

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (8): 1421.doi: 10.7503/cjcu20160126
• Articles Inorganic Chemistry • Previous Articles Next Articles
DONG Gaoyun, LI Rui, FAN Tingting, LI Jiajia, LI Xia*( )
)
Received:2016-03-03
Online:2016-07-14
Published:2016-07-14
Contact:
LI Xia
E-mail:xiali@cnu.edu.cn
Supported by:CLC Number:
TrendMD:
DONG Gaoyun,LI Rui,FAN Tingting,LI Jiajia,LI Xia. Luminescence Sensing of Benzaldehyde and Cation of 3-(2-Carboxy-phenoxy)-phthalicate-based Lanthanide Complexes†[J]. Chem. J. Chinese Universities, 2016, 37(8): 1421.
| Compound | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Empirical formula | C78H46N8O14Sm2 | C27H17N2O8Eu | C27H17N2O8Gd | C27H17N2O8Tb | C27H17N2O8Dy |
| Formula weight | 1619.93 | 649.39 | 654.67 | 656.34 | 659.92 |
| Temperature/K | 296(2) | 296(2) | 296(2) | 296(2) | 296(2) |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | P21/n | P21/n | P21/n | P21/n |
| a/nm | 2.79501(11) | 1.17504(5) | 1.17519(4) | 1.1732(2) | 1.17249(4) |
| b/nm | 1.33737(5) | 1.04560(4) | 1.04434(4) | 1.0427(2) | 1.04125(4) |
| c/nm | 2.30816(15) | 2.01377(8) | 2.01048(7) | 2.0085(4) | 2.00662(7) |
| β/(°) | 121.4560(10) | 96.7414(11) | 96.8519(11) | 96.86(3) | 96.8906(10) |
| V/nm3 | 7.3599(6) | 2.45706(17) | 2.44983(15) | 2.4394(9) | 2.43210(15) |
| Z | 4 | 4 | 4 | 4 | 4 |
| Dc/(Mg·m-3) | 1.462 | 1.755 | 1.775 | 1.787 | 1.802 |
| Absorption coefficient/mm-1 | 1.649 | 2.608 | 2.763 | 2.955 | 3.128 |
| F(000) | 3224 | 1280 | 1284 | 1288 | 1292 |
| Crystal size/mm | 0.328×0.069×0.056 | 0.411×0.359×0.189 | 0.339×0.204×0.156 | 0.296×0.230×0.101 | 0.382×0.116×0.091 |
| θ range for data collection /(°) | 2.98—24.51 | 3.19—27.49 | 3.19—27.55 | 3.451—25.098 | 3.20—27.50 |
| Limiting indices | -36≤h≤36, -17≤k≤17, -29≤l≤30 | -14≤h≤13, -12≤k≤12, -24≤l≤24 | -14≤h≤13, -12≤k≤12, -23≤l≤23 | -14≤h≤13, -12≤k≤12, -23≤l≤23 | -13≤h≤13, -12≤k≤12, -23≤l≤23 |
| Reflections collected/unique | 75545/6539 | 31331/4367 | 31544 / 4354 | 32542 / 4341 | 31358 / 4324 |
| Rint | 0.0920 | 0.0256 | 0.0280 | 0.0291 | 0.0326 |
| Completeness(%) | 99.6 | 99.8 | 99.8 | 99.8 | 99.8 |
| Data/restraints/parameters | 8519/0/460 | 4367/1/353 | 4354/1/353 | 4331/1/353 | 4324/1/353 |
| Goodness-of-fit on F2 | 1.037 | 1.064 | 1.037 | 1.082 | 1.076 |
| Final R indices[I>2σ(I)] | R1=0.1082, wR2=0.0991 | R1=0.0225, wR2=0.0471 | R1=0.0240, wR2=0.0434 | R1=0.0229, wR2=0.0441 | R1=0.0288, wR2=0.0459 |
| R indices(all data) | R1=0.0440, wR2=0.0833 | R1=0.0194, wR2 =0.0451 | R1=0.0191, wR2=0.0411 | R1=0.0182, wR2=0.0420 | R1=0.0210, wR2=0.0432 |
| CCDC No. | 1052818 | 1052814 | 1052845 | 1052846 | 1052875 |
Table 1 Crystallographic data of complexes 1—5
| Compound | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Empirical formula | C78H46N8O14Sm2 | C27H17N2O8Eu | C27H17N2O8Gd | C27H17N2O8Tb | C27H17N2O8Dy |
| Formula weight | 1619.93 | 649.39 | 654.67 | 656.34 | 659.92 |
| Temperature/K | 296(2) | 296(2) | 296(2) | 296(2) | 296(2) |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | P21/n | P21/n | P21/n | P21/n |
| a/nm | 2.79501(11) | 1.17504(5) | 1.17519(4) | 1.1732(2) | 1.17249(4) |
| b/nm | 1.33737(5) | 1.04560(4) | 1.04434(4) | 1.0427(2) | 1.04125(4) |
| c/nm | 2.30816(15) | 2.01377(8) | 2.01048(7) | 2.0085(4) | 2.00662(7) |
| β/(°) | 121.4560(10) | 96.7414(11) | 96.8519(11) | 96.86(3) | 96.8906(10) |
| V/nm3 | 7.3599(6) | 2.45706(17) | 2.44983(15) | 2.4394(9) | 2.43210(15) |
| Z | 4 | 4 | 4 | 4 | 4 |
| Dc/(Mg·m-3) | 1.462 | 1.755 | 1.775 | 1.787 | 1.802 |
| Absorption coefficient/mm-1 | 1.649 | 2.608 | 2.763 | 2.955 | 3.128 |
| F(000) | 3224 | 1280 | 1284 | 1288 | 1292 |
| Crystal size/mm | 0.328×0.069×0.056 | 0.411×0.359×0.189 | 0.339×0.204×0.156 | 0.296×0.230×0.101 | 0.382×0.116×0.091 |
| θ range for data collection /(°) | 2.98—24.51 | 3.19—27.49 | 3.19—27.55 | 3.451—25.098 | 3.20—27.50 |
| Limiting indices | -36≤h≤36, -17≤k≤17, -29≤l≤30 | -14≤h≤13, -12≤k≤12, -24≤l≤24 | -14≤h≤13, -12≤k≤12, -23≤l≤23 | -14≤h≤13, -12≤k≤12, -23≤l≤23 | -13≤h≤13, -12≤k≤12, -23≤l≤23 |
| Reflections collected/unique | 75545/6539 | 31331/4367 | 31544 / 4354 | 32542 / 4341 | 31358 / 4324 |
| Rint | 0.0920 | 0.0256 | 0.0280 | 0.0291 | 0.0326 |
| Completeness(%) | 99.6 | 99.8 | 99.8 | 99.8 | 99.8 |
| Data/restraints/parameters | 8519/0/460 | 4367/1/353 | 4354/1/353 | 4331/1/353 | 4324/1/353 |
| Goodness-of-fit on F2 | 1.037 | 1.064 | 1.037 | 1.082 | 1.076 |
| Final R indices[I>2σ(I)] | R1=0.1082, wR2=0.0991 | R1=0.0225, wR2=0.0471 | R1=0.0240, wR2=0.0434 | R1=0.0229, wR2=0.0441 | R1=0.0288, wR2=0.0459 |
| R indices(all data) | R1=0.0440, wR2=0.0833 | R1=0.0194, wR2 =0.0451 | R1=0.0191, wR2=0.0411 | R1=0.0182, wR2=0.0420 | R1=0.0210, wR2=0.0432 |
| CCDC No. | 1052818 | 1052814 | 1052845 | 1052846 | 1052875 |
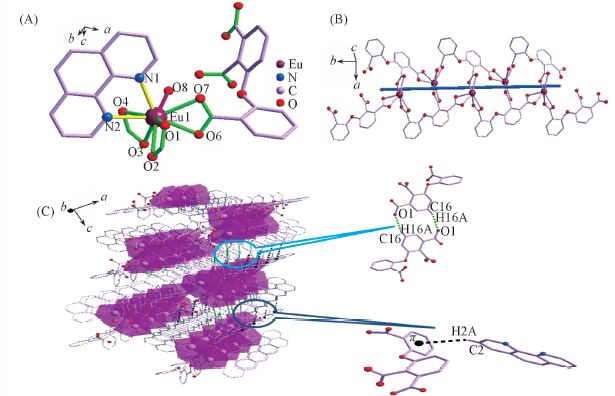
Fig.2 Coordination environment of Eu3+ in complex 2(A), 1D chain structure(B) and 3D supramolecular structure via the hydrogen bonds and C—H…π interactions(C) of complex 2
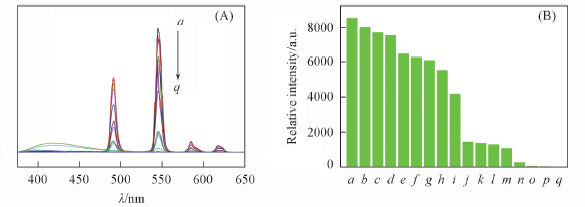
Fig.5 Emission spectra(A) and the 5D4→7F5 transition intensities(B) of complex 4 in the presence of different cations(10-2 mol/L) a. H2O; b. K+; c. Ca2+; d. Li+; e. Mg2+; f. Na+; g. Pb2+; h. Ba2+; i. Zn2+; j. Al3+; k. Cd2+; l. Ni2+; m. Cu2+; n. Ag+; o. Co2+; p. Cr2+; q. Fe3+.
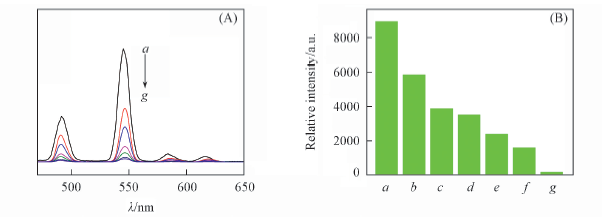
Fig.6 Emission spectra(A) and the 5D4→7F5 transition intensities(B) of complex 4 in the presence of different concentrations of Co2+(λex=350 nm) c(Co2+)/(mol·L-1): a. 0; b. 5×10-6; c. 1×10-5; d. 1×10-4; e. 1×10-3; f. 5×10-3; g. 5×10-2.
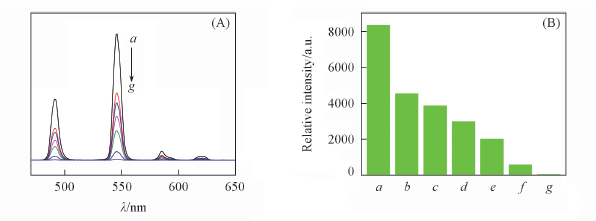
Fig.7 Emission spectra(A) and 5D4→7F5 transition intensities(B) of complex 4 in the presence of different concentrations of Fe3+(λex=350 nm) c(Fe3+)/(mol·L-1): a. 0; b. 5×10-6; c. 1×10-5; d. 1×10-4; e. 1×10-3; f. 5×10-3; g. 5×10-2.
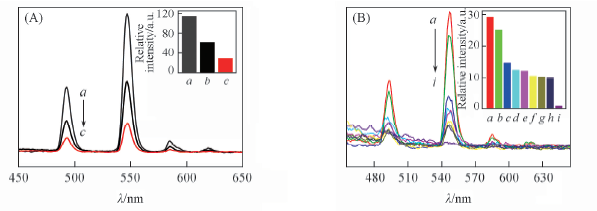
Fig.8 Emission spectra(A, B) and 5D4→7F5 transition intensity(insets) of complex 4 dispersed in different solvents(λex=350 nm) (A) a. EtOAc; b. acetone; c. benzene. (B) a. Benzene; b. dichloromethane; c. methanol; d. formamide; e. dimethylbenzene; f. acetonitrile; g. DMF; h. formaldeltyde; i. benzaldehyde.
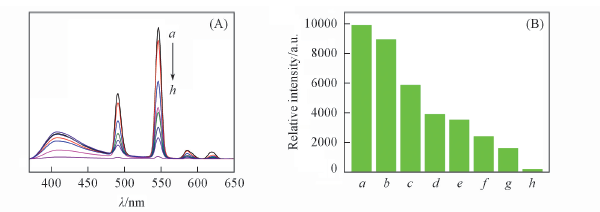
Fig.9 Emission spectra(A) and 5D4→7F5 transition intensity(B) of complex 4 dispersed in EtOAc with different concentrations of benzaldehyde(λex=350 nm) c(Benzaldehyde)/(mol·L-1): a. 10-6; b. 5×10-5; c. 10-4; d. 2×10-4; e. 3×10-4; f. 5×10-4; g. 6×10-4; h. 8×10-4.
| [1] |
Pramanik, S. , Zheng, C. , Zhang, X. , Emge T., J. , Li, J. , J. Am. Chem. Soc., 2011, 133, 4153- 4155
doi: 10.1021/ja106851d URL pmid: 21384862 |
| [2] | Ma L., Q. , Abney, C. , Lin, W. , Chem. Soc. Rev., 2009, 38, 1248- 1256 |
| [3] |
Lee J., Y. , Farha O., K. , Roberts, J. , Scheidt K., A. , Nguyen S., T. , Hupp J., T. , Chem. Soc. Rev., 2009, 38, 1450- 1459
doi: 10.1039/b807080f URL |
| [4] |
Hao J., N. , Yan, B. , Chem. Commun., 2015, 51, 7737- 7740
doi: 10.1007/s12035-015-9439-0 URL pmid: 26392295 |
| [5] |
Zhang Z., J. , Xiang S., C. , Rao X., T. , Zheng, Q. , Fronczek F., R. , Qian G., D. , Chen B., L. , Chem. Commun., 2010, 46, 7205- 7207
doi: 10.1039/c0cc01236j URL pmid: 20737107 |
| [6] | Li J., R. , Kuppler R., J. , Zhou H., C. , Chem. Soc. Rev., 2009, 38, 1477- 1504 |
| [7] |
Wang Y., N. , Zhang, P. , Yu J., H. , Xu J., Q. , Dalton Trans., 2015, 44, 1655- 1663
doi: 10.1186/s12885-015-1887-4 URL pmid: 26541196 |
| [8] | Feng, X. , Wang Y., Y. , Hu Y., C. , Chen S., P. , Zhao W., J. , Yang X., W. , J. Coord. Chem., 2012, 65, 2692- 2704 |
| [9] | Wang, L. , Acta Cryst., 2013, 69, 101- 107 |
| [10] |
Zhang S., Q. , Jiang F., L. , Wu M., Y. , Ma, J. , Bu, Y. , Hong M., C. , Cryst. Growth Des., 2012, 12, 1452- 1463
doi: 10.1021/cg201556b URL |
| [11] |
Wang H., L. , Zhang D., P. , Sun D., F. , Chen Y., T. , Zhang L., F. , Tian L., J. , Jiang J., Z. , Ni Z., H. , Cryst. Growth Des., 2009, 9, 5273- 5282
doi: 10.3959/1536-1098-63.1.27 URL |
| [12] |
Wu, H. , Yang, J. , Su Z., M. , Batten S., R. , Ma J., F. , J. Am. Chem. Soc., 2011, 133, 11406- 11409
doi: 10.1021/ja202303b URL pmid: 21728370 |
| [13] |
Kong C., Y. , J. Inorg. Organomet. Polym. Mater., 2011, 21, 189- 194
doi: 10.1039/C1JM13551A URL |
| [14] |
Celedonio M., A. , Lucia A., M. , Raul G., R. , Eur. J. Inorg. Chem., 2015, 29, 4921- 4934
doi: 10.1002/ejic.201500776 URL |
| [15] |
Marchand, A. , Granzhan, A. , Iida, K. , Tsushima, Y. , Ma, Y. , Nagasawa, K. , Teulade-Fichou, M. , Gabelica, Valerie. , J. Am. Chem. Soc., 2015, 137, 750- 756
doi: 10.1021/ja5099403 URL pmid: 25525863 |
| [16] |
Accorsi, G. , Listorti, A. , Yoosaf, K. , Armaroli, N. , Chem. Soc. Rev., 2009, 38, 1690- 1700
doi: 10.1039/b806408n URL pmid: 19587962 |
| [17] | Bauer C., A. , Timofeeva T., V. , Settersten T., B. , Patterson B., D. , Liu V., H. , Simmons B., A. , Allendorf M., D. , J. Am. Chem. Soc., 2007, 129, 7136- 7144 |
| [18] |
Saleem, M. , Lee K., H. , RSC Adv., 2015, 5, 72150- 72287
doi: 10.1039/C5RA13831K URL |
| [19] |
Novio, F. , Simmchen, J. , Vazquez-Mera, N. , Amorin-Ferre, L. , Ruiz-Molina D., C. , Chem. Rev., 2013, 257, 2839- 2847
doi: 10.1016/j.ccr.2013.04.022 URL |
| [20] |
徐布一, 叶懿, 阮若云, 颜有仪, 廖林川. 高等学校化学学报, 2015, 36( 9), 1667- 1673
doi: 10.7503/cjcu20150228 |
|
Xu B., Y. , Ye, Y. , Ruan R., Y. , Yan Y., Y. , Liao L., C. , Chem. J. Chinese Universities, 2015, 36( 9), 1667- 1673
doi: 10.7503/cjcu20150228 |
|
| [21] |
徐惠, 代艳娜, 单洪岩, 费强, 郇延富, 李光华, 冯国栋. 高等学校化学学报, 2014, 35( 4), 736- 740
doi: 10.7503/cjcu20131096 |
|
Xu, H. , Dai Y., N. , Shan H., Y. , Fei, Q. , Huan Y., F. , Li G., H. , Feng G., D. , Chem. J. Chinese Universities, 2014, 35( 4), 736- 740
doi: 10.7503/cjcu20131096 |
|
| [22] | Zhao, B. , Chen X., Y. , Cheng, P. , Liao D., Z. , Yan S., P. , Jiang Z., H. , J. Am. Chem. Soc., 2004, 126, 15394- 15395 |
| [23] |
Harbuzaru B., V. , Corma, A. , Rey, F. , Atienzar, P. , Ananias, D. , Carlos L., D. , Rocha, J. , Angew. Chem. Int. Ed., 2008, 47, 1080- 1083
doi: 10.1002/anie.200704702 URL pmid: 18183561 |
| [24] |
赵秀巧, 王会, 董丽君, 徐庆红. 高等学校化学学报, 2013, 34( 6), 1318- 1326
doi: 10.7503/cjcu20121156 |
|
Zhao X., Q. , Wang, H. , Dong L., J. , Xun Q., H. , Chem. J. Chinese Universities, 2013, 34( 6), 1318- 1326
doi: 10.7503/cjcu20121156 |
|
| [25] | Zhou, Y. , Chen H., H. , Yan, B. , J. Mater. Chem. A., 2014, 2, 13691- 13697 |
| [26] |
Hao J., N. , Yan, B. , Chem. Commun., 2015, 51, 7737- 7740
doi: 10.1007/s12035-015-9439-0 URL pmid: 26392295 |
| [27] |
Shi B., B. , Zhong Y., H. , Guo L., L. , Dalton Trans., 2015, 44, 4362- 4369
doi: 10.1039/c4dt03326d URL pmid: 25641054 |
| [28] | Sheldrick G., M. , SHELXS 97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, 1997 |
| [29] | Sheldrick G., M. , SHELXL 97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, 1997 |
| [30] |
Gore A., H. , Gunjal D., B. , Kokate M., R. , Sudarsan, V. , Anbhule P., V. , Patil S., R. , Kolekar G., B. , ACS Appl. Mater. Interfaces, 2012, 4, 5217- 5226
doi: 10.1021/am301136q URL pmid: 22948013 |
| [31] | Zhou, Y. , Chen H., H. , Yan, B. , J. Mater. Chem. A, 2014, 2, 13691- 13697 |
| [32] |
Xiang, Z. , Fang, C. , Leng, S. , Cao, D. , J. Mater. Chem. A, 2014, 2, 7662- 7665
doi: 10.1016/j.ica.2014.09.015 URL |
| [1] | HUAN Xinyu, LAI Ganqiang, HUANG Yue, YANG Caiguang. Research Progress on Chemical Intervention of N6-methyladenosine Modification [J]. Chem. J. Chinese Universities, 2022, 43(Album-4): 20220340. |
| [2] | CHEN Jialu, HUANG Shuo. Application of Nanopore Sequencing Technology in the Detection of Nucleic Acid Modifications [J]. Chem. J. Chinese Universities, 2022, 43(Album-4): 20220333. |
| [3] | ZHANG Qingyi, CAO Jie, SHU Xiao, LIU Jianzhao. Effects of Exogenous N6-methyladenosine (m6A) Incorporation on the Expression of Cellular mRNA Transcripts [J]. Chem. J. Chinese Universities, 2022, 43(Album-4): 20220173. |
| [4] | WANG Xintian, LI Pan, CAO Yue, HONG Wenhao, GENG Zhongxuan, AN Zhiyang, WANG Haoyu, WANG Hua, SUN Bin, ZHU Wenlei, ZHOU Yang. Techno-economic Analysis and Industrial Application Prospects of Single-atom Materials in CO2 Catalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220347. |
| [5] | JI Shuangqi, JIN Zhao, GUAN Wenna, PAN Xiangyu, GUAN Tong. Preparation and Chromatographic Performance of Mixed-mode Silica Stationary Phase Modified by Double Cationic Ionic Liquid and Octadecyl Group [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220008. |
| [6] | ZHAO Sheng, HUO Zhipeng, ZHONG Guoqiang, ZHANG Hong, HU Liqun. Preparation of Modified Gadolinium/Boron/Polyethylene Nanocomposite and Its Radiation Shielding Performance for Neutron and Gamma-ray [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220039. |
| [7] | YAN Zhixuan, MA Ji, QU Jinlei, LIU Li, SUN Chong, LIU Jiwen, LIU Guangye, SUN Lishui, HE Lixia. Synthesis and Application of Modified Low Molecular Weight Polyisoprene [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220066. |
| [8] | SHA Meng, XU Weiqing, WU Zhichao, GU Wenling, ZHU Chengzhou. Recent Advances in Single-atom Materials for Enzyme-like Catalysis and Biomedical Applications [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220077. |
| [9] | HU Huimin, CUI Jing, LIU Dandan, SONG Jiaxin, ZHANG Ning, FAN Xiaoqiang, ZHAO Zhen, KONG Lian, XIAO Xia, XIE Zean. Influence of Different Transition Metal Decoration on the Propane Dehydrogenation Performance over Pt/M-DMSN Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210815. |
| [10] | ZHAO Yongmei, MU Yeshu, HONG Chen, LUO Wen, TIAN Zhiyong. Bis-naphthalimide Derivatives for Picronitric Acid Detection in Aqueous Solution [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210765. |
| [11] | MENG Xianglong, YANG Ge, GUO Hailing, LIU Chenguang, CHAI Yongming, WANG Chunzheng, GUO Yongmei. Synthesis of Nano-zeolite and Its Adsorption Performance for Hydrogen Sulfide [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210687. |
| [12] | TANG Qian, DAN Feijun, GUO Tao, LAN Haichuang. Synthesis and Application of Quinolinone-coumarin-based Colorimetric Fluorescent Probe for Recognition of Hg2+ [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210660. |
| [13] | JIA Hongjun, ZHANG Jiatao, MA Zhuoli, WANG Heng, YANG Xinyu, YANG Jiazhi. Preparation of PTFE/PAA/Nafion Composite Membrane by Aqueous Polymerization of Acrylic Acid and Its Properties [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220350. |
| [14] | WANG Di, ZHONG Keli, TANG Lijun, HOU Shuhua, LYU Chunxin. Synthesis of Schiff-based Covalent Organic Framework and Its Recognition of I ‒ [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220115. |
| [15] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||