

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (9): 1660.doi: 10.7503/cjcu20160337
• Physical Chemistry • Previous Articles Next Articles
ZHANG Hui1,2,*( ), ZHANG Hongmei1, WANG Lianjun1, SHEN Jinyou1
), ZHANG Hongmei1, WANG Lianjun1, SHEN Jinyou1
Received:2016-05-12
Online:2016-09-10
Published:2016-08-26
Contact:
ZHANG Hui
E-mail:zhanghui13401@163.com
Supported by:CLC Number:
TrendMD:
ZHANG Hui, ZHANG Hongmei, WANG Lianjun, SHEN Jinyou. Density Functional Theory Studies on the CO2 Absorption by 1-Ethylamine-3-methylimidazolium Tetrafluoroborate†[J]. Chem. J. Chinese Universities, 2016, 37(9): 1660.
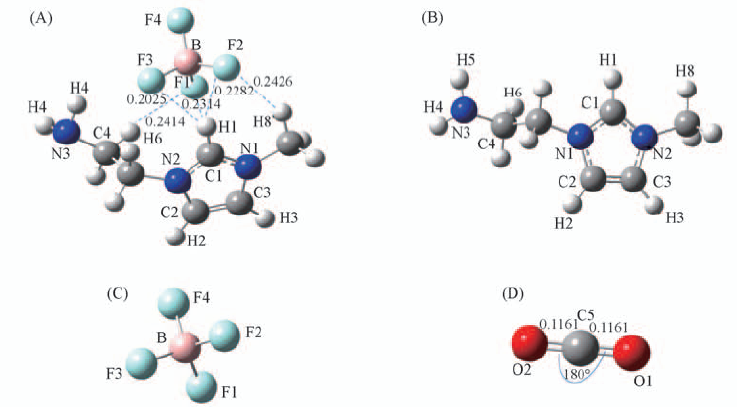
Fig.1 Optimized geometric configurations and some geometrical parameters of [NH2e-mim][BF4](A),[NH2e-mim]+(B), [BF4]-(C) and CO2(D) Bond length or distance/nm, angle/(°).
| Species | [NH2e-mim][BF4] | [NH2e-mim]+ | [BF4]- | CO2 |
|---|---|---|---|---|
| C1—H1 | 0.1078 | 0.1078 | ||
| C4—H6 | 0.1095 | 0.1103 | ||
| N3—H5 | 0.1018 | 0.1013 | ||
| N3—H4 | 0.1014 | 0.1011 | ||
| N3—C4 | 0.1462 | 0.1453 | ||
| C4—H6 | 0.1095 | 0.1103 | ||
| B—F1 | 0.1434 | 0.1413 | ||
| B—F2 | 0.1421 | 0.1414 | ||
| B—F3 | 0.1436 | 0.1413 | ||
| B—F4 | 0.1369 | 0.1414 | ||
| C5—O1 | 0.1161 | |||
| C5—O2 | 0.1161 | |||
| ∠H1—C1—N1 | 126.05 | 125.51 | ||
| ∠H1—C1—N2 | 124.89 | 125.47 | ||
| ∠H4—N3—H5 | 106.88 | 107.88 | ||
| ∠O2—C5—O1 | 180 | |||
| ∠H1—C1—N1—C3 | 176.57 | 179.60 | ||
| ∠N1—C1—N2—C2 | -0.490 | -0.24 | ||
| ∠N1—C3—C2—N2 | -0.37 | -0.17 |
Table 1 Bond lengths(nm) and bond angles(°) of optimized configurations of [NH2e-mim]BF4,[NH2e-mim]+, [BF4]- and CO2
| Species | [NH2e-mim][BF4] | [NH2e-mim]+ | [BF4]- | CO2 |
|---|---|---|---|---|
| C1—H1 | 0.1078 | 0.1078 | ||
| C4—H6 | 0.1095 | 0.1103 | ||
| N3—H5 | 0.1018 | 0.1013 | ||
| N3—H4 | 0.1014 | 0.1011 | ||
| N3—C4 | 0.1462 | 0.1453 | ||
| C4—H6 | 0.1095 | 0.1103 | ||
| B—F1 | 0.1434 | 0.1413 | ||
| B—F2 | 0.1421 | 0.1414 | ||
| B—F3 | 0.1436 | 0.1413 | ||
| B—F4 | 0.1369 | 0.1414 | ||
| C5—O1 | 0.1161 | |||
| C5—O2 | 0.1161 | |||
| ∠H1—C1—N1 | 126.05 | 125.51 | ||
| ∠H1—C1—N2 | 124.89 | 125.47 | ||
| ∠H4—N3—H5 | 106.88 | 107.88 | ||
| ∠O2—C5—O1 | 180 | |||
| ∠H1—C1—N1—C3 | 176.57 | 179.60 | ||
| ∠N1—C1—N2—C2 | -0.490 | -0.24 | ||
| ∠N1—C3—C2—N2 | -0.37 | -0.17 |
| Species | ZPE/(kJ·mol-1) | G/(kJ·mol-1) | H/(kJ·mol-1) |
|---|---|---|---|
| CO2 | 31.51 | -495300.58 | -495237.56 |
| [BF4]- | 39.38 | -1114947.46 | -1114860.81 |
| [NH2e-mim]+ | 488.34 | -1049798.30 | -1049674.90 |
| [NH2e-mim]+[BF4]- | 530.35 | -2165081.82 | -2164924.29 |
| [NHe-mim] | 441.08 | -1048496.05 | -1048375.28 |
| [NHe-mim][BF4]- | 493.59 | -2163359.49 | -2163199.33 |
| [NH3e-mim]2+ | 519.85 | -1050530.81 | -1050257.76 |
| [NH3e-mim]2+[BF4]- | 567.11 | -2166058.50 | -2165900.97 |
| R1(2[NH2e-mim]+[BF4]-+CO2) | 1100.08 | -4824962.74 | -4824584.67 |
| IM1a | 1110.59 | -4825138.65 | -4824834.09 |
| TS1b | 1100.08 | -4825096.64 | -4824857.72 |
| IM2c | 1102.71 | -4825427.45 | -4825149.15 |
| P1d | 1113.21 | -4825411.70 | -4825138.65 |
| R2([NH2e-mim]+[BF4]-+CO2) | 564.48 | -2660461.16 | -2660227.49 |
| TS2e | 556.61 | -2660253.74 | -2660075.21 |
| P2f | 574.98 | -2660324.63 | -2660146.10 |
Table 2 Thermodynamic parameters of optimized configurations in [NH2e-mim]BF4-CO2 reaction system
| Species | ZPE/(kJ·mol-1) | G/(kJ·mol-1) | H/(kJ·mol-1) |
|---|---|---|---|
| CO2 | 31.51 | -495300.58 | -495237.56 |
| [BF4]- | 39.38 | -1114947.46 | -1114860.81 |
| [NH2e-mim]+ | 488.34 | -1049798.30 | -1049674.90 |
| [NH2e-mim]+[BF4]- | 530.35 | -2165081.82 | -2164924.29 |
| [NHe-mim] | 441.08 | -1048496.05 | -1048375.28 |
| [NHe-mim][BF4]- | 493.59 | -2163359.49 | -2163199.33 |
| [NH3e-mim]2+ | 519.85 | -1050530.81 | -1050257.76 |
| [NH3e-mim]2+[BF4]- | 567.11 | -2166058.50 | -2165900.97 |
| R1(2[NH2e-mim]+[BF4]-+CO2) | 1100.08 | -4824962.74 | -4824584.67 |
| IM1a | 1110.59 | -4825138.65 | -4824834.09 |
| TS1b | 1100.08 | -4825096.64 | -4824857.72 |
| IM2c | 1102.71 | -4825427.45 | -4825149.15 |
| P1d | 1113.21 | -4825411.70 | -4825138.65 |
| R2([NH2e-mim]+[BF4]-+CO2) | 564.48 | -2660461.16 | -2660227.49 |
| TS2e | 556.61 | -2660253.74 | -2660075.21 |
| P2f | 574.98 | -2660324.63 | -2660146.10 |
| Reaction | ΔG 0—/(kJ·mol-1) | ΔH 0—/(kJ·mol-1) |
|---|---|---|
| [NH2e-mim]+[BF4]- | 332.65 | 385.16 |
| 2[NH2e-mim]+→[NHe-mim] + [NH3e-mim]2+ | 553.98 | 701.01 |
| [NHe-mim] + [BF4]-→[NHe-mim][BF4]- | 97.93 | 49.88 |
| [NH2e-mim]++[BF4]-→[NH2e-mim]+[BF4]- | -332.65 | -385.16 |
| [NH3e-mim]2++[BF4]-→[NH3e-mim]2+[BF4]- | -571.57 | -773.73 |
| 2[NH2e-mim]+[BF4]-+CO2→IM1 | -175.91 | -248.11 |
| 2[NH2e-mim]+[BF4]-+CO2→TS1 | -133.90 | -272.79 |
| 2[NH2e-mim]+[BF4]-+CO2→TS2 | -463.74 | -565.22 |
| 2[NH2e-mim]+[BF4]-+CO2→P1 | -448.96 | -553.19 |
| [NH2e-mim]+[BF4]-+CO2→TS2 | 207.41 | 152.28 |
| [NH2e-mim]+[BF4]-+CO2→P2 | 136.53 | 81.39 |
Table 3 ΔG 0— and ΔH 0— for each reaction
| Reaction | ΔG 0—/(kJ·mol-1) | ΔH 0—/(kJ·mol-1) |
|---|---|---|
| [NH2e-mim]+[BF4]- | 332.65 | 385.16 |
| 2[NH2e-mim]+→[NHe-mim] + [NH3e-mim]2+ | 553.98 | 701.01 |
| [NHe-mim] + [BF4]-→[NHe-mim][BF4]- | 97.93 | 49.88 |
| [NH2e-mim]++[BF4]-→[NH2e-mim]+[BF4]- | -332.65 | -385.16 |
| [NH3e-mim]2++[BF4]-→[NH3e-mim]2+[BF4]- | -571.57 | -773.73 |
| 2[NH2e-mim]+[BF4]-+CO2→IM1 | -175.91 | -248.11 |
| 2[NH2e-mim]+[BF4]-+CO2→TS1 | -133.90 | -272.79 |
| 2[NH2e-mim]+[BF4]-+CO2→TS2 | -463.74 | -565.22 |
| 2[NH2e-mim]+[BF4]-+CO2→P1 | -448.96 | -553.19 |
| [NH2e-mim]+[BF4]-+CO2→TS2 | 207.41 | 152.28 |
| [NH2e-mim]+[BF4]-+CO2→P2 | 136.53 | 81.39 |
| [1] | Ahmed D., Ludwik A., Guido M., J. Phys. Chem. A, 2000, 104, 2112—2119 |
| [2] | Vincenzo B., J. Phys. Chem. A, 2004, 108, 4146—4150 |
| [3] | Schlu1cker S., Singh R. K., Asthana B. P., Popp J. Kiefer W., J. Phys. Chem. A,2001, 105, 9983—9989 |
| [4] | Wu Y., Xue Y., Xie D. Q., Yan G. S., J. Org. Chem., 2005, 70, 5045—5054 |
| [5] | Liu T. T., Lu X., Zhang M. T., Chem. Res. Chinese Universities,2014, 30(4), 656—660 |
| [6] | Wang P. C., Lu M., Chem. J. Chinese Universities,2014, 35(3), 596—601 |
| (王鹏程, 陆明. 高等学校化学学报,2014, 35(3), 596—601) | |
| [7] | Li Y., Jian L., Chem. Res. Chinese Universities,2014, 30(6), 997—1004 |
| [8] | Yu Y. X., Phys. Chem. Chem. Phys., 2013, 15, 16819—16827 |
| [9] | Wu D. L., Jiang W., Liu X. Q., Qiu N. X., Xue Y., Chem. Res. Chinese Universities,2016, 32(1), 118—126 |
| [10] | Gholizadeh R., Yu Y. X., Appl. Surf. Sci., 2015, 357, 1187—1195 |
| [11] | Li X. L., Chen J. J., Luo M., Chen X. Y., Li P. P., Acta Phys. -Chim. Sin., 2010, 26(5), 1364—1372 |
| [12] | Zhang Y., Chen X. Y., Wang H. J., Diao K. S., Chen J. M., J. Mol. Struc-Theochem., 2010, 952, 16—24 |
| [13] | Sun H., Zhou X.Q., Xue Z. M., Zhou Z. Y., Mu T. C., Int. J. Greenh. Gas Con., 2014, 20, 43—48 |
| [14] | Yu Y. X., ACS Appl. Mater. Interfaces, 2014, 6, 16267—16275 |
| [15] | Yu Y. X., J. Mater. Chem. A, 2014, 2, 8910—8917 |
| [16] | Magalhaes A. L., Madail S. R., Ramos M. J., Theor. Chem. Acc., 2000, 105, 68—76 |
| [17] | Loos P. F., Assfeld X., Rivail J. L., Theor. Chem. Acc., 2007, 118, 165—171 |
| [18] | Sun X. L., Huo R. P., Bu X. Y., Li J. L., Chem. J. Chinese Universities,2015, 36(8), 1570—1575 |
| (孙小丽, 霍瑞萍, 步宇翔, 李吉来. 高等学校化学学报,2015, 36(8), 1570—1575) | |
| [19] | Song Z. X., Wang H. J., Xing L. J., J. Struct. Chem., 2009, 20, 509—515 |
| [20] | Wang C. M., Zheng J. J., Cui G. K., Luo X. Y., Guo Y., Li H. R., Chem. Commun., 2013, 49, 1166—1168 |
| [21] | Gao H. Y., Zhang Y., Wang H. J., Liu J. H., Chen J. M., J. Phys. Chem., 2010, 114, 10243—10252 |
| [22] | Christopher A. Z., Gary S. G., Michael T. B., James D., Tetsuya T., Rika H., J. Am. Soc. Mass Spectrom., 2015, 26, 1559—1569 |
| [23] | Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J., R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam N. J., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E. Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision A. 1, Gaussian Inc., Wallingford CT, 2009 |
| [24] | Herzberg G., Electronic Spectra of Polyatomic Molecules, Van Nostrand, New York, 1996 |
| [25] | Babarao R., Dai S., J. Phys. Chem. B,2011, 115(32), 9789—9794 |
| [26] | Liu K. H., Pu M., Li H. Y., Chen B. H., Chin. J. Chem. Phys., 2005, 18(3), 332—335 |
| (刘坤辉, 蒲敏, 李会英, 陈标华. 化学物理学报, 2005, 18(3), 332—335 ) | |
| [27] | Yu G. R., Zhang S. J., Yao X. Q., Zhang J. M., Dong K., Dai W. B., Mori R. H., Ind. Eng. Chem. Res., 2006, 45(8), 2875—2880 |
| [28] | Katsyuba S. A., Griaznova T. P., Vidiš A., Dyson P. J., J. Phys. Chem. B,2009, 113, 5046—5051 |
| [29] | Bates E. D., Mayton R. D., Ntai I., Davis J. H., J. Am. Chem. Soc., 2002, 124(6), 926—927 |
| [30] | Zhang J. M., Zhang S. J., Dong K., Zhang Y. Q, Shen Y. Q., Lv X. M., Chem. Eur. J., 2006, 12(15), 4021—4026 |
| [31] | Gurkan B. E., de la Fuente J. C., Mindrup E. M., Ficke L. E., Goodrich B. F., Price E. A., Schneider W. F., Brennecke J. F., J. Am. Chem. Soc., 2010, 132(7), 2116—2117 |
| [32] | Zhang Y. Q., Zhang S. J., Lu X. M., Zhou Q., Fan W., Zhang X. P., Chem. Eur. J., 2009, 15, 3003—3011 |
| [33] | Zhang J. M., Zhang S. J., Dong K., Zhang Y. Q., Shen Y. Q., Lv X. M., Chem. Eur. J., 2006, 15, 4021—4026 |
| [34] | Li X. Y., Hou M. Q., Zhang Z. F., Han B. X., Yang G. Y., Wang X. L., Zou L. Z., Green Chem., 2008, 10, 879—884 |
| [1] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [2] | YANG Jingyi, SHI Siqi, PENG Huaitao, YANG Qihao, CHEN Liang. Integration of Atomically Dispersed Ga Sites with C3N4 Nanosheets for Efficient Photo-driven CO2 Cycloaddition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220349. |
| [3] | WANG Xintian, LI Pan, CAO Yue, HONG Wenhao, GENG Zhongxuan, AN Zhiyang, WANG Haoyu, WANG Hua, SUN Bin, ZHU Wenlei, ZHOU Yang. Techno-economic Analysis and Industrial Application Prospects of Single-atom Materials in CO2 Catalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220347. |
| [4] | CUI Wei, ZHAO Deyin, BAI Wenxuan, ZHANG Xiaodong, YU Jiang. CO2 Absorption in Composite of Aprotic Solvent and Iron-based Ionic Liquid [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220120. |
| [5] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [6] | WANG Zhengwen, GAO Fengxiang, CAO Han, LIU Shunjie, WANG Xianhong, WANG Fosong. Synthesis and Property of CO2 Copolymer⁃based UV-curable Polymer [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220236. |
| [7] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| [8] | XIA Wu, REN Yingyi, LIU Jing, WANG Feng. Chitosan Encapsulated CdSe QDs Assemblies for Visible Light-induced CO2 Reduction in an Aqueous Solution [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220192. |
| [9] | ZHANG Xinxin, XU Di, WANG Yanqiu, HONG Xinlin, LIU Guoliang, YANG Hengquan. Effect of Mn Promoter on CuFe-based Catalysts for CO2 Hydrogenation to Higher Alcohols [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220187. |
| [10] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [11] | DING Yang, WANG Wanhui, BAO Ming. Recent Progress in Porous Framework-immobilized Molecular Catalysts for CO2 Hydrogenation to Formic Acid [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220309. |
| [12] | WANG Ruhan, JIA Shunhan, WU Limin, SUN Xiaofu, HAN Buxing. CO2-involved Electrochemical C—N Coupling into Value-added Chemicals [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220395. |
| [13] | ZHAO Yingzhe, ZHANG Jianling. Applications of Metal-organic Framework-based Material in Carbon Dioxide Photocatalytic Conversion [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220223. |
| [14] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [15] | JI Shuangqi, JIN Zhao, GUAN Wenna, PAN Xiangyu, GUAN Tong. Preparation and Chromatographic Performance of Mixed-mode Silica Stationary Phase Modified by Double Cationic Ionic Liquid and Octadecyl Group [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220008. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||