

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (7): 1241.doi: 10.7503/cjcu20160863
• Physical Chemistry • Previous Articles Next Articles
LIU Zile1,2, ZENG Zequan2, YANG Jieyang2, CUI Yan2, WU Ailian1, LI Zhe1,*( ), HUANG Zhanggen2,*(
), HUANG Zhanggen2,*( )
)
Received:2016-11-30
Online:2017-07-10
Published:2017-05-18
Contact:
LI Zhe,HUANG Zhanggen
E-mail:lizhe@tyut.edu.cn;zghuang@sxicc.ac.cn
Supported by:CLC Number:
TrendMD:
LIU Zile, ZENG Zequan, YANG Jieyang, CUI Yan, WU Ailian, LI Zhe, HUANG Zhanggen. Degradation of Phenol with Persulfate Activated by Surface Modified Activated Carbon†[J]. Chem. J. Chinese Universities, 2017, 38(7): 1241.
| Sample | SBET/ (m2·g-1) | Vmic/ (cm3·g-1) | Pore size/nm | pHpzc | Acidity/ (mmol·g-1) | Basicity/ (mmol·g-1) | Smic/ (m2·g-1) |
|---|---|---|---|---|---|---|---|
| AC0 | 631 | 0.14 | 3.05 | 5.01 | 1.88 | 0.52 | 348.87 |
| ACN | 554 | 0.13 | 3.03 | 3.71 | 2.46 | 0.20 | 331.54 |
| ACNH | 730 | 0.16 | 2.86 | 7.74 | 1.45 | 1.04 | 396.31 |
Table 1 Physical properties, surface acidity and basicity of the AC samples
| Sample | SBET/ (m2·g-1) | Vmic/ (cm3·g-1) | Pore size/nm | pHpzc | Acidity/ (mmol·g-1) | Basicity/ (mmol·g-1) | Smic/ (m2·g-1) |
|---|---|---|---|---|---|---|---|
| AC0 | 631 | 0.14 | 3.05 | 5.01 | 1.88 | 0.52 | 348.87 |
| ACN | 554 | 0.13 | 3.03 | 3.71 | 2.46 | 0.20 | 331.54 |
| ACNH | 730 | 0.16 | 2.86 | 7.74 | 1.45 | 1.04 | 396.31 |
| Sample | Mass fraction(%) | ||||
|---|---|---|---|---|---|
| C | H | O | N | S | |
| AC0 | 91.16 | 0.99 | 4.77 | 0.55 | 0.31 |
| ACN | 89.74 | 1.04 | 6.51 | 0.75 | 0.25 |
| ACNH | 95.60 | 0.62 | 2.26 | 0.76 | 0.25 |
Table 2 Elemental analysis of the AC samples
| Sample | Mass fraction(%) | ||||
|---|---|---|---|---|---|
| C | H | O | N | S | |
| AC0 | 91.16 | 0.99 | 4.77 | 0.55 | 0.31 |
| ACN | 89.74 | 1.04 | 6.51 | 0.75 | 0.25 |
| ACNH | 95.60 | 0.62 | 2.26 | 0.76 | 0.25 |
| Sample | Peak area distribution(%) | ||||
|---|---|---|---|---|---|
| C1(Peak Ⅰ) | C2(Peak Ⅱ) | C3(Peak Ⅲ) | C4(Peak Ⅳ) | C5(Peak Ⅴ) | |
| AC0 | 67.01 | 17.32 | 3.73 | 5.28 | 6.66 |
| ACN | 63.87 | 19.77 | 3.37 | 6.69 | 6.30 |
| ACNH | 63.58 | 19.55 | 5.24 | 3.49 | 8.13 |
Table 3 Deconvolution of the C1s XPS profiles of AC samples
| Sample | Peak area distribution(%) | ||||
|---|---|---|---|---|---|
| C1(Peak Ⅰ) | C2(Peak Ⅱ) | C3(Peak Ⅲ) | C4(Peak Ⅳ) | C5(Peak Ⅴ) | |
| AC0 | 67.01 | 17.32 | 3.73 | 5.28 | 6.66 |
| ACN | 63.87 | 19.77 | 3.37 | 6.69 | 6.30 |
| ACNH | 63.58 | 19.55 | 5.24 | 3.49 | 8.13 |
| Sample | Peak area distribution(%) | ||||
|---|---|---|---|---|---|
| O1(PeakⅠ) | O2(Peak Ⅱ) | O3(Peak Ⅲ) | O4(Peak Ⅳ) | O2ads+H2Oads(Peak Ⅴ) | |
| AC0 | 21.42 | 34.73 | 30.59 | 11.62 | 1.65 |
| ACN | 18.26 | 37.85 | 24.26 | 17.02 | 2.61 |
| ACNH | 27.56 | 20.14 | 31.69 | 18.69 | 1.91 |
Table 4 Deconvolution of the O1s XPS profiles of AC samples
| Sample | Peak area distribution(%) | ||||
|---|---|---|---|---|---|
| O1(PeakⅠ) | O2(Peak Ⅱ) | O3(Peak Ⅲ) | O4(Peak Ⅳ) | O2ads+H2Oads(Peak Ⅴ) | |
| AC0 | 21.42 | 34.73 | 30.59 | 11.62 | 1.65 |
| ACN | 18.26 | 37.85 | 24.26 | 17.02 | 2.61 |
| ACNH | 27.56 | 20.14 | 31.69 | 18.69 | 1.91 |
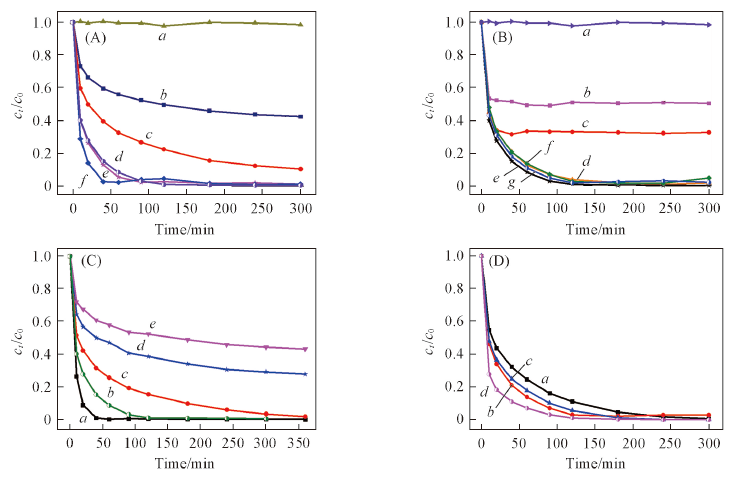
Fig.7 Effects of dosage of ACNH (A), mole ratio of PS/phenol(B), the initial concentration of phenol(C) and the initial pH in the ACNH/PS system(D)(A) Dosage of ACNH/(g·L-1): a. 0; b. 0.16; c. 0.28; d. 0.4; e. 0.48; f. 0.6; (B) molar ratio of PS/phenol: a. PS; b. 0.5∶1; c. 1∶1; d. 2∶1; e. 3∶1; f. 7∶1; g. 15∶1; (C) initial concentration of phenol/(mg·L-1): a. 50; b. 80; c. 100; d. 150; e. 200; (D) pH: a. 3; b. 6; c. 9; d. 11.
| Sample | SBET/(m2·g-1) | Microporous volume/(cm3·g-1) | Pore size/nm | Micropore area/(m2·g-1) |
|---|---|---|---|---|
| Fresh ACNH | 631 | 0.14 | 3.05 | 348.87 |
| Used ACNH | 275 | 0.04 | 4.37 | 90.58 |
| Regenerated ACNH | 435 | 0.07 | 3.89 | 146.98 |
Table 5 Physical properties of fresh ACNH, used ACNH and regenerated ACNH
| Sample | SBET/(m2·g-1) | Microporous volume/(cm3·g-1) | Pore size/nm | Micropore area/(m2·g-1) |
|---|---|---|---|---|
| Fresh ACNH | 631 | 0.14 | 3.05 | 348.87 |
| Used ACNH | 275 | 0.04 | 4.37 | 90.58 |
| Regenerated ACNH | 435 | 0.07 | 3.89 | 146.98 |
| [1] | Neyens E., Baeyens J., J. Hazard. Mater., 2003, 98(1—3), 33—50 |
| [2] | Pera-Titus M., García-Molina V., Baños M. A., Giménez J., Esplugas S., Appl. Catal. B: Environ., 2004, 47(4), 219—256 |
| [3] | Klavarioti M., Mantzavinos D., Kassinos D., Environ. Int., 2009, 35(2), 402—417 |
| [4] | Feng L., van Hullebusch E. D., Rodrigo M. A., Esposito G., Oturan M. A., Chem. Eng. J., 2013, 228, 944—964 |
| [5] | Yang S., Wang P., Yang X., Wei G., Zhang W., Shan L., J. Environ. Sci.-China., 2009, 21(9), 1175—1180 |
| [6] | Neta P., Huie R. E., Ross A. B., J. Phys. Chem. Ref. Data,1988, 17(3), 1027—1284 |
| [7] | Tan C., Gao N., Deng Y., An N., Deng J., Chem. Eng. J., 2012, 203, 294—300 |
| [8] | Lau T. K., Chu W., Graham N. J., Environ. Sci. Technol., 2007, 41(2), 613—619 |
| [9] | An D., Westerhoff P., Zheng M., Wu M., Yang Y., Chiu C. A., Water Res., 2015, 73, 304—310 |
| [10] | Furman O. S., Teel A. L., Watts R. J., Environ. Sci. Technol., 2010, 44(16), 6423—6428 |
| [11] | Anipsitakis G. P., Dionysiou D. D., Environ. Sci. Technol., 2004, 38(13), 3705—3712 |
| [12] | Oh S. Y., Kang S. G., Chiu P. C., Sci. Total Environ., 2010, 408(16), 3464—3468 |
| [13] | Yang S., Yang X., Shao X., Niu R., Wang L., J. Hazard. Mater., 2011, 186(1), 659—666 |
| [14] | Yu G. X., Chen H., Lu S. X., Zhu Z. N., J. Fuel Chem. Technol., 2005, 33(5), 566—570 |
| (余国贤,陈辉,陆善祥,朱中南.燃料化学学报, 2005, 33(5), 566—570) | |
| [15] | Georgi A., Kopinke F. D., Appl. Catal. B: Environ., 2005, 58(1), 9—18 |
| [16] | Oliveira L. C., Silva C. N., Yoshida M. I., Lago R. M., Carbon,2004, 42(11), 2279—2284 |
| [17] | Szymański G. S., Catal. Today, 2008, 137(2), 460—465 |
| [18] | Boehm H., Carbon, 2002, 40(2), 145—149 |
| [19] | Jaramillo J., Álvarez P., Gómez-Serrano V., Appl. Surf. Sci., 2010, 256(17), 5232—5236 |
| [20] | Qiao W., Korai Y., Mochida I., Hori Y., Maeda T., Carbon,2002, 40(3), 351—358 |
| [21] | Li N., Zhu J., Zha Q. F., Chem. J. Chinese Universities,2012, 33(3), 548—554 |
| (李娜, 朱健, 查庆芳.高等学校化学学报, 2012, 33(3), 548—554) | |
| [22] | Cao H., Xing L., Wu G., Xie Y., Shi S., Zhang Y., Minakata D., Crittenden J. C., Appl. Catal. B: Environ., 2014, 146, 169—176 |
| [23] | Ania C., Parra J., Pis J., Fuel Process. Technol., 2002, 79(3), 265—271 |
| [24] | Lopez-Ramon M., Stoeckli F., Moreno-Castilla C., Carrasco-Marin F., Carbon,1999, 37(8), 1215—1221 |
| [25] | Montes-Moran M., Suarez D., Menéndez J., Fuente E., Carbon,2004, 42(7), 1219—1225 |
| [26] | Barroso-Bogeat A. N., Alexandre-Franco M., Fern dez-Gonz lez C., Goómez-Serrano V., Energ.Fuel,2014, 28(6), 4096—4103 |
| [27] | Papirer E., Li S., Donnet J. B., Carbon,1987, 25(2), 243—247 |
| [28] | Biniak S., Szymański G., Siedlewski J., swiatkowski A., Carbon, 1997, 35(12), 1799—1810 |
| [29] | Figueiredo J. L., Pereira M. F. R., Catal.Today,2010, 150(1), 2—7 |
| [30] | Yang S., Li L., Xiao T., Zheng D., Zhang Y., Appl. Surf. Sci., 2016, 383, 142—150 |
| [31] | Shafeeyan M. S., Daud W. M. A. W., Houshmand A., Shamiri A., J. Anal. Appl. Pyrol., 2010, 89(2), 143—151 |
| [32] | Sun H., Kwan C., Suvorova A., Ang H. M., Tadé M. O., Wang S., Appl. Catal. B: Environ., 2014, 154, 134—141 |
| [33] | Zielke U., Hüttinger K., Hoffman W., Carbon,1996, 34(8), 983—998 |
| [34] | Zhang S. Y., Lü J. F., Yue G. X., Wang Y., J. Environ.Sci. ,2003, 23(3), 317—321 |
| (张守玉, 吕俊复, 岳光溪, 王洋.环境科学学报, 2003, 23(3), 317—321) | |
| [35] | Suárez D., Menéndez J. A., Fuente E., Montes-Morán M. A., Langmuir,1999, 15(11), 3897—3904 |
| [36] | Suárez D., Menéndez J. A., Montes-Morán M. A., Angew. Chem., 2000, 112(7), 1376—1379 |
| [37] | Kong X. K., Sun Z. Y., Chen M., Chen Q. W., Energ. Environ. Sci., 2013, 6(11), 3260—3266 |
| [38] | Kong X. K., Chen C. L., Chen Q. W., Chem. Soc. Rev., 2014, 43(8), 2841—2857 |
| [39] | Sun H., Wang Y., Liu S., Ge L., Wang L., Zhu Z., Wang S., Chem. Commun., 2013, 49(85), 9914—9916 |
| [1] | ZHAO Sheng, HUO Zhipeng, ZHONG Guoqiang, ZHANG Hong, HU Liqun. Preparation of Modified Gadolinium/Boron/Polyethylene Nanocomposite and Its Radiation Shielding Performance for Neutron and Gamma-ray [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220039. |
| [2] | SHI Ying, HU Guangjian, WU Minjie, LI Feng. Applications of Low Temperature Plasma for the Materials in Li-ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(5): 1315. |
| [3] | JI Shaobo, CHEN Xiaodong. Surface and Interface Chemistry in Flexible Electronics [J]. Chem. J. Chinese Universities, 2021, 42(4): 1074. |
| [4] | YAN Fanyong, SUN Zhonghui, PANG Jiping, JIANG Yingxia, CHEN Yuan. Functionalized Carbon Dots of Benzothiazine Derivatives for Detection of Quercetin in Ginkgo Biloba Tea [J]. Chem. J. Chinese Universities, 2020, 41(8): 1768. |
| [5] | PENG Xinyan, LIU Yunhong, LI Jiawen, FENG Yilong, WANG Hanchun. Preparation and Characterization of Tannin/Zwitterionic Modified Oil-water Separation Membrane † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1337. |
| [6] | CHEN Liangdan,ZOU Wei,WU Liang,XIA Fanjie,HU Zhiyi,LI Yu,SU Baolian. Nano-Al2O3 Coated Li-rich Cathode Material Li1. 2Ni0.13Co0.13Mn0.54O2 for Highly Improved Lithium-ion Batteries † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1329. |
| [7] | LIU Shuaizhuo,ZHANG Qian,LIU Ning,XIAO Wenyan,FAN Leiyi,ZHOU Ying. One-step Synergistic Hydrophobic Modification of Melamine Sponge and Its Application † [J]. Chem. J. Chinese Universities, 2020, 41(3): 521. |
| [8] | ZHANG Xinyu, WANG Hong, FANG Yun, FAN Ye. Stimuli-responsive Fe3O4 Nanoparticle Modified by Conjugated Linoleic Acid [J]. Chem. J. Chinese Universities, 2020, 41(11): 2519. |
| [9] | MAO Long, LIU Yuejun, FAN Shuhong. Preparation and Properties of Polypyrrole Modified Layered Clay/poly(ε-caprolactone) Antibacterial Nanocomposites [J]. Chem. J. Chinese Universities, 2019, 40(8): 1726. |
| [10] | ZHOU Hai, CHEN Hao, GUO Ya, KANG Min. Synthesis of Meso-porous Co3O4 Polyhedra and Their Electrochemical Properties† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1374. |
| [11] | SUI Jiayang, LIU Xiaoyang, QIAN Miaomiao, ZHU Yanchao, XUE Beichen, FENG Yi, TIAN Yumei, WANG Xiaofeng. Surface Modification of Silica/carbon Black Derived from Rice Husks and Its Influence on Natural Rubber Composites† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1561. |
| [12] | LI Xiangnan,YU Mingming,FAN Yong,WANG Qiuxian,ZHANG Huishuang,YANG Shuting. Study on Electrochemical Performances of N-doped P/C Composite as Anode Material of Lithium Ion Batteries † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2360. |
| [13] | LIU Yanhua, JIN Lu, XUE Beichen, GUO Yupeng. Preparation and Electrochemical Properties of Rice Husk Based Activated Carbon Modified by Pitch† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1242. |
| [14] | GAO Jun, HU Hui, LIU Xueyan. Preparation and Evaluation of Modified Cyanobacteria-derived Activated Carbon for CO2 Adsorption† [J]. Chem. J. Chinese Universities, 2018, 39(2): 284. |
| [15] | WANG Danfeng, YANG Haiyan, NING Yuesheng, ZHAO Binyuan. Morphosynthesis of Porous Silver Cubes on the Surface of Hydrogen-pretreated Monolithic Activated Carbon [J]. Chem. J. Chinese Universities, 2017, 38(9): 1503. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||