

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (5): 706.doi: 10.7503/cjcu20170061
• Articles: Inorganic Chemistry • Previous Articles Next Articles
HAN Linhuan, LI Kaifeng, CHEN Yanwei*( )
)
Received:2017-01-23
Online:2017-05-10
Published:2017-04-20
Contact:
CHEN Yanwei
E-mail:yanweichen@nenu.edu.cn
Supported by:CLC Number:
TrendMD:
HAN Linhuan, LI Kaifeng, CHEN Yanwei. Growth of Gold Nanorods and Their Interaction with Ferric Chloride†[J]. Chem. J. Chinese Universities, 2017, 38(5): 706.
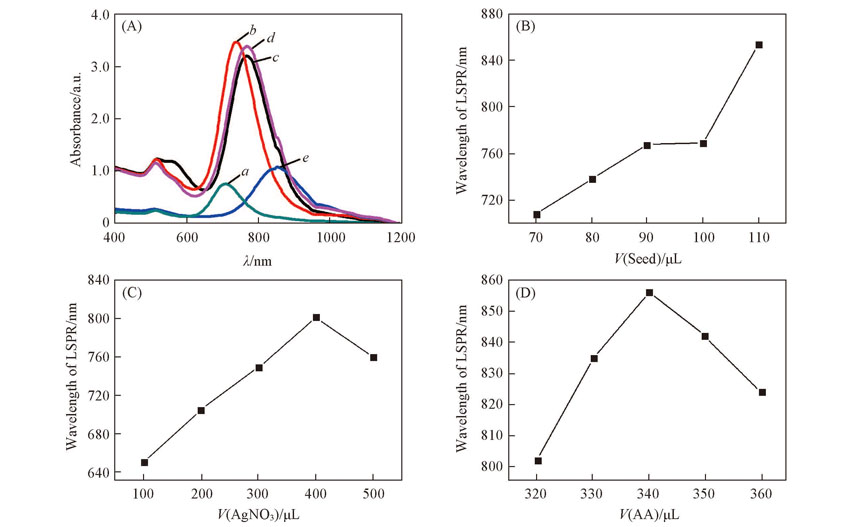
Fig.1 UV-Vis-NIR spectra of AuNRs adding different amounts of seed(A), and effects of seed(B), AgNO3(C) and AA amounts(D) on LSPR absorption peak position
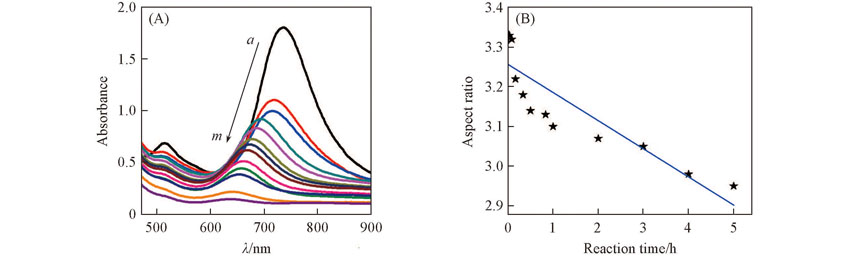
Fig.2 UV-Vis absorption spectra of FeCl3 etching AuNRs at different reaction time(A) and relationship between the aspect ratios of AuNRs and the reaction time(B)Time/min from a to m: 0, 2, 5, 10, 20, 30, 50, 60, 120, 180, 240, 300, 360.
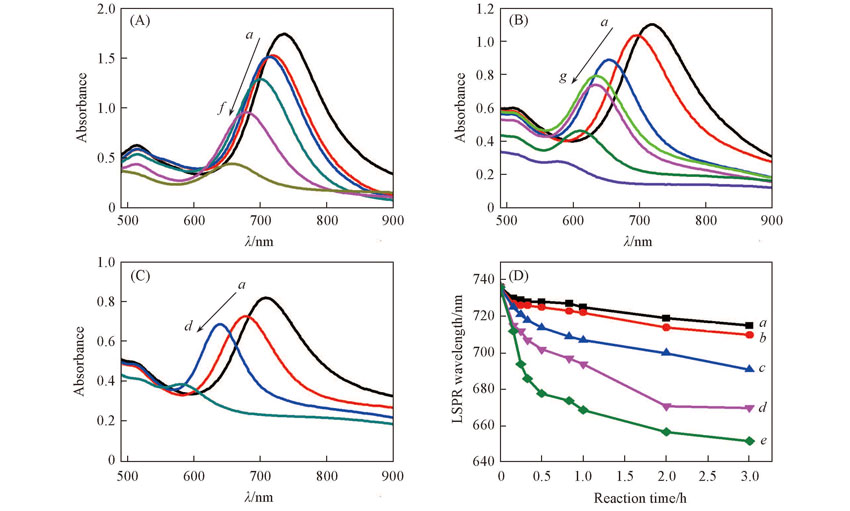
Fig.6 UV-Vis absorption spectra of FeCl3 etching AuNRs at different concentrations of FeCl3 for 2 h(A), at different concentrations of CTAB for 5 min(B) and under different reaction temperature for 30 min(C) and time dependence of the LSPR peak position of the AuNRs at different concentrations of FeCl3(D)(A) c(FeCl3)/(mol/L) from a to f: 0, 0.01, 0.02, 0.05, 0.10, 0.20. (B) c(CTAB)/(mmol/L) from a to g: 0, 5, 25, 35, 40, 60, 70. (C) Temperature/℃ from a to d: 20, 25, 40, 60. (D) c(FeCl3)/(mol/L) from a to e: 0.01, 0.02, 0.05, 0.10, 0.20.
| [1] | Jong B., Kim Y. S., Choi Y., Small, 2011, 7(2), 265—270 |
| [2] | Huang C. P., Yin X. G., Huang H., Zhu Y. Y., Opt. Express, 2009, 17(8), 6407—6413 |
| [3] | Eustis S., El-Sayed M. A., Chem. Soc. Rev., 2006, 35(3), 209—217 |
| [4] | Jiang X. C., Pileni M. P., Colloid Surface A, 2007, 295(1—3), 228—232 |
| [5] | Woo K. C., Shao L., Chen H. J., Liang Y., Wang J. F., Lin H. Q., ACS Nano, 2011, 5(7), 5976—5986 |
| [6] | Jin P., Dai Z., Guo W. J., Chen G. P., Chem. J. Chinese Universities, 2015, 36(5), 844—849 |
| (金萍, 代昭, 郭文娟, 陈广平.高等学校化学学报, 2015,36(5), 844—849) | |
| [7] | Zhang L. Y., Chi Y. N., Shan G. Y., Chen Y. W., Liu N., Chem. J. Chinese Universities, 2016, 37(7), 1239—1244 |
| (张龄月, 迟娅楠, 单桂晔, 陈艳伟, 刘娜.高等学校化学学报, 2016,37(7), 1239—1244) | |
| [8] | Zheng Z. K., Tachikawa T., Majima T., J. Am. Chem. Soc., 2015, 137(2), 948—957 |
| [9] | Lohse S. E., Murphy C. J., Chem. Mater., 2013, 25(8), 1250—1261 |
| [10] | Terentyuk G., Panfilova E., Khanadeev V., Chumakov D., Genina E., Bashkatov A., Tuchin V., Bucharskaya A., Maslyakova G., Khlebtsov N., Khlebtsov B., Nano Res., 2014, 7(3), 325—337 |
| [11] | Maltzahn G. V., Park J. H., Agrawal A., Bandaru N. K., Das S. K., Sailor M. J., Bhatia S. N., Cancer Res., 2009, 69(9), 3892—3900 |
| [12] | Sugiura T., Matsuki D., Okajima J., Komiya A., Mori S., Maruyama S., Kodama T., Nano Res., 2015, 8(12), 3842—3852 |
| [13] | Kirui D. K., Krishnan S., Strickland A. D., Batt C. A., Macromol. Biosci., 2011, 11(6), 779—788 |
| [14] | Kang H. Z., Trondoli A. C., Zhu. G., Chen Y., Chang Y. J., Liu H. P., Huang Y. F., Zhang X. L., Tan W. H., ACS Nano, 2011, 5(6), 5094—5099 |
| [15] | Wu H. Y., Huang W. L., Huang M. H., Cryst. Growth Des., 2007, 7(4), 831—835 |
| [16] | Jean R. D., Larsson M., Cheng W. D., Hsu Y. Y., Bow J. S., Liu D. M., Appl. Surf. Sci., 2016, 390, 675—680 |
| [17] | Han B., Zhu Z. N., Li Z. T., Zhang W., Tang Z. Y., J. Am. Chem. Soc., 2014, 136(46), 16104—16107 |
| [18] | Slaughter L. S., Wu Y. P., Willingham B. A., Nordlander P., Link S., ACS Nano, 2010, 4(8), 4657—4666 |
| [19] | Murphy C. J., Sau T. K., Gole A. M., Orendorff C. J., Gao J. X., Gou L. F., Hunyadi S. E., Li T., J. Phys. Chem. B, 2005, 109(29), 13857—13870 |
| [20] | Wen T., Zhang H., Tang X. P., Chu W. G., Liu W. Q., Ji Y. L., Hu Z. J., Hou S., Hu X. N., Wu X. C., J. Phys. Chem. C, 2013, 117(48), 25769—25777 |
| [21] | Yang R., Song D., Wang C., Zhu A., Xiao R., Liu J., Long F., RSC. Adv., 2015, 5(124), 102542—102549 |
| [22] | Shen X. M., Liu W. Q., Gao X. J., Lu Z. H., Wu X. C., Gao X. F., J. Am. Chem. Soc., 2015, 137(50), 15882—15891 |
| [23] | Tsung C. K., Kou X. S., Shi Q. H., Zhang J. P., Yeung M. H., Wang J. F., Stucky G. D., J. Am. Chem. Soc., 2006, 128(16), 5352—5353 |
| [24] | Sreeprasad T. S., Samal A. K., Pradeep T., Langmuir, 2007, 23(18), 9463—9471 |
| [25] | Thota S., Chena S., Zhao J., Chem. Commun., 2016, 52, 5593—5596 |
| [26] | Chen S., Thota S., Wang X. D., Zhao J., J. Mater. Chem. A, 2016, 4, 9038—9043 |
| [27] | Liu W. Q., Hou S., Yan J., Zhang H., Jia Y. L., Wu X. C., Nanoscale, 2016, 8(2), 780—784 |
| [28] | Wilson A., Bernard R., Borensztein Y., Croset B., Cruguel H., Vlad A., Coati A., Garreau Y., Prévot G., J. Phys. Chem. Lett., 2015, 6(11), 2050—2055 |
| [29] | Jiao Z., Xia H., Tao X., J. Phys. Chem. C, 2011, 115, 7887—7895 |
| [30] | Bai T. T., Sun J. F., Che R. C., Xu L. N., Yin C. Y., Guo Z. R., Gu N., ACS Appl. Mater. Interfaces, 2014, 6(5), 3331—3340 |
| [31] | Zheng Z. K., Tachikawa T., Majima T., J. Am. Chem. Soc., 2014, 136(19), 6870—6873 |
| [32] | Stewart I. E., Ye S. R., Chen Z. F., Flowers P. F., Wiley B. J., Chem. Mater.2015, 27(22), 7788—7794 |
| [33] | Yao T. J., Cui T. Y., Fang X., Cui F., Wu J., Chem. J. Chinese Universities, 2013, 34(10), 2421—2426 |
| (姚同杰, 崔铁钰, 方雪, 崔放, 吴杰.高等学校化学学报, 2013,34(10), 2421—2426) | |
| [34] | Xiong L., Li S. W., Zhang B., Du Y. C., Miao P., Ma Y., Han Y. X., Zhao H. T., Xu P., RSC Adv., 2015, 5(93), 76101—76106 |
| [35] | Liu C., Jiang Q., Chen L., Zhang H., Chen H. X., Zhou J., Ye Y., Chem. J. Chinese Universities, 2013, 34(11), 2488—2492 |
| (刘婵, 江茜, 陈蕾, 张侯, 陈怀侠, 周吉, 叶勇.高等学校化学学报, 2013,34(11), 2488—2492) | |
| [36] | Jiao L. S., Wang Z. J., Niu L., Shen J., You T. Y., Dong S. J., Ivaska A., J. Solid State Electrochem., 2006, 10(11), 886—893 |
| [37] | Quynh L. M., Nam N. H., Kong K., Nhung N. T., Notingher I., Henini M., Luong N. H., J. Electron. Mater., 2016, 45(5), 2563—2568 |
| [1] | GAO Yifei, XIAO Changfa, JI Dawei, HUANG Yangzheng. Preparation of PVDF Hollow Fiber Membranes via Melt Spinning-stretching Method and Its Oil-water Separation Performance [J]. Chem. J. Chinese Universities, 2021, 42(6): 2065. |
| [2] | GE Haoying, DU Jianjun, LONG Saran, SUN Wen, FAN Jiangli, PENG Xiaojun. Surface Functionalized Gold Nanomaterials in Tumor Diagnosis and Treatment [J]. Chem. J. Chinese Universities, 2021, 42(4): 1202. |
| [3] | WANG Yawen, LI Dong, LIANG Wenkai, SUN Yinghui, JIANG Lin. Multiplex Structures of Plasmonic Metal Nanoparticles and Their Applications [J]. Chem. J. Chinese Universities, 2021, 42(4): 1213. |
| [4] | LU Feng, GONG Yi, ZHAO Ting, ZHAO Ning, JU Wenwen, FAN Quli, HUANG Wei. Seedless Synthesis of Gold Nanorods with Narrow Absorption Using Binary Surfactant Mixture [J]. Chem. J. Chinese Universities, 2021, 42(3): 700. |
| [5] | ZHANG Aiqin, WANG Man, SHEN Gangyi, JIN Jun. Interactions Between Polybrominated Diphenyl Ethers and Human Serum Albumin Using SPR and Molecular Docking [J]. Chem. J. Chinese Universities, 2020, 41(9): 2054. |
| [6] | LI Dong,SUN Yinghui,WANG Zhongshun,HUANG Jing,Lü Nan,JIANG Lin. Large-scale Multiplexed Surface Plasmonic Gold Nanostructures Based on Nanoimprint and Self-assembly † [J]. Chem. J. Chinese Universities, 2020, 41(2): 221. |
| [7] | SUN Weixin, LIU Jia, WANG Jiazheng, ZHANG Yimiao, JIN Lei, ZHOU Jianzhang, YANG Fangzu, WU Deyin, TIAN Zhongqun. Preparation of Platinum-modified Uniform Gold Nanopillar Electrodes and Photoelectrocatalytic Oxidation of Methanol [J]. Chem. J. Chinese Universities, 2020, 41(12): 2788. |
| [8] | CHEN Yaoyan,ZHAO Xin,WANG Zhe,DONG Jie,ZHANG Qinghua. Effects of Preparation Conditions on the Morphologies, Structures and Electrochemical Properties of MXene† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1249. |
| [9] | SONG Ziqi,GONG Baijuan,WANG Lu,FENG Jing,WANG Bo,YAN Hongjing,LI Zhimin,SUN Hongchen. Polydopamine-coated Gold Nanorods for Rat Submandibular Gland Radiography† [J]. Chem. J. Chinese Universities, 2019, 40(1): 166. |
| [10] | WU Dan, LI Man, ZHONG Yao, AN Kuisheng, CHEN Yanwei. Property of Au Nanoshuttles with Arrow-head Prepared via Wet Chemical Synthetic Method† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1617. |
| [11] | LUO Dajun, SHAO Huiju, JIN Jinbo, XIE Gaoyi, CUI Zhenyu, YU Jie, QIN Shuhao. Preparation of Hydrophilic Polypropylene/Polyvinyl Butyral Hollow Fiber Membranes by Melt-spinning-stretching† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1838. |
| [12] | ZHANG Shuming, LUO Jianhui, XIA Bibo, LI Yuanyang, HE Meiying, JIANG Bo. Sol-gel Preparation of Superhydrophilic Silica Coating-materials with Low Refractive Index† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1342. |
| [13] | HUANG Jipei,LI Yi,YANG Shenhui,ZHOU Yazhou,CHENG Xiaonong,ZHU Jia,YANG Juan. Synthesis of Three-dimensional Pt-Ag Aerogels and Their Electrocatalytic Performance Toward Oxygen Reduction Reaction† [J]. Chem. J. Chinese Universities, 2018, 39(5): 1063. |
| [14] | LI Kaifeng,WU Dan,CHEN Yanwei. Effect of Copper Doping on the Growth and Optical Properties of Au Nanorods† [J]. Chem. J. Chinese Universities, 2018, 39(5): 855. |
| [15] | LI Ren, ZHAO Jianwei, HOU Jin, HE Yuanyuan, CHENG Na. Effect of the Convex and the Concave Microstructures in the Metallic Nanowires on the Initial Deformation Behavior† [J]. Chem. J. Chinese Universities, 2018, 39(3): 514. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||