

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (11): 2118.doi: 10.7503/cjcu20170101
• Polymer Chemistry • Previous Articles Next Articles
LI Zhigang1, ZHANG Yixuan1, ZHANG Qingsong1,*( ), MA Youwei2, HU Tao1, BAI Haihui2, LIU Pengfei3, WANG Ke4, ZHANG Xiaoyong4
), MA Youwei2, HU Tao1, BAI Haihui2, LIU Pengfei3, WANG Ke4, ZHANG Xiaoyong4
Received:2017-02-22
Online:2017-11-10
Published:2017-10-30
Contact:
ZHANG Qingsong
E-mail:zqs8011@163.com
Supported by:CLC Number:
TrendMD:
LI Zhigang, ZHANG Yixuan, ZHANG Qingsong, MA Youwei, HU Tao, BAI Haihui, LIU Pengfei, WANG Ke, ZHANG Xiaoyong. Adsorption Kinetics/Thermodynamic Behavior and Adsorption/Desorption Mechanism of Crystal Violet by Semi-interpenetrating Sodium Alginate/Polyacrylamide Hydrogel†[J]. Chem. J. Chinese Universities, 2017, 38(11): 2118.
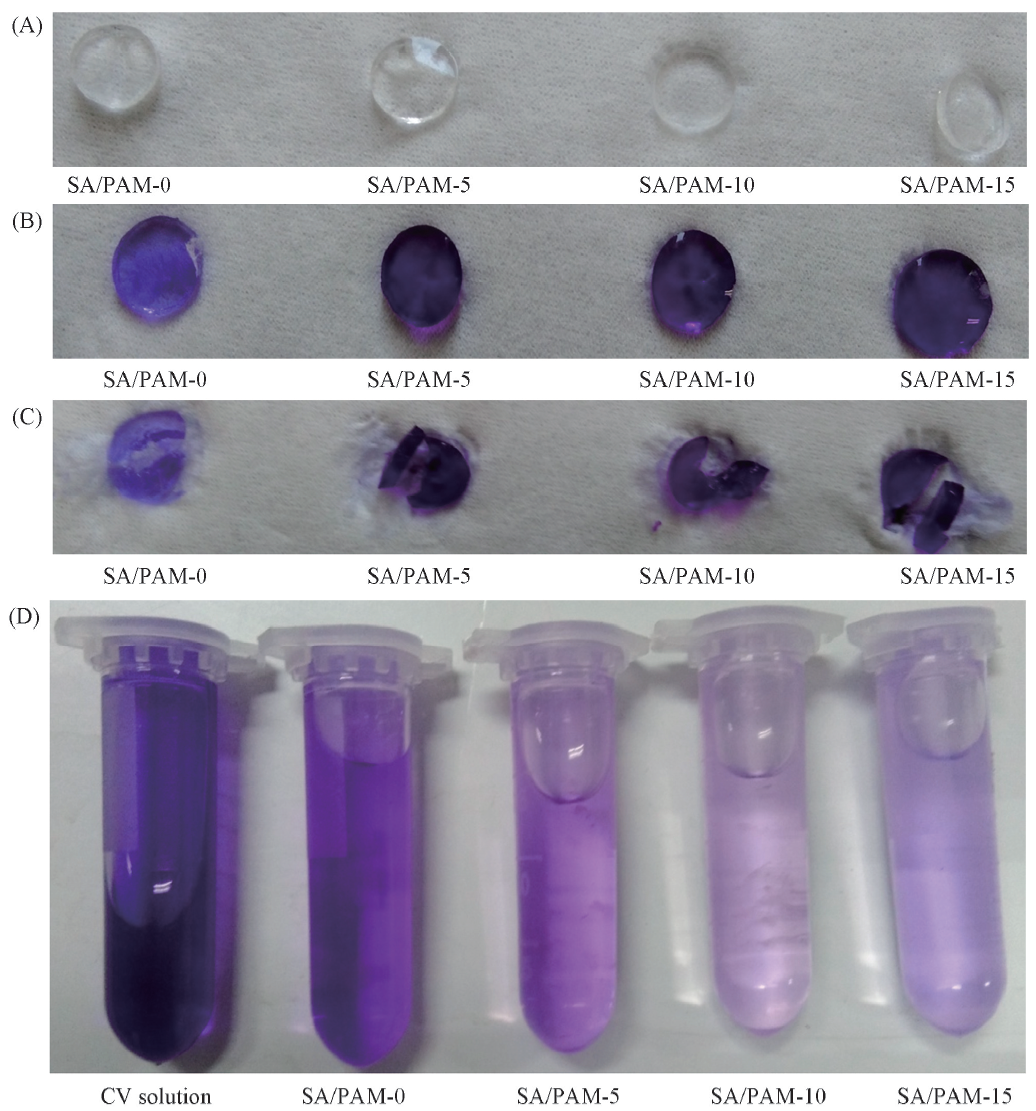
Fig.1 Appearance of SA/PAM hydrogels before(A) and after(B—D) adsorption of CV molecules(A) Surface appearances before adsorption of SA/PAM hydrogels; (B) surface appearances after adsorption of SA/PAM hydrogels; (C) cross-section appearances after adsorption of SA/PAM hydrogels; (D) appearances of CV solution after adsorption of SA/PAM hydrogels.
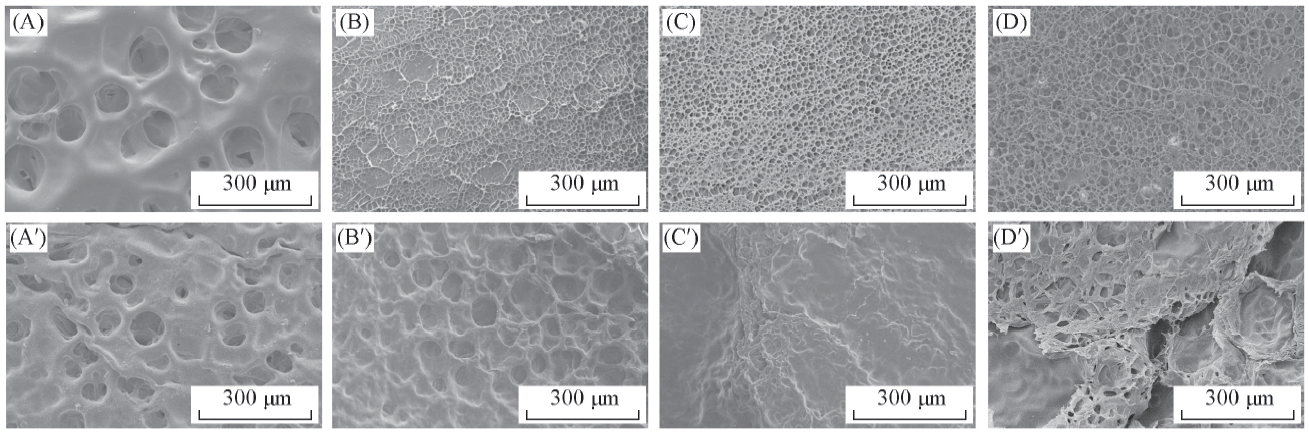
Fig.2 Surface morphology and pore size of SA/PAM hydrogels before(A—D) and after(A'—D') adsorption of CV molecules (A, A') SA/PAM-0; (B, B') SA/PAM-5; (C, C') SA/PAM-10; (D, D') SA/PAM-15.
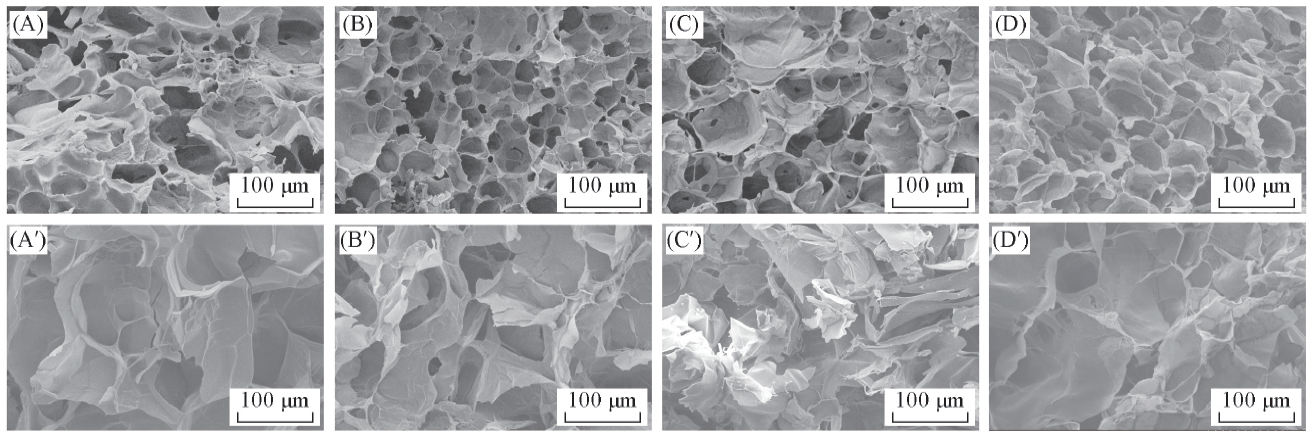
Fig.3 Cross-section morphology of SA/PAM hydrogels before(A—D) and after(A'—D') adsorption of CV molecules(A, A') SA/PAM-0; (B, B') SA/PAM-5; (C, C') SA/PAM-10; (D, D') SA/PAM-15.
| Hydrogel | Pseudo-first-order | Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | R2 | qe/(mg·g-1) | K2(h·g/mg) | R2 | qe/(mg·g-1) | ||
| SA/PAM-0 | 0.005377 | 0.9936 | 1.9516 | 0.003320 | 0.8230 | 2.2049 | |
| SA/PAM-5 | 0.002498 | 0.9985 | 8.8436 | 0.01495 | 0.7479 | 10.7631 | |
| SA/PAM-10 | 0.002323 | 0.9983 | 13.5838 | 0.006666 | 0.6634 | 18.2515 | |
| SA/PAM-15 | 0.004227 | 0.9981 | 8.2453 | 0.02581 | 0.9071 | 10.3616 | |
Table 1 Parameters of pseudo-first-order and pseudo-second-order model for the adsorption of CV by SA/PAM hydrogels
| Hydrogel | Pseudo-first-order | Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | R2 | qe/(mg·g-1) | K2(h·g/mg) | R2 | qe/(mg·g-1) | ||
| SA/PAM-0 | 0.005377 | 0.9936 | 1.9516 | 0.003320 | 0.8230 | 2.2049 | |
| SA/PAM-5 | 0.002498 | 0.9985 | 8.8436 | 0.01495 | 0.7479 | 10.7631 | |
| SA/PAM-10 | 0.002323 | 0.9983 | 13.5838 | 0.006666 | 0.6634 | 18.2515 | |
| SA/PAM-15 | 0.004227 | 0.9981 | 8.2453 | 0.02581 | 0.9071 | 10.3616 | |
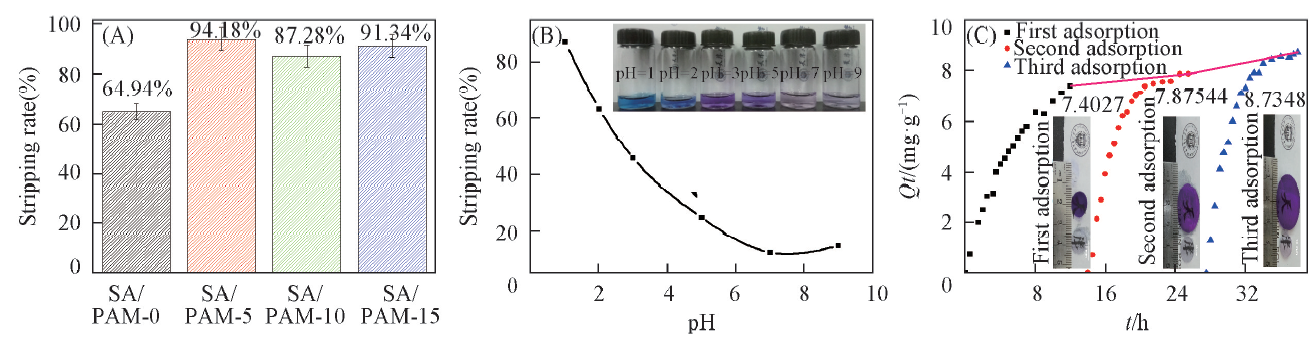
Fig.7 Stripping rates of CV on SA/PAM-x hydrogels(A), SA/PAM-10 hydrogel with different pH value(B) and cyclic adsorption dynamics on CV by SA/PAM-15 hydrogels(C) at 20 ℃Inset of (B): appearances of CV solution after alesorption in different pH; inset of (C): surface appearances of SA/PAM-15 hydrogel through three cyclic adsorption and desorption.
| Hydrogel | Intra-particle diffusion model(qt=kidt0.5+C) | Film diffusion[In(1-F)=-kft] | |||
|---|---|---|---|---|---|
| kid/(mg·g-1·min-1/2) | C | R2 | kf/min-1 | R2 | |
| SA/PAM-0 | 0.09546 | -0.1076 | 0.9724 | 0.005377 | 0.9936 |
| SA/PAM-5 | 0.3226 | -0.9807 | 0.9757 | 0.002499 | 0.9985 |
| SA/PAM-10 | 0.4789 | -1.515 | 0.9731 | 0.002323 | 0.9983 |
| SA/PAM-15 | 0.3437 | -0.3554 | 0.9800 | 0.004227 | 0.9980 |
Table 2 Rate parameters for the adsorption of CV by SA/PAM hydrogels
| Hydrogel | Intra-particle diffusion model(qt=kidt0.5+C) | Film diffusion[In(1-F)=-kft] | |||
|---|---|---|---|---|---|
| kid/(mg·g-1·min-1/2) | C | R2 | kf/min-1 | R2 | |
| SA/PAM-0 | 0.09546 | -0.1076 | 0.9724 | 0.005377 | 0.9936 |
| SA/PAM-5 | 0.3226 | -0.9807 | 0.9757 | 0.002499 | 0.9985 |
| SA/PAM-10 | 0.4789 | -1.515 | 0.9731 | 0.002323 | 0.9983 |
| SA/PAM-15 | 0.3437 | -0.3554 | 0.9800 | 0.004227 | 0.9980 |
| Equilibrium isotherms model | Parameter of model | Slope | Intercept | R2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| SA/PAM-x | x | 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 |
| Langmuir | Qm/(mg·g-1) | 36.75 | 11.03 | -0.02721 | -0.09066 | 4.960 | 3.687 | 0.2023 | 0.5216 |
| b/(L·mg-1) | -5.486×10-3 | -24.789×10-3 | |||||||
| Freundlic | n | 0.8715 | 0.5808 | 1.147 | 1.721 | -1.888 | -2.584 | 0.9733 | 0.9542 |
| 1/n | 1.147 | 1.721 | |||||||
| Kf/(mg·g-1)· (L·mg-1 | 0.1513 | 0.07547 | |||||||
| Temkin | B/(L·mg-1) | 5.028 | 11.00 | 5.028 | 11.00 | -8.929 | -27.28 | 0.9967 | 0.9948 |
| A | 0.1693 | 0.08377 | |||||||
| D-R | Qm/(mg·g-1) | 61.45 | 1938 | -1.272×10-6 | -1.618×10-6 | 11.03 | 14.48 | 0.9976 | 0.9999 |
| B1/(mol2·kJ-2) | -1.272×10-6 | -1.618×10-6 |
Table 3 Equilibrium isotherms parameters for the adsorption of crystal violet by SA/PAM-0 and SA/PAM-10 at 35 ℃
| Equilibrium isotherms model | Parameter of model | Slope | Intercept | R2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| SA/PAM-x | x | 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 |
| Langmuir | Qm/(mg·g-1) | 36.75 | 11.03 | -0.02721 | -0.09066 | 4.960 | 3.687 | 0.2023 | 0.5216 |
| b/(L·mg-1) | -5.486×10-3 | -24.789×10-3 | |||||||
| Freundlic | n | 0.8715 | 0.5808 | 1.147 | 1.721 | -1.888 | -2.584 | 0.9733 | 0.9542 |
| 1/n | 1.147 | 1.721 | |||||||
| Kf/(mg·g-1)· (L·mg-1 | 0.1513 | 0.07547 | |||||||
| Temkin | B/(L·mg-1) | 5.028 | 11.00 | 5.028 | 11.00 | -8.929 | -27.28 | 0.9967 | 0.9948 |
| A | 0.1693 | 0.08377 | |||||||
| D-R | Qm/(mg·g-1) | 61.45 | 1938 | -1.272×10-6 | -1.618×10-6 | 11.03 | 14.48 | 0.9976 | 0.9999 |
| B1/(mol2·kJ-2) | -1.272×10-6 | -1.618×10-6 |
| Hydrogel | ΔS/(kJ·mol-1·K-1) | ΔH/(kJ·mol-1) | ΔG/(kJ·mol-1) |
|---|---|---|---|
| SA/PAM-0 | 11.86 | ||
| SA/PAM-5 | -0.003645 | -1.388 | <0 |
| SA/PAM-10 | -0.001904 | -0.8510 | <0 |
| SA/PAM-15 | -0.003249 | -1.655 | <0 |
Table 4 Adsorption thermodynamic parameters for the adsorption of CV by SA/PAM-x hydrogels
| Hydrogel | ΔS/(kJ·mol-1·K-1) | ΔH/(kJ·mol-1) | ΔG/(kJ·mol-1) |
|---|---|---|---|
| SA/PAM-0 | 11.86 | ||
| SA/PAM-5 | -0.003645 | -1.388 | <0 |
| SA/PAM-10 | -0.001904 | -0.8510 | <0 |
| SA/PAM-15 | -0.003249 | -1.655 | <0 |
| [1] | Han R. P., Ding D., Xu Y. F., Zou W. H., Wang Y. F., Li Y. F., Zou L. N., Bioresour. Technol., 2008, 99(8), 2938-2946 |
| [2] | Kunz A., Mansilla H., Duran N., Environ. Technol., 2002, 23(8), 911-918 |
| [3] | Kang Q., Sep Purif. Technol., 2007, 57(2), 356-365 |
| [4] | Vijayabalan A., Selvam K., Velmurugan R., Swaminathan M., J. Hazard. Mater., 2009, 172(2), 914-921 |
| [5] | Kurousumi A., Kaneko E., Nakamura Y., Biodegradation,2008, 19(4), 489-494 |
| [6] | Fang R., He W. X., Xue H. Y, Chen W. J., React. Funct Polym., 2016, 102, 1-10 |
| [7] | Zhang W., Chen M., Diao G. W., Chem. J. Chinese Universities,2010, 31(9), 2227-2230 |
| (张旺, 陈铭, 刁国旺. 高等学校化学学报, 2011, 32(9), 2227-2230) | |
| [8] | Ali I., Chem. Rev., 2012, 112(10), 5073-5091 |
| [9] | Suo L. L., Li S. J., Li Y. T., Zhang L., Zhang X., Chem. J. Chinese Universities,2010, 31(11), 2043-2049 |
| (索路路, 李生娟, 李应涛, 张莉, 张熙. 高等学校化学学报, 2016, 37(11), 2043-2049) | |
| [10] | Li Y., Liu X., Yuan W. C., Brown L. J., Wang D. Y., Langmuir,2015, 31(23), 6351-6366 |
| [11] | Wang X. J., Zhang K. S., Ren Y. X., Yao J., Sci. Techn. Food Ind., 2008, 29(2), 259-262 |
| (王秀娟, 张坤生, 任云霞, 姚俊. 食品工业科技, 2008, 29(2), 259-262) | |
| [12] | Sui K. Y., Xie D., Gao S., Wu Z. M., Wu W. W., Xia Y. Z., J. Funct. Mater., 2010, 41(2), 268-270 |
| (隋坤艳, 谢丹, 高耸, 吴智明, 吴文文, 夏延致. 功能材料, 2010, 41(2), 268-270) | |
| [13] | Wu D. B., Gao Y. W, Li W. J., Zheng X. N., Chen Y. G., Wang Q. Q., Chem. Eng. J., 2016, 102, 1-10 |
| [14] | Meng Q. R., Wu C. Y., Leng S., Xie L. N., Liu X. P., Environ. Pollu. Control., 2015, 37(8), 58-63 |
| (孟倩茹, 吴称意, 冷珊, 谢丽娜, 刘信平. 环境污染及治防, 2015, 37(8), 58-63) | |
| [15] | Zhang Q. S., Peng Z., Zhao Y. P., Chen L., Ma J., J.Mater. Eng., 2011, 12, 20-24 |
| (张青松, 彭喆, 赵义平, 陈莉, 马静. 材料工程, 2011, 12, 20-24) | |
| [16] | Wang X. B., Cai W. P.,Liu S.W, Wang G. Z., Wu Z. K., Zhao H. J., Chem. J. Chinese Universities,2010, 31, 199-205 |
| [17] | Zhang Q. S., Li X. W., Zhao Y. P., Chen L., Appl. Clay Sci., 2009, 46(4), 346-350 |
| [18] | Kamal H., Hegazy E. S. A., Sharada H. M., Abd elhalim S. A., Lotfy S., Mohamed R. D., J. Nucl. Technol. Appl. Sci., 2014, 2(5), 523-537 |
| [19] | Jeon Y. S., Jing L. J., Kim J. H., J. Ind. Eng. Chem., 2008, 14(6), 726-731 |
| [20] | Li S. F., Bioresour. Technol., 2010, 101(7), 2197-2202 |
| [1] | WANG Xuebin, XUE Yuan, MAO Hua’nyu, XIANG Yanxin, BAO Chunyan. Preparation of Photo/reduction Dual-responsive Hydrogel Microspheres and Their Application in Three-dimensional Cell Culture [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220116. |
| [2] | HUANG Yi, LYU Lingling, PAN Xiaopeng, SUN Guangdong, LI Yongqiang, YAO Juming, SHAO Jianzhong. Three-dimensional Printing of Photocrosslinked Self-supporting Silk Fibroin Hydrogels [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210841. |
| [3] | ZHOU Yonghui, LI Yao, WU Yuxuan, TIAN Jing, XU Longquan, FEI Xu. Synthesis of A Novel Photoluminescence Self-healing Hydrogel [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210606. |
| [4] | YAN Shuting, YAO Yuan, TAO Xinfeng, LIN Shaoliang. Synthesis and Properties of Polypeptoid Hydrogels Containing Sulfonium Groups [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220381. |
| [5] | GAO Huiling, CAO Zhenzhen, GU Fang, WANG Haijun. Monte Carlo Simulation on Self-healing Behaviour of Hydrogen-bonded Hydrogel [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220482. |
| [6] | CAO Meiqi, LIU Xia, CUI Shuxun. Single-molecule Mechanics of Polyacrylamide Under Different Liquid Environments [J]. Chem. J. Chinese Universities, 2021, 42(9): 2982. |
| [7] | XU Yan, YANG Hongguo, NIU Huibin, TIAN Hailin, PIAO Hongguang, HUANG Yingping, FANG Yanfen. Preparation Mechanism and Application of Alcohol⁃modified Fe3O4 Magnetic Nanoparticles [J]. Chem. J. Chinese Universities, 2021, 42(8): 2564. |
| [8] | CAI Yaqian, ZHANG Jiahuai, LIU Fangzhe, LI Haichao, SHI Jianping, GUAN Shuang. Protein-based Hydrogel Assisted by Hofmeister Effect for Strain Sensor [J]. Chem. J. Chinese Universities, 2021, 42(8): 2609. |
| [9] | LI Peihong, ZHANG Chunling, DAI Xueyan, SUI Yanlong. Progress of Graphene Oxide/Polymer Composite Hydrogel [J]. Chem. J. Chinese Universities, 2021, 42(6): 1694. |
| [10] | LUO Chunhui, ZHAO Yufei. Facile Synthesis and Properties of Robust and Anti-swelling Hydrogels [J]. Chem. J. Chinese Universities, 2021, 42(6): 2024. |
| [11] | WANG Aqiang, ZHU Yuzhang, JIN Jian. Preparation of Carboxyl-betaine Polyurethane Hydrogel and Study on Its Underwater Anti-crude-oil-adhesion Property [J]. Chem. J. Chinese Universities, 2021, 42(4): 1246. |
| [12] | WANG Jie, LI Ying, SHAO Liang, BAI Yang, MA Zhonglei, MA Jianzhong. Preparation and Properties of Poly(vinyl alcohol)/polypyrrole Composite Conductive Hydrogel Strain Sensor [J]. Chem. J. Chinese Universities, 2021, 42(3): 929. |
| [13] | WANG Mingxia, LIU Zhihui, ZHU Zhen, LI Lingfeng, WANG Bowei. Preparation and Properties of Nano Lithium Magnesium Silicate-chitosan-sodium Alginate Composite Scaffold Materials [J]. Chem. J. Chinese Universities, 2021, 42(10): 3240. |
| [14] | ZHANG Juan, HU Xinyue, WANG Hongbo, LIAN Ying, LE Jinyu, YANG Zihao. Crystal-like Hydrogels Consisting of Parallel Hexahedrons Obtained from the Self-assembly ofβ⁃Cyclodextrin/perfluorononanoic Acid Inclusion Complexes [J]. Chem. J. Chinese Universities, 2021, 42(10): 3187. |
| [15] | WANG Bowei, MA Rui, WU Fan, LIU Zhihui, LI Lingfeng, ZHANG Xiao, LIU Dingkun, YANG Nan, LI Meihui, YANG Defeng, SUN Qi. Preparation and Characterization of Graphene Oxide-sodium Alginate-chitosan Composite Scaffold [J]. Chem. J. Chinese Universities, 2020, 41(9): 2099. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||