

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (9): 2003.doi: 10.7503/cjcu20180167
• Physical Chemistry • Previous Articles Next Articles
WANG Yao1, WANG Yunpeng3, CAI Meirong2, LI Huanrong1,*( ), YANG Zhongqiang3,*(
), YANG Zhongqiang3,*( )
)
Received:2018-03-04
Online:2018-09-07
Published:2018-04-28
Contact:
LI Huanrong,YANG Zhongqiang
E-mail:hrli@ruc.edu.cn;zyang@tsinghua.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Yao,WANG Yunpeng,CAI Meirong,LI Huanrong,YANG Zhongqiang. Preparation and Growth of Interfacial Ultrasmall Water Droplets Between Alkanes and Hydrophobic Solid†[J]. Chem. J. Chinese Universities, 2018, 39(9): 2003.
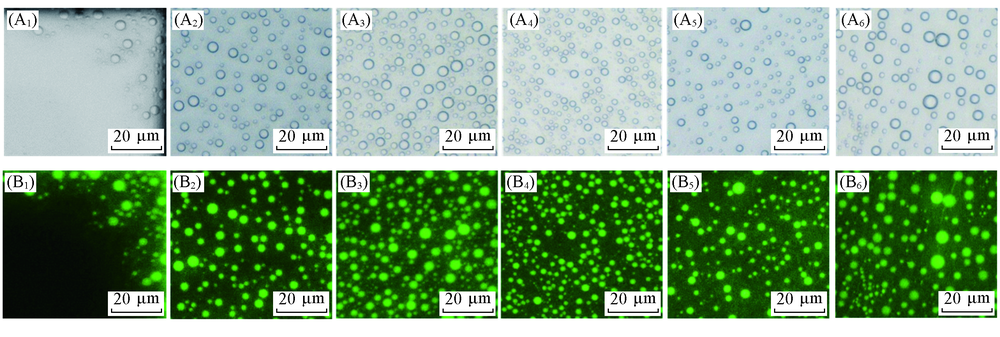
Fig.2 Optical(A1—A6) and fluorescent(B1—B6) micrographs of ultrasmall water droplets formed between alkanes and OTS-coated glass interfaces after 3 d incubation(A1, B1) n-C8H18; (A2, B2) n-C10H22; (A3, B3) n-C13H28; (A4, B4) n-C14H30; (A5, B5) n-C15H32; (A6, B6) n-C16H34.
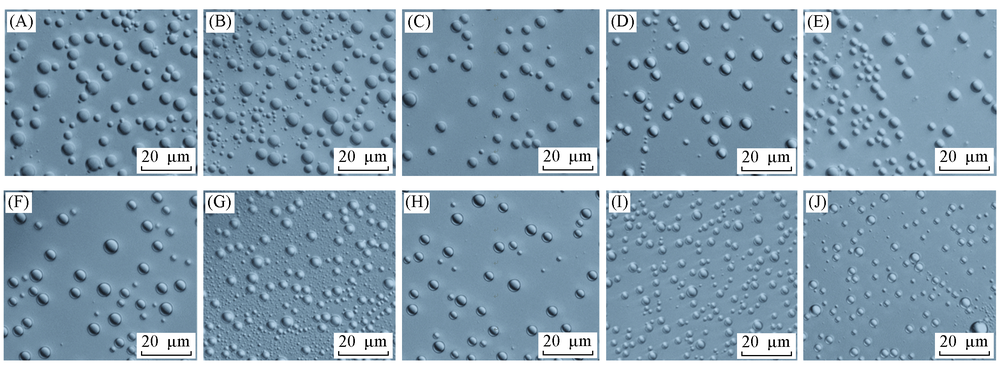
Fig.3 Optical micrographs of ultrasmall water droplets between alkanes and OTS-coated glass interfaces after 3 d incubation (A)—(J) C7—C16, respectively.
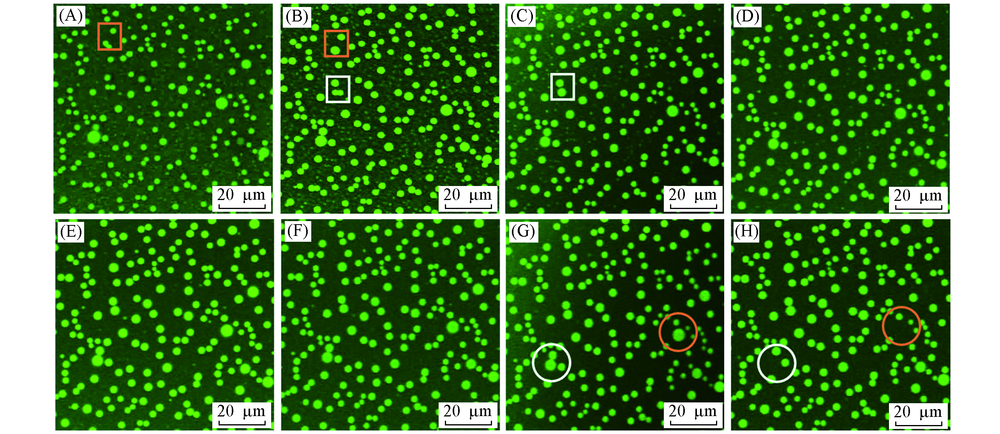
Fig.5 Confocal fluorescent images of ultrasmall water droplets formed between n-hexadecane and OTS-coated glass over timeWater droplets can coalesce(squares)or disappear(circles). (A) Day 1; (B) day 3; (C) day 6; (D) day 9; (E) day 12; (F) day 15; (G) day 20; (H) day 22.
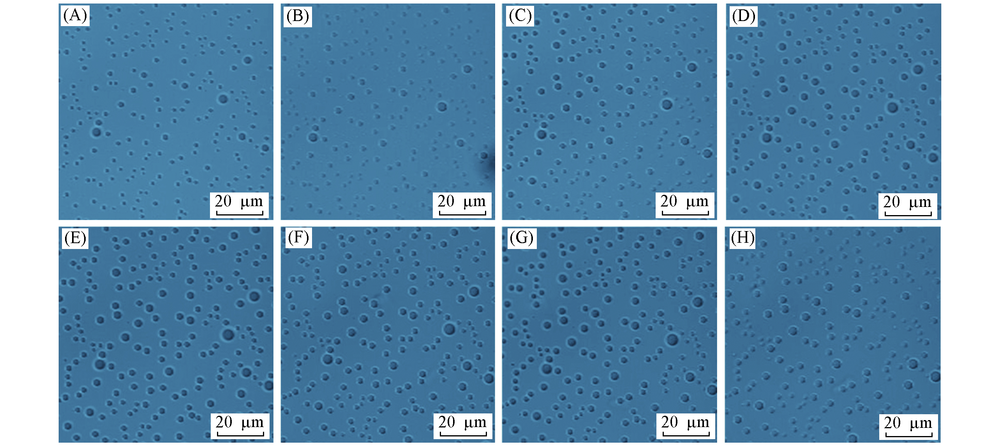
Fig.6 Confocal bright field images of ultrasmall water droplets formed between n-hexadecane and OTS-coated glass over time (A) Day 1; (B) day 3; (C) day 6; (D) day 9; (E) day 12; (F) day 15; (G) day 20; (H) day 22.
| [1] | Méndez-Vilas A., Jódar-Reyes A. B., González-Martín M. L., Small, 2009, 5(12), 1366—1390 |
| [2] | Boreyko J. B., Polizos G., Datskos P. G., Sarles S. A., Collier C. P., Proc. Natl. Acad. Sci., 2014, 111(21), 7588—7593 |
| [3] | Gu Y., Colloids and Surfaces A: Physicochem. Eng. Aspects, 2001, 181(1), 215—224 |
| [4] | Park J., Han H. S., Kim Y. C., Ahn J. P., Ok M. R., Lee K. E., Lee J., Cha P., Seok H., Jeon H., Sci. Rep., 2015, 5, 18150 |
| [5] | Zhang H. F., Zhao Y. G., Lv R., Yang C., Int. J. Therm. Sci., 2016, 101, 59—67 |
| [6] | Kajiya T., Schellenberger F., Papadopoulos P., Vollmer D Sci. Rep., 2016, 6, 23687 |
| [7] | Kim P., Wong T. S., Alvarenga M. J., Kreder M. J., Adorno-martinez W. E., Aizenberg J., ACS Nano, 2012, 6(8), 6569—6577 |
| [8] | Mishchenko L., Hatton B., Bahadur V., Taylor J. A., Krupenkin T., Aizenberg J., ACS Nano, 2010, 4(12), 7699—7707 |
| [9] | Paxson A. T., Yagüe J. L., Gleason K. K., Varanasi K. K., Adv. Mater., 2014, 26(3), 418—423 |
| [10] | Rodolfa K. T., Bruckbauer A., Zhou D., Schevchuk A. I., Korchev Y. E., Klenerman D., Nano. Lett., 2006, 6(2), 252—257 |
| [11] | You B., Shi L., Wen N. G., Liu X. H., Wu L. M., Zi J., Macromolecules, 2008, 41(18), 6624—6626 |
| [12] | Sun H., Wu. L. X., Prog. Chem., 2010, 22(9), 1784—1798 |
| (孙航, 吴立新. 化学进展, 2010, 22(9), 1784—1798) | |
| [13] | Wang R., Kido M., Surf. Interface Anal., 2005, 37(12), 1105—1110 |
| [14] | Wang R., Kido M., Mater. Lett., 2003, 57(16), 2360—2365 |
| [15] | Wang R., Cong L., Kido M., Appl. Surf. Sci., 2002, 191(1), 74—84 |
| [16] | Ku B. K., Kim S. S., J. Aerosol Sci., 2002, 33, 1361—1378 |
| [17] | Jayasinghe S. N., Edirisinghe M. J., J. Aerosol Sci., 2002, 33(10), 1379—1388 |
| [18] | Boreyko J. B., Chen C., Phys. Rev. Lett., 2009, 103, 184501 |
| [19] | Anand S., Paxson A. T., Dhiman R., Smith J. D., Varanasi K. K., ACS Nano, 2012, 6(11), 10122—10129 |
| [20] | Zhang X. H., Ducker W., Langmuir, 2008, 24(1), 110—115 |
| [21] | Xu H. L., Zhang X. H., Adv. Colloid Interface Sci., 2015, 224, 17—32 |
| [22] | Yang Z. Q., Abbott N. L., Langmuir, 2010, 26(17), 13797—13804 |
| [23] | Zhang L., Zhang Y., Zhang X., Li Z., Shen G., Ye M., Fan C., Fang H., Hu J., Langmuir, 2006, 22(19), 8109—8113 |
| [24] | An H. J., Liu G. M., Atkin R., Craig V. S. J., ACS Nano, 2015, 9(7), 7596—7607. |
| [25] | Li D. Y., Wang W. J., Zhao X. Z., Prog. Chem., 2012, 8(24), 1447—1455 |
| (李大勇, 王伟杰, 赵学增. 化学进展, 2012, 24(8), 1447—1455) | |
| [26] | Lohse D., Zhang X. H., Rev. Mod. Phys., 2015, 87(3), 981—1035 |
| [27] | Lou S. T., Ouyang Z. Q., Zhang Y., Li X. J., Hu J., Li M. Q., Yang F. J., J. Vac. Sci. Technol. B, 2000, 18(5), 2573—2575 |
| [28] | Ishida N., Inoue T., Miyahara M., Higashitani K., Langmuir, 2000, 16(16), 6377—6380 |
| [29] | Zhang P., Xu Z., Liu Q., Yuan S., J. Chem. Phys., 2014, 140, 164702 |
| [30] | Tian C. S., Shen Y. R., Proc. Natl. Acad. Sci., 2009, 106(36), 15148—15153 |
| [31] | Liu X. K., Leng C., Yu L., He K., Brown L. J., Chen Z., Cho J. H., Wang D. Y., Angew. Chem. Int. Ed., 2015, 54(16), 4851—4856 |
| [32] | Guo F., Yang S. Y., Ma C., Wong P. L., Tribol. Lett., 2014, 54(1), 81—88 |
| [33] | Hu S. W., Guo Q. Z., Xia G. F., Nie H., Li D. D., Acta Petrol. Sin.: Pet. Process Section, 2015, 4, 831—835 |
| (胡松伟, 郭庆洲, 夏国富, 聂红, 李大东. 石油学报: 石油加工, 2015, 31(4), 831—835) | |
| [34] | Li Y., Yang Q. M., Mei R. A., Cai M. R., Heng J. Y. Y., Yang Z. Q., J. Phys. Chem. B, 2017, 121, 6766—6772 |
| [1] | LI Wei, LUO Piao, HUANG Lianzhan, CUI Zhiming. Lithium Polystyrene Sulfonate Based Interfacial Protective Layer for Lithium Metal Anodes [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220166. |
| [2] | TIAN Zhen, DU Na, LI Haiping, SONG Shue, HOU Wanguo. Points of Zero Charge and Surface Acid-base Reaction Equilibrium Constants of Mg-Al-Ti Layered Double Hydroxides [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210833. |
| [3] | QIAO Zhenghua, FAN Qi, HAO Jingcheng. Silicone Surfactant-enhanced Dual Networks and High Temperature Resistance Porous Silicone Elastomers [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220384. |
| [4] | WANG Jie, HUO Haiyan, WANG Yang, ZHANG Zhong, LIU Shuxia. General Strategy for In situ Synthesis of NENU-n Series Polyoxometalate-based MOFs on Copper Foil [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210557. |
| [5] | XUE Jin, CAO Xiaowei, LIU Yifan, WANG Min. Preparation of Paper Hollow Gold Nanocage SERS Sensor and Its Rapid and Highly Sensitive Detection for miRNAs in Sputum of Patients with Non-small Cell Lung Cancer [J]. Chem. J. Chinese Universities, 2021, 42(8): 2393. |
| [6] | LIU Shasha, ZHANG Heng, YUAN Shiling, LIU Chengbu. Molecular Dynamics Simulation of Pulsed Electric Field O/W Emulsion Demulsification [J]. Chem. J. Chinese Universities, 2021, 42(7): 2170. |
| [7] | GAO Yifei, XIAO Changfa, JI Dawei, HUANG Yangzheng. Preparation of PVDF Hollow Fiber Membranes via Melt Spinning-stretching Method and Its Oil-water Separation Performance [J]. Chem. J. Chinese Universities, 2021, 42(6): 2065. |
| [8] | WANG Yimeng, LIU Kai, WANG Baoguo. Coating Strategies of Ni-rich Layered Cathode in LIBs [J]. Chem. J. Chinese Universities, 2021, 42(5): 1514. |
| [9] | WANG Zengqiang, SUN Yiling, QIAN Zhengfang, WANG Renheng. Advances in Lithium Metal Batteries Based on Surface Interface Reaction and Optimization [J]. Chem. J. Chinese Universities, 2021, 42(4): 1017. |
| [10] | WANG Aqiang, ZHU Yuzhang, JIN Jian. Preparation of Carboxyl-betaine Polyurethane Hydrogel and Study on Its Underwater Anti-crude-oil-adhesion Property [J]. Chem. J. Chinese Universities, 2021, 42(4): 1246. |
| [11] | XIA Jiahao, XU Huaping. Selenium-containing Surface/interface Chemistry [J]. Chem. J. Chinese Universities, 2021, 42(4): 997. |
| [12] | ZOU Junyan, ZHANG Yanyan, CHEN Shi, SHAO Huaiyu, TANG Yuxin. Recent Development on Surface-interface Chemistry of All-solid-state Lithium Batteries [J]. Chem. J. Chinese Universities, 2021, 42(4): 1005. |
| [13] | FAN Wenqian, ZHONG Zhengxiang, TIAN Gongwei, WANG Yu, GONG Guifen, QI Dianpeng. Application of Conductive Polymer in Nerve Interface Electrode [J]. Chem. J. Chinese Universities, 2021, 42(4): 1146. |
| [14] | ZHANG Jun, LIU Yixuan, DU Xiaohui, YANG Hui. Highly Adhesive and Stretchable Polymers for the Interface of Cyber-human Interaction [J]. Chem. J. Chinese Universities, 2021, 42(4): 1093. |
| [15] | BA Zhichen, LIANG Daxin, XIE Yanjun. Progress of MXenes Composites: Interface Modification and Structure Design [J]. Chem. J. Chinese Universities, 2021, 42(4): 1225. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||