n=10—28, m=0—5 | 2011[ |
| Pd6 | O2 | C | Theory | 2012[ |
| A | O2 | C | n=7, 9, 11 | 2004[ |
| Ba+, BaO+ | N2O | A | 2005[ | |
| Eu+, EuO+ | N2O | A | 2005[ | |
| Yb+, YbO+ | N2O | A | 2005[ | |
| Os | N2O | A | 2005[ | |
| Ir | N2O | A | 2005[ | |
| Pt | N2O | A | 2005[ | |
| Pt+, Pt | N2O | D | 2001[ | |
| Pt7 | N2O | D | 2004[ | |
| Pt4 | N2O | D | 2007[ | |
| Ptn | O2/N2O | B | n=3—6, m=0—2 | 1998[ |
| A | O2 | C | 2003[ | |
| Au3(CO)2—5 | O2 | A | Theory | 2011[ |
| A | O2 | A | 2002[ | |
| AlV | N2O | D | Theory | 2011[ |
| YAl | N2O | D | Theory | 2013[ |
| [1] | Lide D.R., CRC Handbook of Chemistry and Physics, 87th ed.: Physical Constants of Inorganic Compounds, CRC Taylor & Francis Group, Boca Raton, 2006, 4—56 |
| [2] | Jones R. A., Strickland J. A., Stunkard J. A., Siegel J., Toxicol. Appl. Pharmacol., 1971, 19, 46 |
| [3] | Royer S., Duprez D., Chem. Cat. Chem., 2011, 3, 24—65 |
| [4] | Freund H. J., Meijer G., Scheffler M., Schlogl R., Wolf M., Angew. Chem. Int. Ed., 2011, 50, 10064—10094 |
| [5] | Cotton F.A., Wilkinson G., Advanced Inorganic Chemistry, A Comprehensive Text, 4th ed., John Wiley & Sons, New York, 1980, 1049—1079 |
| [6] | Zhou M.F., Andrews L., Bauschlicher C. W. Jr., Chem. Rev., 2001, 101, 1931—1961 |
| [7] | Roithova J. , Schröder D., Chem. Rev., 2010, 110, 1170—1211 |
| [8] | Zhai H. J., Wang L. S., Chem. Phys. Lett., 2010, 500, 185—195 |
| [9] | Castleman A. W. Jr., Catal. Lett., 2011, 141, 1243—1253 |
| [10] | Yin S., Bernstein E. R., Int. J. Mass Spectrom., 2012, 321/322, 49—65 |
| [11] | Lang S. M., Bernhardt T. M., Phys. Chem. Chem. Phys., 2012, 14, 9255—9269 |
| [12] | Asmis K. R., Phys. Chem. Chem. Phys., 2012, 14, 9270—9281 |
| [13] | Ding X. L., Wu X. N., Zhao Y. X., He S. G., Acc. Chem. Res., 2012, 45, 382—390 |
| [14] | Schlangen M., Schwarz H., Catal. Lett., 2012, 142, 1265—1278 |
| [15] | Qiao B. T., Wang A. Q., Yang X. F., Allard L. F., Jiang Z., Cui Y. T., Liu J. Y., Li J., Zhang T., Nat. Chem., 2011, 3, 634—641 |
| [16] | Yoon B., Häkkinen H., Landman U., Worz A. S., Antonietti J. M., Abbet S., Judai K., Heiz U., Science,2005, 307, 403—407 |
| [17] | Valden M., Lai X., Goodman D. W., Science,1998, 281, 1647—1650 |
| [18] | Min B. K., Friend C. M., Chem. Rev., 2007, 107, 2709—2724 |
| [19] | Fierro-Gonzalez J.C., Gates B. C., Chem. Soc. Rev., 2008, 37, 2127—2134 |
| [20] | O'Hair R. A. J., Khairallah G. N., J. Cluster Sci., 2004, 15, 331—363 |
| [21] | Böhme D. K., Schwarz H., Angew. Chem. Int. Ed., 2005, 44, 2336—2354 |
| [22] | Johnson G. E., Mitrić R., BonaČić-Koutecky V., Castleman A. W. Jr., Chem. Phys. Lett., 2009, 475, 1—9 |
| [23] | Bernhardt T. M., Int. J. Mass Spectrom., 2005, 243, 1—29 |
| [24] | Zhao Y.X., Wu X. N., Ma J. B., He S. G., Ding X. L., Phys. Chem. Chem. Phys., 2011, 13, 1925—1938 |
| [25] | Lang S. M., Fleischer I., Bernhardt T. M., Barnett R. N., Landman U., J. Am. Chem. Soc., 2012, 134, 20654—20659 |
| [26] | Lang S. M., Schnabel T., Bernhardt T. M., Phys. Chem. Chem. Phys., 2012, 14, 9364—9370 |
| [27] | Wu X. N., Zhao Y. X., Xue W., Wang Z. C., He S. G., Ding X. L., Phys. Chem. Chem. Phys., 2010, 12, 3984—3997 |
| [28] | Wu X. N., Ding X. L., Bai S. M., Xu B., He S. G., Shi Q., J. Phys. Chem. C,2011, 115, 13329—13337 |
| [29] | Wang Z. C., Yin S., Bernstein E. R., J. Phys. Chem. Lett., 2012, 3, 2415—2419 |
| [30] | Xu B., Zhao Y. X., Ding X. L., He S. G., Int. J. Mass Spectrom., 2013, 334, 1—7 |
| [31] | Ma J. B., Wang Z. C., Schlangen M., He S. G., Schwarz H., Angew. Chem. Int. Ed., 2013, 52, 1226—1230 |
| [32] | Yuan Z., Zhao Y. X., Li X. N., He S. G., Int. J. Mass Spectrom., 2013, 354/355, 105—112 |
| [33] | Ma J. B., Xu B., Meng J. H., Wu X. N., Ding X. L., Li X. N., He S. G., J. Am. Chem. Soc., 2013, 135, 2991—2998 |
| [34] | Wang Z. C., Yin S., Bernstein E. R., Phys.Chem. Chem. Phys., 2013, 15, 10429—10434 |
| [35] | Yamada A., Miyajima K., Mafuné F., Phys. Chem. Chem. Phys., 2012, 14, 4188—4195 |
| [36] | Hirabayashi S., Kawazoe Y., Ichihashi M., Eur. Phys. J. D,2013, 67, 1—6 |
| [37] | Sakuma K., Miyajima K., Mafuné F., J. Phys. Chem. A,2013, 117, 3260—3265 |
| [38] | Yin S., Wang Z. C., Bernstein E. R., J. Chem Phys., 2013, 139, 084307(9) |
| [39] | Dietz T. G., Duncan M. A., Powers D. E., Smalley R. E., J. Chem. Phys., 1981, 74, 6511—6512 |
| [40] | Himeno H., Miyajima K., Yasuike T., Mafuné F., J. Phys. Chem. A,2011, 115, 11479—11485 |
| [41] | Blagojevic V., Orlova G., Bohme D. K., J. Am. Chem. Soc., 2005, 127, 3545—3555 |
| [42] | He S. G., Xie Y., Dong F., Heinbuch S., Jakubikova E., Rocca J. J., Bernstein E. R., J. Phys. Chem. A,2008, 112, 11067—11077 |
| [43] | Xue W., Wang Z. C., He S. G., Xie Y., Bernstein E. R., J. Am. Chem. Soc., 2008, 130, 15879—15888 |
| [44] | Geusic M.E., Morse M. D., O’brien S. C., Smalley R. E., Rev. Sci. Instrum., 1985, 56, 2123—2130 |
| [45] | Wu X. N., Ma J. B., Xu B., Zhao Y. X., Ding X. L., He S. G., J. Phys. Chem. A,2011, 115, 5238—5246 |
| [46] | Bell R. C., Zemski K. A., Justes D. R., Castleman A. W. Jr., J. Chem. Phys., 2001, 114, 798—811 |
| [47] | Socaciu L. D., Hagen J., Heiz U., Bernhardt T. M., Leisner T., Wöste L., Chem. Phys. Lett., 2001, 340, 282—288 |
| [48] | Eller K., Schwarz H., Int. J. Mass Spectrom. Ion Processes,1989, 93, 243—257 |
| [49] | Berg C., Schindler T., Niednerschatteburg G., Bondybey V. E., J. Chem. Phys., 1995, 102, 4870—4884 |
| [50] | Wallace W. T., Whetten R. L., J. Am. Chem. Soc., 2002, 124, 7499—7505 |
| [51] | Bondybey V. E., Beyer M. K., J. Phys. Chem. A,2001, 105, 951—960 |
| [52] | Cramer C. J., Truhlar D. G., Phys. Chem. Chem. Phys., 2009, 11, 10757—10816 |
| [53] | Burke K., J. Chem. Phys., 2012, 136, 150901-1—150901-9 |
| [54] | Steinfeld J.I., Francisco J. S., Hase W. L., Chemical Kinetics and Dynamics, Prentice Hall, Upper Saddle River, NJ, 1999, 340—345 |
| [55] | Beyer T., Swinehart D. R., Commun. ACM,1973, 16, 379—379 |
| [56] | Baxter R. J., Hu P., J. Chem. Phys., 2002, 116, 4379—4381 |
| [57] | Johnson G. E., Tyo E. C., Castleman. A. W. Jr., J. Phys. Chem. A,2008, 112, 4732—4735 |
| [58] | Broadley S., Vondrak T., Wright T. G., Plane J. M. C., Phys. Chem. Chem. Phys., 2008, 10, 5287—5298 |
| [59] | Tyo E. C., Nöβler M., Mitrić R., BonaČić-Koutecky V., Castleman A. W. Jr., Phys. Chem. Chem. Phys., 2011, 13, 4243—4249 |
| [60] | Kappes M. M., Staley R. H., J. Am. Chem. Soc., 1981, 103, 1286—1287 |
| [61] | Reilly N. M., Reveles J. U., Johnson G. E., Del Campo J. M., Khanna S. N., Köster A. M., Castleman. A. W. Jr., J. Phys. Chem. C,2007, 111, 19086—19097 |
| [62] | Reilly N. M., Reveles J. U., Johnson G. E., Khanna S. N., Castleman A. W. Jr., Chem. Phys. Lett., 2007, 435, 295—300 |
| [63] | Reilly N. M., Reveles J. U., Johnson G. E., Khanna S. N., Castleman A. W. Jr., J. Phys. Chem. A,2007, 111, 4158—4166 |
| [64] | Reveles J. U., Johnson G. E., Khanna S. N., Castleman. A. W. Jr., J. Phys. Chem. C,2010, 114, 5438—5446 |
| [65] | Xie Y., Dong F., Heinbuch S., Rocca J. J., Bernstein E. R., Phys. Chem. Chem. Phys., 2010, 12, 947—959 |
| [66] | Johnson G. E., Reveles J. U., Reilly N. M., Tyo E. C., Khanna S. N., Castleman A. W. Jr., J. Phys. Chem. A,2008, 112, 11330—11340 |
| [67] | Johnson G. E., Reilly N. M., Castleman A. W. Jr., Int. J. Mass Spectrom., 2009, 280, 93—100 |
| [68] | Johnson G. E., Mitrić R., Tyo E. C., BonaČić-Koutecky V., Castleman A. W. Jr., J. Am. Chem. Soc., 2008, 130, 13912—13920 |
| [69] | Johnson G. E., Mitrić R., Nössler M., Tyo E. C., BonaČić-Koutecky V., Castleman A. W. Jr., J. Am. Chem. Soc,2009, 131, 5460—5470 |
| [70] | Wyrwas R. B., Jarrold C. C., J. Am. Chem. Soc., 2006, 128, 13688—13689 |
| [71] | Wyrwas R. B., Robertson E. M., Jarrold C. C., J. Chem. Phys., 2007, 126, 214309-1—214309-8 |
| [72] | Reber A. C., Khanna S. N., Tyo E. C., Harmon C. L., Castleman A. W. Jr., J. Chem. Phys., 2011, 135, 234303-1—234303-7 |
| [73] | Popolan D. M., Bernhardt T. M., J. Chem. Phys., 2011, 134, 091102-1—091102-3 |
| [74] | Socaciu L.D., Hagen J., Le Roux J., Popolan D., Bernhardt T. M., Wöste L., Vajda S., J. Chem. Phys., 2004, 120, 2078—2081 |
| [75] | Bernhardt T. M., Socaciu-Siebert L. D., Hagen J., Wöste L., Appl. Catal. A-Gen., 2005, 291, 170—178 |
| [76] | Hagen J., Socaciu-Siebert L. D., Le Roux J., Popolan D., Vajda S., Bernhardt T. M., Wöste L., Int. J. Mass Spectrom., 2007, 261, 152—158 |
| [77] | Johnson G. E., Tyo E. C., Castleman A. W. Jr., Proc. Natl. Acad. Sci. USA,2008, 105, 18108—18113 |
| [78] | Beyer M. K., Berg C. B., Bondybey V. E., Phys. Chem. Chem. Phys., 2001, 3, 1840—1847 |
| [79] | Brönstrup M., Schröder D., Kretzschmar I., Schwarz H., Harvey J. N., J. Am. Chem. Soc., 2001, 123, 142—147 |
| [80] | Balaj O. P., Balteanu I., Rossteuscher T. T. J., Beyer M. K., Bondybey V. E., Angew. Chem. Int. Ed., 2004, 43, 6519—6522 |
| [81] | Siu C. K., Reitmeier S. J., Balteanu I., Bondybey V. E., Beyer M. K., Eur. Phys. J. D,2007, 43, 189—192 |
| [82] | Shi Y., Ervin K. M., J. Chem. Phys., 1998, 108, 1757—1760 |
| [83] | Kimble M. L., Castleman A. W. Jr., Mitrić R., Bürgel C., BonaČić-Koutecky V., J. Am. Chem. Soc., 2004, 126, 2526—2535 |
| [84] | Kimble M. L., Moore N. A., Johnson G. E., Castleman A. W. Jr., Burgel C., Mitrić R., BonaČić-Koutecky V., J. Chem. Phys., 2006, 125, 204311-1—204311-14 |
| [85] | Kimble M. L., Castleman A. W. Jr., Bürgel C., BonaČić-Koutecky V., Int. J. Mass Spectrom., 2006, 254, 163—167 |
| [86] | Kimble M. L., Moore N. A., Castleman A. W. Jr., Bürgel C., Mitrić R., BonaČić-Koutecky V., Eur. Phys. J. D,2007, 43, 205—208 |
| [87] | Socaciu L. D., Hagen J., Bernhardt T. M., Wöste L., Heiz U., Hakkinen H., Landman U., J. Am. Chem. Soc., 2003, 125, 10437—10445 |
| [88] | Kimble M. L., Castleman A. W. Jr., Int. J. Mass Spectrom., 2004, 233, 99—101 |
| [89] | Bürgel C., Reilly N. M., Johnson G. E., MitrićR., Kimble M. L., Castleman A. W. Jr., BonaČić-Koutecky V., J. Am. Chem. Soc., 2008, 130, 1694—1698 |
| [90] | Johnson G. E., Reilly N. M., Tyo E. C., Castleman A. W. Jr., J. Phys. Chem. C,2008, 112, 9730—9736 |
| [91] | Wang Z. C., Dietl N., Kretschmer R., Weiske T., Schlangen M., Schwarz H., Angew. Chem. Int. Ed., 2011, 50, 12351—12354 |
| [92] | Baranov V., Javahery G., Hopkinson A. C., Bohme D. K., J. Am. Chem. Soc., 1995, 117, 12801—12809 |
| [93] | Che M., Tench A. J., Adv. Catal., 1982, 31, 77—133 |
| [94] | Lee J., Grabowski J. J., Chem. Rev., 1992, 92, 1611—1647 |
| [95] | Tang D. Y., Hu J. P., Zhang Y. Q., Hu C. W., Acta Chim. Sin., 2009, 67, 1859—1864 |
| [96] | Tang D. Y., Hu J. P., Zhang Y. Q., Hu C. W., Acta Chim. Sin., 2010, 68, 1379—1384 |
| [97] | Grigorieva A. V., Goodilin E. A., Dubova K. L., Anufrieva T. A., Derlyukova L. E., Vyacheslavov A. S., Tretyakov Y. D., Solid State Sci., 2010, 12, 1024—1028 |
| [98] | Liu X. W., Zhou K. B., Wang L., Wang B. Y., Li Y. D., J. Am. Chem. Soc., 2009, 131, 3140—3141 |
| [99] | Gonzalez-Rovira L., Sanchez-Amaya J. M., Lopez-Haro M., del Rio E., Hungria A. B., Midgley P., Calvino J. J., Bernal S., Botana F. J., Nano Lett., 2009, 9, 1395—1400 |
| [100] | Haruta M., Kobayashi T., Sano H., Yamada N., Chem. Lett., 1987, 2, 405—408 |
| [101] | Konova P., Naydenov A., Venkov C., Mehandjiev D., Andreeva D., Tabakova T., J. Mol. Catal. A: Chem., 2004, 213, 235—240 |
| [102] | Konova P., Naydenov A., Tabakova T., Mehandjiev D., Catal. Commun., 2004, 5, 537—542 |
| [103] | Carrettin S., Concepción P., Corma A., Nieto J. M. L., Puntes V. F., Angew. Chem. Int. Ed., 2004, 43, 2538—2540 |
| [104] | Widmann D., Behm R. J., Angew. Chem. Int. Ed., 2011, 50, 10241—10245 |
| [105] | Kotobuki M., Leppelt R., Hansgen D. A., Widmann D., Behm R. J., J. Catal., 2009, 264, 67—76 |
| [106] | Widmann D., Liu Y., Schüth F., Behm R. J., J. Catal., 2010, 276, 292—305 |
| [107] | Zhao Y. X., Ding X. L., Ma Y. P., Wang Z. C., He S. G., Theor. Chem. Acc., 2010, 127, 449—465 |
| [108] | Wu X. N., Xu B., Meng J. H., He S. G., Int. J. Mass Spectrom., 2012, 310, 57—64 |
| [109] | Yin S., Ma Y. P., Du L., He S. G., Ge M. F., Chin. Sci. Bull., 2008, 53, 3829—3838 |
| [110] | Ma Y. P., Zhao Y. X., Li Z. Y., Ding X. L., He S. G., Chin. J. Chem. Phys., 2011, 24, 586—596 |
| [111] | Tian L. H., Zhao Y. X., Wu X. N., Ding X. L., He S. G., Ma T. M., Chem. Phys. Chem., 2012, 13, 1282—1288 |
| [112] | Panov G. I., Dubkov K. A., Starokon E. V., Catal. Today,2006, 117, 148—155 |
| [113] | Zhao C., Wachs I. E., Catal. Today,2006, 118, 332—343 |
| [114] | Feyel S., Döbler J., Schröder D., Sauer J., Schwarz H., Angew. Chem. Int. Ed., 2006, 45, 4681—4685 |
| [115] | Feyel S., Döbler J., Höckendorf R., Beyer M.K., Sauer J., Schwarz H., Angew. Chem. Int. Ed., 2008, 47, 1946—1950 |
| [116] | Schlangen M., Schwarz H., Dalton Trans., 2009, 46, 10155—10165 |
| [117] | Dietl N., Höckendorf R. F., Schlangen M., Lerch M., Beyer M. K., Schwarz H., Angew. Chem. Int. Ed., 2011, 50, 1430—1434 |
| [118] | Zhao Y. X., Wu X. N., Wang Z. C., He S. G., Ding X. L., Chem. Commun., 2010, 46, 1736—1738 |
| [119] | Ding X. L., Zhao Y. X., Wu X. N., Wang Z. C., Ma J. B., He S. G., Chem.-Eur. J., 2010, 16, 11463—11470 |
| [120] | Wang Z. C., Wu X. N., Zhao Y. X., Ma J. B., Ding X. L., He S. G., Chem. Phys. Lett., 2010, 489, 25—29 |
| [121] | Ma J. B., Wu X. N., Zhao X. X., Ding X. L., He S. G., Phys. Chem. Chem. Phys., 2010, 12, 12223—12228 |
| [122] | Ma J. B., Wu X. N., Zhao Y. X., Ding X. L., He S. G., J. Phys. Chem. A,2010, 114, 10024—10027 |
| [123] | Zhao Y. X., Wu X. N., Ma J. B., He S. G., Ding X. L., J. Phys. Chem. C,2010, 114, 12271—12279 |
| [124] | Ma J. B., Wu X. N., Zhao Y. X., Ding X. L., He S. G., Chin. J. Chem. Phys., 2010, 23, 133—137 |
| [125] | Zhao Y. X., Yuan J. Y., Ding X. L., He S. G., Zheng W. J., Phys. Chem. Chem. Phys., 2011, 13, 10084—10090 |
| [126] | Wang Z. C., Wu X. N., Zhao Y. X., Ma J. B., Ding X. L., He S. G., Chem.-Eur. J., 2011, 17, 3449—3457 |
| [127] | Meng J. H., Zhao Y. X., He S. G., J. Phys. Chem. C,2013, 117, 17548—17556 |
| [128] | Dietl N., Schlangen M., Schwarz H., Angew. Chem. Int. Ed., 2012, 51, 5544—5555 |
| [129] | Dong F., Heinbuch S., He S. G., Xie Y., Rocca J. J., Bernstein E. R., J. Chem. Phys., 2006, 125, 164318—164318 |
| [130] | Wang W. G., Wang Z. C., Yin S., He S. G., Ge M. F., Chin. J. Chem. Phys., 2007, 20, 412—418 |
| [131] | Dong F., Heinbuch S., Xie Y., Rocca J.J., Bernstein E. R., Wang Z. C., Deng K., He S. G., J. Am. Chem. Soc., 2008, 130, 1932—1943 |
| [132] | Yin S., Xue W., Ding X. L., Wang W. G., He S. G., Ge M. F., Int. J. Mass Spectrom., 2009, 281, 72—78 |
| [133] | Li X. N., Xu B., Ding X. L., He S. G., Dalton Trans., 2012, 41, 5562—5570 |
| [134] | Ma J.B., Zhao Y. X., He S. G., Ding X. L., J. Phys. Chem. A, 2012, 116, 2049—2054 |
| [135] | Manzoli M., Boccuzzi F., Chiorino A., Vindigni F., Deng W., Flytzani-Stephanopoulos M., J. Catal., 2007, 245, 308—315 |
| [136] | Liu H., Kozlov A. I., Kozlova A. P., Shido T., Asakura K., Iwasawa Y., J. Catal., 1999, 185, 252—264 |
| [137] | Guzman J., Carrettin S., Fierro-Gonzalez J. C., Hao Y. L., Gates B. C., Corma A., Angew. Chem. Int. Ed., 2005, 44, 4778—4781 |
| [138] | Walker J. S., Straguzzi G. I., Manogue W. H., Schuit G. C. A., J. Catal., 1988, 110, 298—309 |
| [139] | Uddin M. A., Komatsu T., Yashima T., J. Catal., 1994, 146, 468—475 |
| [140] | Li P., Miser D. E., Rabiei S., Yadav R. T., Hajaligol M. R., Appl. Catal. B: Environ., 2003, 43, 151—162 |
| [141] | Xiong Y. J., Li Z. Q., Li X. X., Hu B., Xie Y., Inorg. Chem., 2004, 43, 6540—6542 |
| [142] | Lin H. Y., Chen Y. W., Wang W. J., J. Nanopart. Res., 2005, 7, 249—263 |
| [143] | Szegedi A., Hegedüs M., Margitfalvi J.L., Kiricsi I.,Chem. Commun., 2005, (11), 1441—1443 |
| [144] | Khedr M. H., Halim K. S. A., Nasr M. I., El-Mansy A. M., Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process., 2006, 430, 40—45 |
| [145] | Hu C. Q., Gao Z. H., Yang X. R., Chem. Lett., 2006, 35, 1288—1289 |
| [146] | Zheng Y. H., Cheng Y., Wang Y. S., Bao F., Zhou L. H., Wei X. F., Zhang Y. Y., Zheng Q., J. Phys. Chem. B,2006, 110, 3093—3097 |
| [147] | Foster J. P., Weinhold F., J. Am. Chem. Soc., 1980, 102, 7211—7218 |
| [148] | Reed A. E., Curtiss L. A., Weinhold F., Chem. Rev., 1988, 88, 899—926 |
| [149] | Li J., Li X., Zhai H. J., Wang L. S., Science,2003, 299, 864—867 |
| [150] | Wang L. S., Phys. Chem. Chem. Phys., 2010, 12, 8694—8705 |
| [151] | Woodham A. P., Meijer G., Fielicke A., Angew. Chem. Int. Ed., 2012, 51, 4444—4447 |
| [152] | Häkkinen H., Landman U., J. Am. Chem. Soc., 2001, 123, 9704—9705 |
| [153] | Hagen J., Socaciu L. D., Elijazyfer M., Heiz U., Bernhardt T. M., Wöste L., Phys. Chem. Chem. Phys., 2002, 4, 1707—1709 |
| [154] | Tang D. Y., Zhang Y. Q., Hu C. W., Acta Chim. Sin., 2008, 66, 1501—1507 |
| [155] | Xie Y., Dong F., Bernstein E. R., Catal. Today,2011, 177, 64—71 |
| [156] | Lira E., Hansen J.Ø., Huo P., Bechstein R., Galliker P., Lægsgaard E., Hammer B., Wendt S., Besenbacher F., Surf. Sci., 2010, 604, 1945—1960 |
| [157] | Blagojevic V., Flaim E., Jarvis M. J. V., Koyanagi G. K., Bohme D. K., J. Phys. Chem. A,2005, 109, 11224—11235 |
| [158] | Blagojevic V., Bohme D. K., Int. J. Mass Spectrom., 2006, 254, 152—154 |
| [159] | DašićA., Zhao X., Bohme D. K., Int. J. Mass Spectrom., 2006, 254, 155—162 |
| [160] | Blagojevic V., BožovićA., Orlova G., Bohme D. K., J. Phys. Chem. A,2008, 112, 10141—10146 |
| [161] | Tian L. H., Ma T. M., Li X. N., He S. G., Dalton Trans., 2013, 42, 11205—11211 |
| [162] | Coquet R., Howard K.L., Willock D. J., Chem. Soc. Rev., 2008, 37, 2046—2076 |
| [1] | TENG Zhenyuan, ZHANG Qitao, SU Chenliang. Charge Separation and Surface Reaction Mechanisms for Polymeric Single-atom Photocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220325. |
| [2] | YANG Jingyi, SHI Siqi, PENG Huaitao, YANG Qihao, CHEN Liang. Integration of Atomically Dispersed Ga Sites with C3N4 Nanosheets for Efficient Photo-driven CO2 Cycloaddition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220349. |
| [3] | WANG Ruyue, WEI Hehe, HUANG Kai, WU Hui. Freezing Synthesis for Single Atom Materials [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220428. |
| [4] | WANG Xintian, LI Pan, CAO Yue, HONG Wenhao, GENG Zhongxuan, AN Zhiyang, WANG Haoyu, WANG Hua, SUN Bin, ZHU Wenlei, ZHOU Yang. Techno-economic Analysis and Industrial Application Prospects of Single-atom Materials in CO2 Catalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220347. |
| [5] | YANG Jingyi, LI Qinghe, QIAO Botao. Synergistic Catalysis Between Ir Single Atoms and Nanoparticles for N2O Decomposition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220388. |
| [6] | LIN Gaoxin, WANG Jiacheng. Progress and Perspective on Molybdenum Disulfide with Single-atom Doping Toward Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220321. |
| [7] | WANG Sicong, PANG Beibei, LIU Xiaokang, DING Tao, YAO Tao. Application of XAFS Technique in Single-atom Electrocatalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220487. |
| [8] | TANG Quanjun, LIU Yingxin, MENG Rongwei, ZHANG Ruotian, LING Guowei, ZHANG Chen. Application of Single-atom Catalysis in Marine Energy [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220324. |
| [9] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [10] | YAO Qing, YU Zhiyong, HUANG Xiaoqing. Progress in Synthesis and Energy-related Electrocatalysis of Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220323. |
| [11] | LIN Zhi, PENG Zhiming, HE Weiqing, SHEN Shaohua. Single-atom and Cluster Photocatalysis: Competition and Cooperation [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220312. |
| [12] | HAN Fuchao, LI Fujin, CHEN Liang, HE Leiyi, JIANG Yunan, XU Shoudong, ZHANG Ding, QI Lu. Enhance of CoSe2/C Composites Modified Separator on Electrochemical Performance of Li-S Batteries at High Sulfur Loading [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220163. |
| [13] | HUANG Qiuhong, LI Wenjun, LI Xin. Organocatalytic Enantioselective Mannich-type Addition of 5H-Oxazol-4-ones to Isatin Derived Ketimines [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220131. |
| [14] | TAN Yan, YU Shen, LYU Jiamin, LIU Zhan, SUN Minghui, CHEN Lihua, SU Baolian. Efficient Preparation of Mesoporous γ-Al2O3 Microspheres and Performance of Pd-loaded Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220133. |
| [15] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||