

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (9): 1643.doi: 10.7503/cjcu20160237
• Organic Chemistry • Previous Articles Next Articles
ZHENG Xi, LIANG Guodong, WANG Chao*( ), LIU Keliang*(
), LIU Keliang*( )
)
Received:2016-04-13
Online:2016-09-10
Published:2016-08-26
Contact:
WANG Chao,LIU Keliang
E-mail:chaow301@gmail.com;keliangliu55@126.com
Supported by:CLC Number:
TrendMD:
ZHENG Xi, LIANG Guodong, WANG Chao, LIU Keliang. Construction of Isopeptide Bridge-tethered NHR-trimeric Coiled-coil in MERS-CoV Membrane Fusion Protein†[J]. Chem. J. Chinese Universities, 2016, 37(9): 1643.
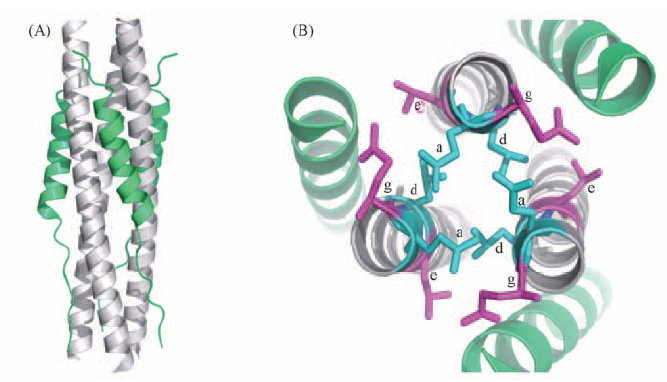
Scheme 1 Structure of 6-helix bundle(6-HB) (A) Side view of MERS-CoV S2 subunit 6-HB structure(PDB: 4mod), peripheral helix present the C-terminal heptad repeat(CHR) in MERS-CoV, while the central helix trimer are N-terminal heptad repeat(NHR); (B) top view of 6-HB show the amino acid in a, d site(cyan) and e, g site.
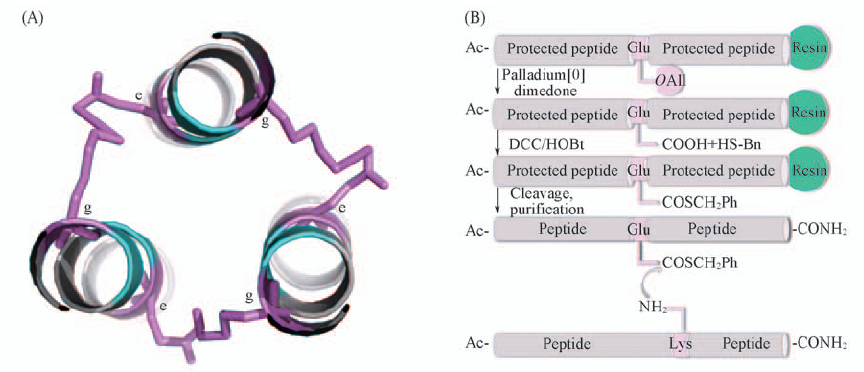
Scheme 2 N-helix trimer stabled by isopeptide (A) Isopeptide bond was formed between lysine in e site and glutamic acid in g' site of adjacent peptide chain; (B) schematic representation of isopeptide bond formation via an interhelical acyl transfer reaction.
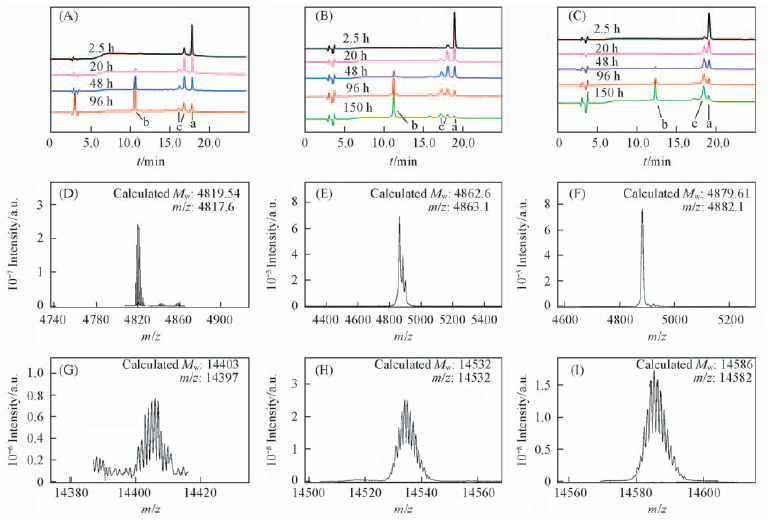
Fig.1 RP-HPLC traces(A—C) and mass spectrometry(D—I) of isopeptide bond forming reaction RP-HPLC traces for the acyl transfer reaction of (IZN21L)3(A), (IZN21M)3(B) and (IZN21R)3(C) from t=2.5 to 150 h. Absorbance was monitored at 210 nm. (A—C): a. The reaction material; b. the reaction product; c. by-products. Mass spectrometry of monomer peptide, the reaction materal of (IZN21L)3(D), (IZN21M)3(E), (IZN21R)3(F) and reaction products, the trimeric peptide (IZN21L)3(G), (IZN21M)3(H) and (IZN21R)3(I).
| Name | Sequence* |
|---|---|
| e f g a b c de f g | |
| HR1P(998—1039) | ANK FNQALGA MQTGFTTTNEAFQKVQDAVNNNAQALSKLASE |
| (IZN21L)3 | (IZm---ANK FNQALGA MQTGFTTTNEA)3 |
| (IZN21M)3 | (IZm-----------------LGA MQTGFTTTNEAFQKVQDA)3 |
| (IZN21R)3 | (IZm-------------------------------------------FQKVQDAVNNNAQALSKLASE)3 |
Table 1 Designed peptide sequences derived from HR1P
| Name | Sequence* |
|---|---|
| e f g a b c de f g | |
| HR1P(998—1039) | ANK FNQALGA MQTGFTTTNEAFQKVQDAVNNNAQALSKLASE |
| (IZN21L)3 | (IZm---ANK FNQALGA MQTGFTTTNEA)3 |
| (IZN21M)3 | (IZm-----------------LGA MQTGFTTTNEAFQKVQDA)3 |
| (IZN21R)3 | (IZm-------------------------------------------FQKVQDAVNNNAQALSKLASE)3 |
| [1] | Zaki A. M., Van B. S., Bestebroer T. M., Osterhaus A. D., Fouchier R. A., N. Engl. J. Med., 2012, 367(19), 1814—1820 |
| [2] | Price S. M., Miazgowicz K. L., Munster V. J.,Path. Dis., 2014, 71(2), 121—136 |
| [3] | Bermingham A., Chand M. A., Brown C. S., Aarons E., Tong C., Langrish C., Hoschler K., Brown K., Galiano M., Myers R., Pebody R. G., Green H. K., Boddington N. L., Gopal R., Price N., Newsholme W., Drosten C., Fouchier R. A., Zambon M., Euro. Surveill., 2012, 17(40), 20290 |
| [4] | Abdel-Moneim A. S., Arch. Virol., 2014, 159(7), 1575—1584 |
| [5] | Memish Z. A., Zumla A. I., Assiri A., N. Engl. J. Med., 2013, 369(9), 884—886 |
| [6] | World Health Organization(WHO), Outbreaks and Emergencies-Middle East Respiratory Syndrome Coronavirus(MERS-CoV). |
| [7] | Drosten C., Günther S., Preiser W., van der Werf S., Brodt H. R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R. A. M., Berger A., Burguiere A. M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J. C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H. D., Osterhaus A., Schmitz H., Doerr H. W.,N. Engl. J. Med., 2003, 348(20), 1967—1976 |
| [8] | Majumder M. S., Kluberg S. A., Mekaru S. R., Brownstein J. S., Emerg. Infect. Dis., 2015, 21(11), 2088—2090 |
| [9] | Chan J. F., Chan K. H., Kao R. Y., To K. K., Zheng B. J., Li C. P. Y., Li P. T. W., Dai J., Mok F. K. Y., Chen H., Hayden F. G., Yuen K., J. Infection,2013, 67(6), 606—616 |
| [10] | Kilianski A., Baker S. C., Antivir. Res., 2014, 101(1), 105—112 |
| [11] | Chen Y., Rajashankar K. R., Yang Y., Agnihothram S. S., Liu C., Lin Y. L., Baric R. S., Li F., J. Virol., 2013, 87(19), 10777—10783 |
| [12] | Gao J., Lu G. W., Qi J. X., Li Y., Wu Y., Deng Y., Geng H. Y., Li H. B., Wang Q. H., Xiao H. X., Tan W. J., Yan J. H., Gao G. F., J. Virol., 2013, 87(24), 13134—13140 |
| [13] | Lu L., Liu Q., Zhu Y., Chan K. H., Qin L., Li Y., Wang Q., Chan J. F. W., Du L. Y., Yu F., Ma C. Q., Ye S., Yuen K. Y., Zhang R. G., Jiang S. B., Nat. Commun., 2014, 5(5), 3067—3078 |
| [14] | Chan J. F. W., Chan K. H., Choi G. K. Y., To K. K. W., Tse H., Cai J. P., Yeung M. L., Cheng V. C., Chen H., Che X. Y., Lau S. K., Woo P. C., Yuen K. Y., J. Infect. Dis., 2013, 207(11), 1743—1752 |
| [15] | Lu G. W., Hu Y. W., Wang Q. H., Qi J. X., Gao F., Li Y., Zhang Y. F., Zhang W., Yuan Y., Bao J. K., Zhang B. C., Shi Y., Yan J. H., Gao G. F., Nature,2013, 500(7461), 227—231 |
| [16] | Gierer S., Bertram S., Kaup F., Wrensch F., Heurich A., Krämer-Kühl A., Welsch K., Winkler M., Meyer B., Drosten C., Dittmer U., von Hahn T., Simmons G., Hofmann H., Poehlmann S., J. Virol., 2013, 87(10), 5502—5511 |
| [17] | Masters P. S., Adv. Virus Res., 2006, 66, 193—292 |
| [18] | Lu M., Blacklow S. C., Kim P. S., Nat. Struct. Biol., 1995, 2(12), 1075—1082 |
| [19] | Ma G., Feng Y., Gao F., Wang J., Liu C., Li Y., Biochem. Bioph. Res. Commun., 2005, 337(4), 1301—1307 |
| [20] | Liu S., Zhao Q., Jiang S., Peptides,2003, 24(9), 1303—1313 |
| [21] | Apellániz B., Huarte N., Largo E., Nieva J. L., Chem. Phys. Lipids,2014, 181, 40—55 |
| [22] | Tripet B., Howard M. W., Jobling M., Holmes R. K., Holmes K. V., Hodges R. S., J. Biol. Chem., 2004, 279(20), 20836—20849 |
| [23] | Eckert D. M., Kim P. S., Annu. Rev. Biochem., 2001, 70(1), 777—810 |
| [24] | Korazim O., Sackett K., Shai Y., J. Mol. Biol., 2006, 364(5), 1103—1117 |
| [25] | Jiang S., Lin K., Strick N., Neurath A. R., Nature,1993, 365, 113 |
| [26] | Kilby J. M., Hopkins S., Venetta T. M., DiMassimo B., Cloud G. A., Lee J. Y., Alldredge L., Hunter E., Lambert D., Bolognesi D., Mathews T., Johnson M. R., Nowak M. A., Shaw G. M., Saag M. S., Nat. Med., 1998, 4(11), 1302—1307 |
| [27] | Muppidi A., Zhang H., Curreli F., Li N., Debnath A. K., Lin Q., Bioorg. Med. Chem. Lett., 2014, 24(7), 1748—1751 |
| [28] | Bianchi E., Finotto M., Ingallinella P., Hrin R., Carella A. V., Hou X. S., Schleif W. A., Miller M. D., Geleziunas R., Pessi A., PNAS,2005, 102(36), 12903—12908 |
| [29] | Eckert D. M., Kim P. S., PNAS,2001, 98(20), 11187—11192 |
| [30] | Chen X., Lu L., Qi Z., Lu H., Wang J., Yu X., Chen Y. H., Jiang S., J. Biol. Chem., 2010, 285(33), 25506—25515 |
| [31] | Kang H. J., Baker E. N., Trends Biochem. Sci., 2011, 36(4), 229—237 |
| [32] | Wang C., Lai W. Q., Yu F., Zhang T., Lu L., Jiang X. F. , Zhang Z., Xu X., Bai Y., Jiang S., Liu K. L., Chem. Sci., 2015, 6(11), 6505—6509 |
| [33] | Liang G. D., Wang C., Shi W. G., Wang K., Jiang X. F., Xu X. Y., Liu K. L., Chem. J. Chinese Universities,2014, 35(10), 2100—2103 |
| (梁国栋, 王潮, 史卫国, 王昆, 姜喜凤, 许笑宇, 刘克良. 高等学校化学学报, ,2014, 35(10), 2100—2103) | |
| [34] | Li X., Lai W. Q., Jiang X. F., Wang C., Liu K. L., Chem. J. Chinese Universities,2016, 37(5), 881—885 |
| (李雪, 来文庆, 姜喜凤, 王潮, 刘克良. 高等学校化学学报,2016, 37(5), 881—885) | |
| [35] | Zhang X. Y., Deng. D. J., Tan J. J., He Y., Li C. H., Wang C. X., Chem. Res. Chinese Universities,2014, 30(2), 297—305 |
| [36] | Suzuki K., Hiroaki H., Kohda D., Tanaka T., Protein Eng., 1998, 11(11), 1051—1055 |
| [37] | Huyghues-Despointes B. M., Klingler T. M., Baldwin R. L., Biochemistry,1995, 34(41), 13267—13271 |
| [38] | Clinton T. R., Weinstock M. T., Jacobsen M. T., Szabo-Fresnais N., Pandya M. J., Whitby F. G., Herbert A. S., Prugar L. I., McKinnon R., Hill C. P., Welch B. D., Dye J. M., Eckert D. M., Kay M. S., Protein Sci., 2015, 24(4), 446—463 |
| [39] | Bai Y., Xue H., Wang K., Cai L., Qiu J., Bi S., Lai L., Cheng M., Liu S., Liu K., Amino Acids,2013, 44, 701—713 |
| [40] | BaiY., Ling Y., Shi W. G., Cai L. F., Jia Q., Jiang S. B., Liu K. L., Chem. Biochem., 2011, 12(17), 2647—2658 |
| [41] | Lai W. Q., Wang C., Yu F., Lu L., Wang Q., Jiang X., Xu X., Zhang T., Wu S., Zheng X., Zhang Z., Dong F., Jiang S., Liu K., Chem. Sci., 2016, 7, 2145—2150 |
| [1] | WANG Baichun, YUAN Yuxin, YAN Yinghua, DING Chuanfan, TANG Keqi. Glucose-6-phosphate Functionalized Hydrophilic Magnetic Probe: a Dual-purpose Affinity Material for Effective Separation and Enrichment of Glycopeptides/Phosphopeptides [J]. Chem. J. Chinese Universities, 2021, 42(10): 3062. |
| [2] | HOU Chunxi, LI Yijia, WANG Tingting, LIU Shengda, YAN Tengfei, LIU Junqiu. Application of Elastin-like Polypeptides in Supramolecular Assembly † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1163. |
| [3] | A Li, WANG Yong. Fluorescent Peptide Sensor for Detection of Copper Ions [J]. Chem. J. Chinese Universities, 2020, 41(12): 2736. |
| [4] | WEN Jing, XU Zhimin, QI Desheng, WANG Jiayu, YU Shuangjiang, HE Chaoliang, HAN Bing. PLG-g-TA/RGD Enzyme-catalyzed Crosslinked Hydrogel for Adhesion and Three-dimensional Culture of Hyaline Chondrocytes † [J]. Chem. J. Chinese Universities, 2019, 40(9): 2020. |
| [5] | LIU Bingtong, ZHUANG Yongliang. Structural Characterization of Peptide Calcium Chelate VGLPNSR-Ca and Its Calcium Absorption Ability in Caco-2 Cell Monolayer [J]. Chem. J. Chinese Universities, 2019, 40(8): 1643. |
| [6] | LIU Guomin, LU Tiancheng, JI Xuan, JIA Wenyuan, LI Yalong, ZHAO Yian, LUO Yungang. Preparation and Osteogenic Induction Activity of CBD-BMP2-MP/PLGA 3D Printed Composite Scaffolds† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1552. |
| [7] | CHANG Junpeng,ZHAO Jiarui,CHEN Sijia,MENG Kai,SHI Weini,LI Ruifang. Structure-activity Relationship of Antimicrobial Peptide SAMP1 and Its Analog Peptides† [J]. Chem. J. Chinese Universities, 2019, 40(4): 705. |
| [8] | ZHANG Ye,WU Weihui,ZONG Liang,DONG Junjun. Carbonate Handle Modified Wang Resin and Its Function† [J]. Chem. J. Chinese Universities, 2019, 40(3): 462. |
| [9] | WU Fangling,CHU Yanqiu,CHEN Xin,WEI Wanghui,DING Chuanfan. Critical Factors Affecting Noncovalent Interaction Between Pentapeptides Explored by Electrospray Ionization Mass Spectrometry† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1927. |
| [10] | LU Tong, WANG Chunyang, ZHU Zhihui, JIANG Wei, HUO Mingnan, LI Fei. Polyethyleneimine Functionalized Graphene Oxide Against hIAPP Amyloid Aggregation† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1274. |
| [11] | CAO Hongyu,JIN Xiaojun,GUO Wei,YU Yaxian,SHI Longfei,TANG Qian,ZHENG Xuefang. Investigation on Binding Interactions Between Extracellular Amino-terminal Domain of GLP-1 Receptor Mutations and GLP-1 by Molecular Dynamics Simulations† [J]. Chem. J. Chinese Universities, 2018, 39(5): 1026. |
| [12] | ZHAO Wencai, HAN Lili, PENG Yingjun, WANG Xiaojing, LIU Shengyu, LI Pengfei, HUANG Yibing, CHEN Yuxin. Effect of Basic Amino Acids on the Biological Activity of Helical Antimicrobial Peptide† [J]. Chem. J. Chinese Universities, 2018, 39(4): 681. |
| [13] | YU Min, HUANG Jingjing, MA Min, FU Ruiyan, YAN Yan, ZHANG Fusheng, YIN Junfeng, XIE Ningning. Zinc Chelating Activity and Quantitative Structure-activity Relationship of Tripeptides† [J]. Chem. J. Chinese Universities, 2018, 39(2): 234. |
| [14] | WANG Song, GUAN Shanshan, WAN Yongfeng, SHAN Yaming, ZHANG Hao. Molecular Dynamics Simulation Study on the Binding Modes of Angiotensin-converting Enzyme with Inhibitory Peptides† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1216. |
| [15] | ZHAO Qi, HE Wanying, DUAN Lijie, ZHANG Yu, YU Shuangjiang, GAO Guanghui. Fabrication and Characterization of Injectable Polysaccharide-polypeptide Hydrogel Based on Schiff’s Base† [J]. Chem. J. Chinese Universities, 2016, 37(9): 1750. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||