

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (4): 681.doi: 10.7503/cjcu20170596
• Organic Chemistry • Previous Articles Next Articles
ZHAO Wencai, HAN Lili, PENG Yingjun, WANG Xiaojing, LIU Shengyu, LI Pengfei, HUANG Yibing*( ), CHEN Yuxin
), CHEN Yuxin
Received:2017-09-01
Online:2018-04-10
Published:2018-03-26
Contact:
HUANG Yibing
E-mail:huangyibing@jlu.edu.cn
Supported by:CLC Number:
TrendMD:
ZHAO Wencai, HAN Lili, PENG Yingjun, WANG Xiaojing, LIU Shengyu, LI Pengfei, HUANG Yibing, CHEN Yuxin. Effect of Basic Amino Acids on the Biological Activity of Helical Antimicrobial Peptide†[J]. Chem. J. Chinese Universities, 2018, 39(4): 681.
| Peptide | Amino acid sequence | Mw | Hydrophobicity tR0/min | PA |
|---|---|---|---|---|
| HPRP-A1 | Ac-FKKLKKLFSKLWNWK-amide | 2036.54 | 46.93 | 2.342 |
| HPRP-A1-2R | Ac-FRKLKKLFSKLWNWR-amide | 2092.56 | 47.28 | 2.205 |
| HPRP-A1-4R | Ac-FRKLKRLFSRLWNWR-amide | 2148.59 | 47.65 | 1.229 |
| HPRP-A1-6R | Ac-FRRLRRLFSRLWNWR-amide | 2204.62 | 48.45 | 1.092 |
Table 1 Biophysical properties of HPRP-A1 and its derivatives*
| Peptide | Amino acid sequence | Mw | Hydrophobicity tR0/min | PA |
|---|---|---|---|---|
| HPRP-A1 | Ac-FKKLKKLFSKLWNWK-amide | 2036.54 | 46.93 | 2.342 |
| HPRP-A1-2R | Ac-FRKLKKLFSKLWNWR-amide | 2092.56 | 47.28 | 2.205 |
| HPRP-A1-4R | Ac-FRKLKRLFSRLWNWR-amide | 2148.59 | 47.65 | 1.229 |
| HPRP-A1-6R | Ac-FRRLRRLFSRLWNWR-amide | 2204.62 | 48.45 | 1.092 |
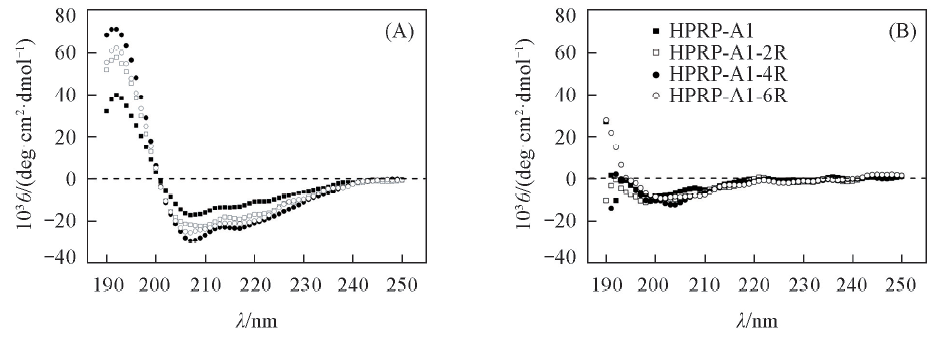
Fig.1 CD spectra of HPRP-A1 and its derivatives(A) In the presence of PBS buffer and 2,2,2-Trifluoroethanol(TFE)(volume ratio 1:1);(B) in PBS buffer(50 mmol/L KH2PO4/K2HPO4+100 mmol/L KCl, pH=7.4) at pH=7.4, 25 ℃(B).
| Peptide | MIC/(μmol·L-1) | MHC/(μmol·L-1) | Therapeutic index | |||
|---|---|---|---|---|---|---|
| P. aeruginosa | S. aereus | E. coil | B. subtilis | |||
| HPRP-A1 | 16 | 16 | 8 | 8 | 64 | 5.33 |
| HPRP-A1-2R | 32 | 32 | 8 | 4 | 64 | 3.37 |
| HPRP-A1-4R | 64 | 16 | 16 | 4 | 32 | 1.28 |
| HPRP-A1-6R | 64 | 32 | 32 | 8 | 32 | 0.94 |
Table 2 Biological activities of HPRP-A1 and its derivatives*
| Peptide | MIC/(μmol·L-1) | MHC/(μmol·L-1) | Therapeutic index | |||
|---|---|---|---|---|---|---|
| P. aeruginosa | S. aereus | E. coil | B. subtilis | |||
| HPRP-A1 | 16 | 16 | 8 | 8 | 64 | 5.33 |
| HPRP-A1-2R | 32 | 32 | 8 | 4 | 64 | 3.37 |
| HPRP-A1-4R | 64 | 16 | 16 | 4 | 32 | 1.28 |
| HPRP-A1-6R | 64 | 32 | 32 | 8 | 32 | 0.94 |
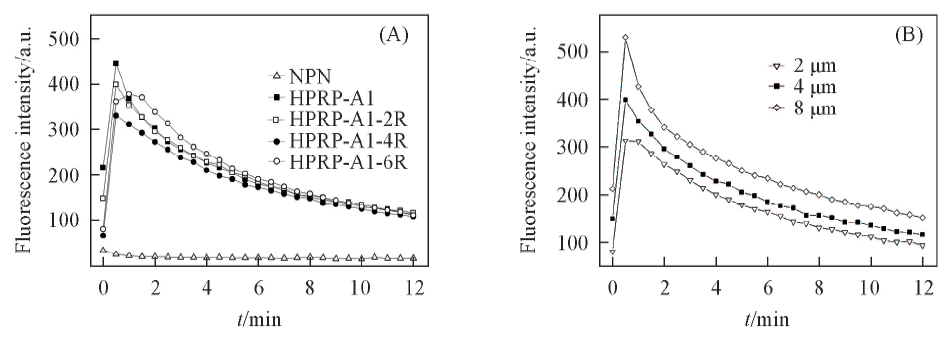
Fig.2 Outer membrane permeabilization assay of HPRP-A1 and its derivativesNPN uptake in P. aeruginosa ATCC 27853 was continuously measured by fluorescence spectrophotometer for 12 min at 25 ℃ endued by 4 μmol/L HPRP-A1 and its derivatives(A) and 2, 4 and 8 μmol/L HPRP-A1(B).
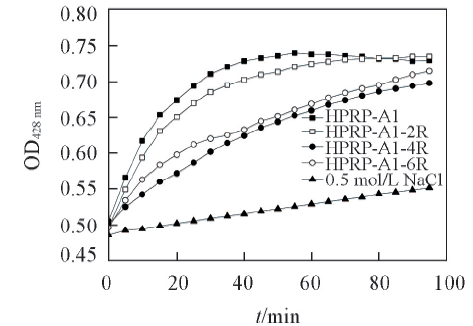
Fig.3 Inner membrane permeabilization assay of HPRP-A1 and its derivativesRelease of cytoplasmic β-galactosidase activity were measured by UV-visible spectrophotometer at 420 nm after adding ONPG(30 mmol/L) and various concentrations peptides into E. coli ML-35 bacterial suspension.
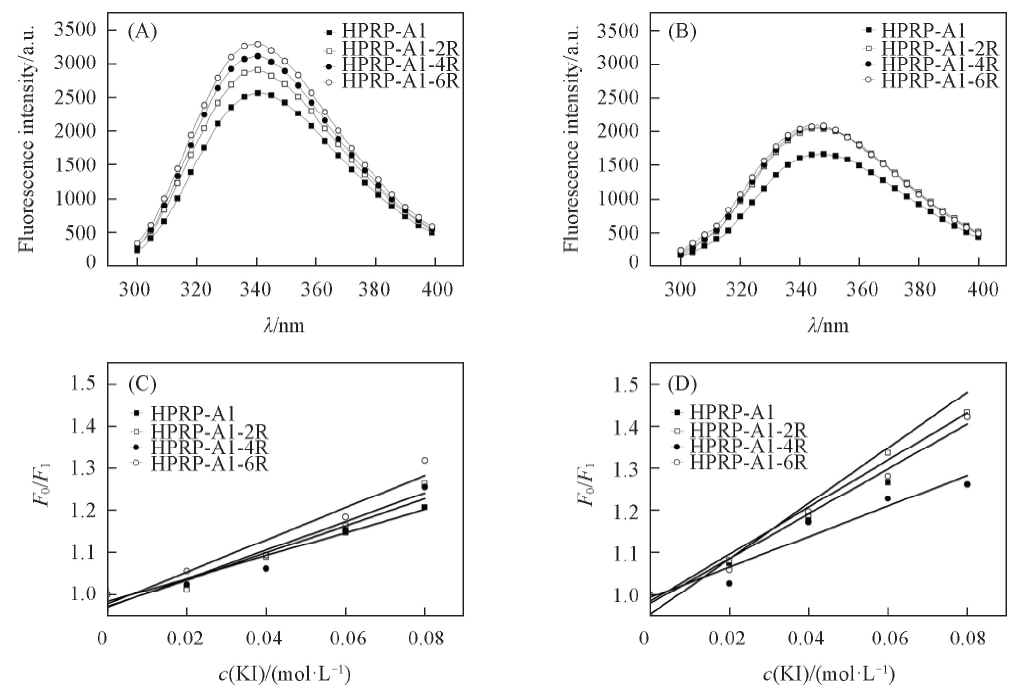
Fig.4 Fluorescence emission spectra(A, B) and Stern-Volmer plot(C, D) of peptides with various liposome models at 25 ℃Stern-Volmer plots were obtained by the sequential addition of the fluorescence quencher KI. PC/PG liposomes(mass fraction 7:3) were used to mimic prokaryotic membrane in (A, C) and PC/cholesterol(mass fraction 8:1) liposomes were used to mimic eukaryotic membrane in (B, D), respectively.
| [1] | Zhang Y. H., Li L. C., Yuan W. C., Zhang X. M., Chem. Res. Chinese Universities, 2015, 31(3), 381-387 |
| [2] | Feng H. Y., Gao L., Ye X. H., Wang L., Xue Z. C., Kong J. M., Li L. Z., Chem. Res. Chinese Universities, 2017, 33(1), 155-159 |
| [3] | Cheng S. Q., Liang G. D., Jiang X. F., Wang C., Liu K. L., Chem. J. Chinese Universities, 2016, 37(7), 1287-1292 |
| (程思绮, 梁国栋, 姜喜凤, 王潮, 刘克良. 高等学校化学学报, 2016, 37(7), 1287-1292) | |
| [4] | Andersson D. I., Hughes D., Kubicek-Sutherland J. Z., Drug Resist. Updates, 2016, 26, 43-57 |
| [5] | Hancock R. E. W., Sahl H. G., Nat. Biotechnol., 2006, 24, 1551-1557 |
| [6] | Huang Y. B., Zhai N. C., Gao G., Chen Y. X., Chem. J. Chinese Universities, 2012, 33(6), 1252-1258 |
| (黄宜兵, 翟乃翠, 高贵, 陈育新. 高等学校化学学报, 2012, 33(6), 1252-1258) | |
| [7] | Huang Y. B., He L. Y., Li G. R., Zhai N. C., Jiang H. Y., Chen Y. X., Protein & Cell, 2014, 5, 631-642 |
| [8] | Huang Y. B., Huang J. F., Chen Y. X., Protein & Cell, 2010, 1, 143-152 |
| [9] | Bechara C., Sagan S., Febs Lett., 2013, 587, 1693-1702 |
| [10] | Vasconcelos L., Parn K., Langel U., Ther. Deliv., 2013, 4, 573-591 |
| [11] | Hao X. Y., Yan Q. Y., Zhao J., Wang W. R., Huang Y. B., Chen Y. X., PLoS One, 2015, 10, e0138911 |
| [12] | Schmidt N. W., Tai K. P., Kamdar K., Mishra A., Lai G. H., Zhao K., Ouellette A. J., Wong G. C., J. Biol. Chem., 2012, 287, 21866-21872 |
| [13] | Zou G. Z., de Leeuw E., Li C., Pazgier M., Li C. Q., Zeng P., Lu W. Y., Lubkowski J., Lu W., J. Biol. Chem., 2007, 282, 19653-19665 |
| [14] | Huang Y. B., Wang X. F., Wang H. Y., Liu Y., Chen Y. X., Mol. Cancer Ther., 2011, 10, 416-426 |
| [15] | Mant C. T., Chen Y., Hodges R. S., J. Chromatogr. A, 2003, 1009, 29-43 |
| [16] | Chen Y. X., Guarnieri M. T., Vasil A. I., Vasil M. L., Mant C. T., Hodges R. S., Antimicrob. Agents Chemother., 2007, 51, 1398-1406 |
| [17] | Yi T. H., Huang Y. B., Chen Y. X., Journal of Peptide Science, 2015, 21, 46-52 |
| [18] | Sun S. Y., Zhao G. X., Huang Y. B, Cai M. J., Shan Y. P., Wang H. D., Chen Y. X., Sci. Rep., 2016, 6, e29145 |
| [19] | Chen Y. X., Mant C. T., Farmer S. W., Hancock R. E. W., Vasil M. L., Hodges R. S., J. Biol. Chem., 2005, 280, 12316-12329 |
| [20] | Huang H. W., Biochemistry, 2000, 39, 8347-8352 |
| [21] | Yi T. H., Huang Y. B., Chen Y. X., Chem. Bio. Drug Des., 2015, 85, 598-607 |
| [22] | Eriksson M., Nielsen P. E., Good L., J. Biol. Chem., 2002, 277, 7144-7147 |
| [23] | Li L. B., Vorobyov I., Allen T. W., J. Phys. Chem. B, 2013, 117, 11906-11920 |
| [24] | Harris F., Dennison S. R., Singh J., Phoenix D. A., Med. Res. Rev., 2013, 33, 190-234 |
| [25] | Lopez-Exposito I., Amigo L., Recio I., BBA-Biomembranes,2008, 1778, 2444-2449 |
| [26] | Mant C. T., Kovacs J. M., Kim H. M., Pollock D. D., Hodges R. S., Biopolymers,2009, 92, 573-595 |
| [27] | Qiao Y., Gao Z. H., Liu Y., Cheng Y., Yu M. X., Duan Y., Liu Y., Biomed. Res. Int., 2014, 2014, e869186 |
| [1] | ZHENG Haijiao, JIANG Liyan, JIA Qiong. Arginine Functionalized Magnetic Nanoparticles and Its Application in Phosphopeptides Enrichment [J]. Chem. J. Chinese Universities, 2021, 42(3): 717. |
| [2] | WANG Yuyao, ZHANG Qiang, YU Jihong. Synthesis of Hierarchical NaX Zeolite and Its CO2 Adsorption Performance † [J]. Chem. J. Chinese Universities, 2020, 41(4): 616. |
| [3] | CHANG Junpeng,ZHAO Jiarui,CHEN Sijia,MENG Kai,SHI Weini,LI Ruifang. Structure-activity Relationship of Antimicrobial Peptide SAMP1 and Its Analog Peptides† [J]. Chem. J. Chinese Universities, 2019, 40(4): 705. |
| [4] | ZHAO Qi, HE Wanying, DUAN Lijie, ZHANG Yu, YU Shuangjiang, GAO Guanghui. Fabrication and Characterization of Injectable Polysaccharide-polypeptide Hydrogel Based on Schiff’s Base† [J]. Chem. J. Chinese Universities, 2016, 37(9): 1750. |
| [5] | LI Lei,HUANG Cuiying,JIANG Xiaonan,GAO Xichan,WANG Changsheng. Ionic Hydrogen Bonding Between Arginine Side Chain and Nucleic Acid Bases† [J]. Chem. J. Chinese Universities, 2016, 37(8): 1460. |
| [6] | AN Huiqin, YU Yucai, YAN Lin, WU Tingting, LI Xiaofeng, HE Xiaoling, ZHAO Lizhi, HUANG Weiping. Synthesis of Highly Dispersed Au Nanoparticles Modified N-Doped TiO2 Nanotubes by the Assist of Lysine and Their Photocatalytic Activity† [J]. Chem. J. Chinese Universities, 2016, 37(11): 2034. |
| [7] | WANG Mei, WANG Jun, BU Yuxiang. Metastable Hydrogen-bonds Featuring Negative Dissociation Energies in Protein-bound DNA in Hole Migration [J]. Chem. J. Chinese Universities, 2015, 36(11): 2271. |
| [8] | YU Lan-Lan, MAO Ye-Xuan, BAI Xi-Xi, RAN Yu, LI Ai-Rong, ZHU Yan-Yan, YU Fei, QU Ling-Bo. Design and Investigation of Novel Antimicrobial Peptides with Dual Active Sequences [J]. Chem. J. Chinese Universities, 2013, 34(5): 1166. |
| [9] | ZHAO Zhi-Qiang, QIU Hui-Hua, YAO Jun, CHEN Yong-Feng. Novel Method for Identification of Protein Arginine-ADP-ribosylation Based on Chemical Derivatization and CID Fragmentation [J]. Chem. J. Chinese Universities, 2013, 34(12): 2704. |
| [10] | LIANG Ya-Qin, HU Zhi-Yong, CAO Duan-Lin, LIANG Dong. Surface Activity Properties of Chiral L-Lysine Based Gemini Surfactants [J]. Chem. J. Chinese Universities, 2013, 34(12): 2783. |
| [11] | ZHANG Jian-Cheng, DING Jian-Xun, XIAO Chun-Sheng, HE Chao-Liang, ZHUANG Xiu-Li, YANG Ya-Nan, CHEN Xue-Si. Synthesis and Characterization of Tumor-acidity-sensitive Poly(L-lysine)-doxorubicin Conjugates [J]. Chem. J. Chinese Universities, 2012, 33(12): 2809. |
| [12] | YU Lan-Lan, RAN Yu, BAI Xi-Xi, LI Ai-Rong, ZHU Yan-Yan, QIN Yun, QU Ling-Bo. Design and Bioactivity of Novel Antimicrobial Peptides and Its Computer Simulation with Phospholipid [J]. Chem. J. Chinese Universities, 2012, 33(12): 2681. |
| [13] | SU Man-Xiu, WANG Li-Feng, DAI Zhi-Jun, YUAN Zhe-Ming, BAI Lian-Yang. Primary Structural Characterizations of Polypeptide and Antimicrobial Peptides QSAM Modeling [J]. Chem. J. Chinese Universities, 2012, 33(11): 2526. |
| [14] | MA Dong-Dong, LIN Ping-Ping, CHEN Li-Li, WANG Yu-Hua, HE Dan-Dan, CHEN Wan-Ling, ZHANG Tian-Tian, CHEN Kui-Zhi, PENG Yi-Ru. Synthesis of Poly(ethylene glycol)-poly(L-lysine) Diblock Copolymer Incorporating Tetra-(p-sulfoazophenyl-4-aminosulfonyl)phthalocyanine Chloride Aluminum(Ⅲ) Polyion Nanoparticles and Its in vitro Photodynamic Therapy Efficacy [J]. Chem. J. Chinese Universities, 2012, 33(07): 1456. |
| [15] | LIU Feng-Jun, ZHANG Bing-Bo, SONG Ge, CHENG Ying-Sheng. Gd3+ and RGD Functionalized Quantum Dots for Fluorescent and MR Dual-modality Imaging of Pancreatic Cancer Cells [J]. Chem. J. Chinese Universities, 2012, 33(02): 378. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||