

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (4): 706.doi: 10.7503/cjcu20150790
• Physical Chemistry • Previous Articles Next Articles
DING Jiyong, SHEN Hongchen, LIU Fufeng*( )
)
Received:2015-10-13
Online:2016-04-10
Published:2016-03-18
Contact:
LIU Fufeng
E-mail:fufengliu@tju.edu.cn
Supported by:TrendMD:
DING Jiyong, SHEN Hongchen, LIU Fufeng. Virtual Screening of Small Molecular Stabilizer for Y220C Mutant of p53†[J]. Chem. J. Chinese Universities, 2016, 37(4): 706.
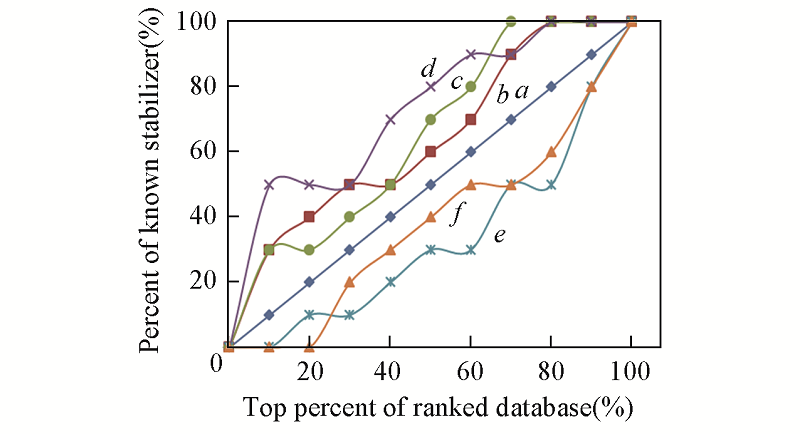
Fig.3 Enrichment curve analysis of the five scoring functions in FlexXa. Random; b. F_Score; c. ChemScore; d. D_Score; e. G_Score; f. PMF_Score. A diagonal represents a random selection. Curves above the diagonal represent the corresponding scoring functions can select the known stabilizers from the library of small molecular compounds.
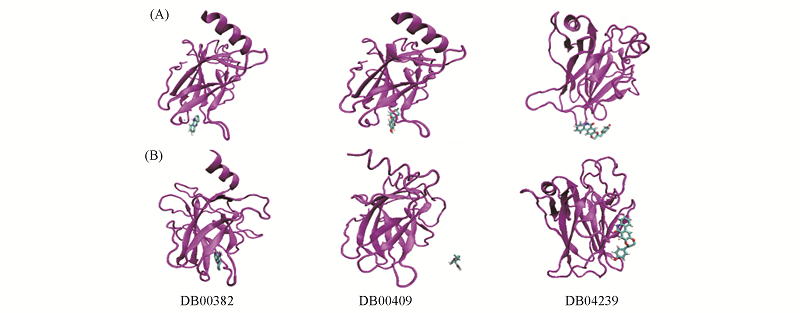
Fig.4 Comparison of binding sites of the 3 compounds on p53C mutant Y220C before and after MD simulations (A) Binding models obtained by docking(i.e., initial binding conformations in MD simulation); (B) binding models obtained after 100 ns MD simulations.
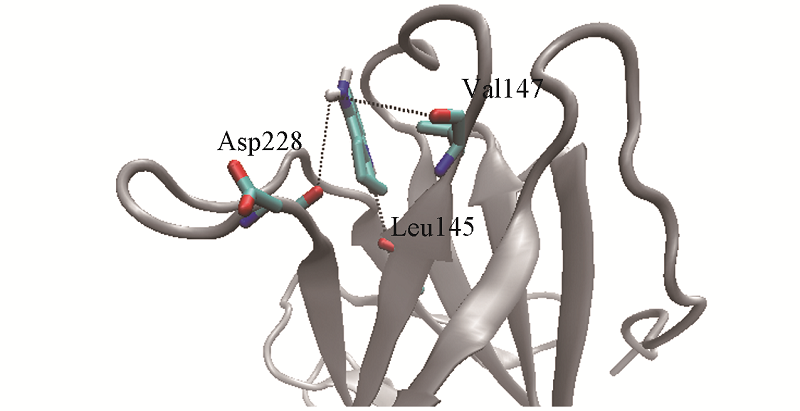
Fig.6 Snapshot of the hydrogen bonds between tacrine and the residues of p53C-Y220CHydrogen bonds between tacrine and the residues Leu145, Val147 and Asp228 of p53C-Y220C are shown in black dotted lines. The residue type and its sequence number which interacted with tacrine via hydrogen bonds are also shown.
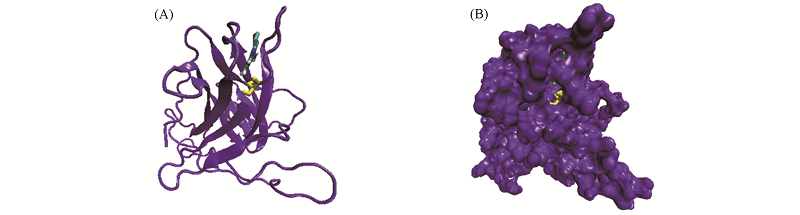
Fig.9 Representative binding modes of tacrine and the hydrophobic pocket created by Y220C mutation on p53C after 100 ns MD simulation (A) Secondary structure; (B) surface representation. The Y220C mutant and tacrine are shown in yellow and green dynamics bond models.
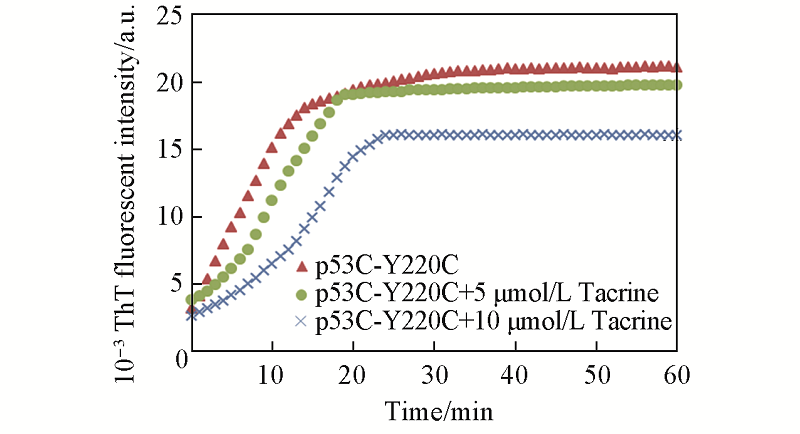
Fig.10 Effect of different concentrations of tarcrine on p53C-Y220C aggregation measured by ThT fluorescence The concentration of p53C-Y220C was 5 μmol/L.
| [1] | Levine A. J., Oren M., Nat. Rev. Cancer, 2009, 9(10), 749—758 |
| [2] | Olivier M., Hollstein M., Hainaut P., CSH Perspect. Biol., 2010, 2(1), a001008 |
| [3] | Xu Z. Y., Zhao L. L., Cao Z. X., Wang J. H., Acta Phys-Chim. Sinica, 2012, 28(7), 1665—1675 |
| (许朝莹, 赵立岭, 曹赞霞, 王吉华. 物理化学学报, 2012, 28(7), 1665—1675) | |
| [4] | Cho Y., Gorina S., Jeffrey P. D., Pavletich N. P., Science, 1994, 265(5170), 346—355 |
| [5] | Joerger A. C., Fersht A. R., Annu. Rev. Biochem., 2008, 77, 557—582 |
| [6] | Joerger A. C., Ang H. C., Fersht A. R., P. Natl. Acad. Sci. USA, 2006, 103(41), 15056—15061 |
| [7] | Wang G., Fersht A. R., P. Natl. Acad. Sci. USA, 2015, 112(8), 2437—2442 |
| [8] | Wilcken R., Liu X., Zimmermann M. O., Rutherford T. J., Fersht A. R., Joerger A. C., Boeckler F. M., J. Am. Chem. Soc., 2012, 134(15), 6810—6818 |
| [9] | Bykov V. J., Wiman K. G., FEBS Lett., 2014, 588(16), 2622—2627 |
| [10] | Friedler A., Hansson L. O., Veprintsev D. B., Freund S. M., Rippin T. M., Nikolova P. V., Proctor M. R., Rudiger S., Fersht A. R., P. Natl. Acad. Sci. USA, 2002, 99(2), 937—942 |
| [11] | Boeckler F. M., Joerger A. C., Jaggi G., Rutherford T. J., Veprintsev D. B., Fersht A. R., P. Natl. Acad. Sci. USA, 2008, 105(30), 10360—10365 |
| [12] | Wassman C. D., Baronio R., Demir O., Wallentine B. D., Chen C. K., Hall L. V., Salehi F., Lin D. W., Chung B. P., Hatfield G. W., Richard Chamberlin A., Luecke H., Lathrop R. H., Kaiser P., Amaro R. E., Nat. Commun., 2013, 4, 1407 |
| [13] | Law V., Knox C., Djoumbou Y., Jewison T., Guo A. C., Liu Y. F., Maciejewski A., Arndt D., Wilson M., Neveu V., Tang A., Gabriel G., Ly C., Adamjee S., Dame Z. T., Han B. S., Zhou Y., Wishart D. S., Nucleic Acids Res., 2014, 42(D1), D1091—D1097 |
| [14] | Trott O., Olson A. J., J. Comput. Chem., 2010, 31(2), 455—461 |
| [15] | Rarey M., Kramer B., Lengauer T., Klebe G., J. Mol. Biol., 1996, 261(3), 470—489 |
| [16] | Schuttelkopf A. W., van Aalten D. M., Acta Crystallogr. D. Biol. Crystallogr., 2004, 60(8), 1355—1363 |
| [17] | Oostenbrink C., Villa A., Mark A. E., van Gunsteren W. F., J. Comput. Chem., 2004, 25(13), 1656—1676 |
| [18] | Pronk S., Pall S., Schulz R., Larsson P., Bjelkmar P., Apostolov R., Shirts M. R., Smith J. C., Kasson P. M., van der Spoel D., Hess B., Lindahl E., Bioinformatics, 2013, 29(7), 845—854 |
| [19] | Darden T., York D., Pedersen L., J. Chem. Phys., 1993, 98, 10089—10092 |
| [20] | Hess B., Bekker H., Berendsen H. J. C., J. Comput. Chem., 1997, 18, 1463—1472 |
| [21] | Beredsen H. J. C., Postma J. P. M., van Gunsteren W. F., Di Nola A., Haak J. R., J. Chem. Phys., 1984, 81, 3684—3690 |
| [22] | Bussi G., Donadio D., Parrinello M., J. Chem. Phys., 2007, 126(1), 014101 |
| [23] | Humphrey W., Dalke A., Schulten K., J. Mol. Graph. Model., 1996, 14(1), 33—38 |
| [24] | Liu F. F., Wang T., Dong X. Y., Sun Y., J. Chromatogr. A, 2007, 1146(1), 41—50 |
| [25] | Zhao W. W., Liu F. F., Shi Q. H., Dong X. Y., Sun Y., Biochem. Eng. J., 2014, 88, 1—11 |
| [26] | Marsh L., Plos One, 2011, 6(8), e23215 |
| [27] | Du W. J., Guo J. J., Gao M. T., Hu S. Q., Dong X. Y., Han Y. F., Liu F. F., Jiang S., Sun Y., Sci. Rep., 2015, 5, 7992 |
| [28] | Chen Y. C., Trends Pharmacol. Sci., 2015, 36(2), 78—95 |
| [29] | Hou T. J., Wang J. M., Li Y. Y., Wang W., J. Comput. Chem., 2011, 32(5), 866—877 |
| [30] | Wu Y. J., Cui Y. L., Zheng Q. C., Zhang H. X., Chem. J. Chinese Universities, 2014, 35(12), 2605—2611 |
| (吴云剑, 崔颖璐, 郑清川, 张红星. 高等学校化学学报, 2014, 35(12), 2605—2611) | |
| [31] | Dong L., Yi Z. S., Wu Z. W., Wang H. Y., Zhang A. Q., Chem. J. Chinese Universities, 2015, 36(3), 516—522 |
| (董露, 易忠胜, 伍智蔚, 王海洋, 张爱茜. 高等学校化学学报, 2015, 36(3), 516—522) | |
| [32] | Jian W. J., Zeng Y., Xiong H. J., Pang J., Carbohydr. Polym., 2011, 85 , 452—456 |
| [33] | Bai S., Zhou R., Liu F. F., Acta Phys-Chim. Sinica, 2013, 29(2), 439—448 |
| (白姝, 周荣, 刘夫锋. 物理化学学报, 2013, 29(2), 439—448) | |
| [34] | Lin D. Q., Tong H. F., Wang H. Y., Yao S. J., J. Phys. Chem. B, 2012, 116(4), 1393—1400 |
| [35] | Wu F., Yang Z. W., Yuan X. H., Chem. J. Chinese Universities, 2013, 34(4), 931—938 |
| (武菲, 杨志伟, 袁晓辉. 高等学校化学学报,2013, 34(4), 931—938) | |
| [36] | Zhang N., Liu F. F., Dong X. Y., Sun Y., J. Phys. Chem. B, 2012, 116(24), 7040—7047 |
| [37] | Liu F. F., Dong X. Y., Sun Y., J. Mol. Graph. Model., 2008, 27(4), 421—429 |
| [38] | Wang G., Fersht A. R., P. Natl. Acad. Sci. USA, 2015, 112(8), 2443—2448 |
| [39] | Liu F. F., Dong X. Y., Sun Y., Acta Phys-Chim. Sinica, 2010, 26(6), 1643—1650 |
| (刘夫锋, 董晓燕, 孙彦. 物理化学学报, 2010, 26(6), 1643—1650) | |
| [40] | Xiong N., Dong X. Y., Zheng J., Liu F. F., Sun Y., ACS Appl. Mater. Inter., 2015, 7(10), 5650—5662 |
| [1] | GAO Zhiwei, LI Junwei, SHI Sai, FU Qiang, JIA Junru, AN Hailong. Analysis of Gating Characteristics of TRPM8 Channel Based on Molecular Dynamics [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220080. |
| [2] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [3] | WANG Xueli, SONG Xiangwei, XIE Yanning, DU Niyang, WANG Zhenxin. Preparation, Characterization of Partially Reduced Graphene Oxide and Its Killing Effect on Human Cervical Cancer Cells [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210595. |
| [4] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [5] | ZHAO Ying, QIAO Ling, ZHAO Guofeng, CHEN Li. Synthesis and Biological Activity of Lycorine Derivatives Containing Malate Ester [J]. Chem. J. Chinese Universities, 2021, 42(9): 2789. |
| [6] | LI Congcong, LIU Minghao, HAN Jiarui, ZHU Jingxuan, HAN Weiwei, LI Wannan. Theoretical Study of the Catalytic Activity of VmoLac Non-specific Substrates Based on Molecular Dynamics Simulations [J]. Chem. J. Chinese Universities, 2021, 42(8): 2518. |
| [7] | LEI Xiaotong, JIN Yiqing, MENG Xuanyu. Prediction of the Binding Site of PIP2 in the TREK-1 Channel Based on Molecular Modeling [J]. Chem. J. Chinese Universities, 2021, 42(8): 2550. |
| [8] | ZENG Yonghui, YAN Tianying. Vibrational Density of States Analysis of Proton Hydration Structure [J]. Chem. J. Chinese Universities, 2021, 42(6): 1855. |
| [9] | QI Renrui, LI Minghao, CHANG Hao, FU Xueqi, GAO Bo, HAN Weiwei, HAN Lu, LI Wannan. Theoretical Study on the Unbinding Pathway of Xanthine Oxidase Inhibitors Based on Steered Molecular Dynamics Simulation [J]. Chem. J. Chinese Universities, 2021, 42(3): 758. |
| [10] | LIU Aiqing, XU Wensheng, XU Xiaolei, CHEN Jizhong, AN Lijia. Molecular Dynamics Simulation of Polymer/rod Nanocomposite [J]. Chem. J. Chinese Universities, 2021, 42(3): 875. |
| [11] | SHUAI Die, ZHAO Meijuan, CHEN Bingnian, WANG Li. Inhibitory Effect of Four Kinds of Keegin-type Phosphomolybdate on Tyrosinase and Melanin Formation and Its Antioxidant Activities [J]. Chem. J. Chinese Universities, 2021, 42(12): 3579. |
| [12] | DUAN Jun, ZHOU Pingping, ZHANG Liqian, MA Lin, YU Wen, ZHU Meiqi, ZHUANG Minyan, YANG Fenglei, CAO Changsheng, ZHANG Peng, SHI Yanhui. Syntheses of Imidazole-coordinated Ru(II) Half-sandwich Macrocyclic Complexes and Their Anti-cancer Activity [J]. Chem. J. Chinese Universities, 2021, 42(12): 3589. |
| [13] | ZHAO Zhuo, WANG Xueqiang. Investigations upon the Bioconjugation-based Construction Technologies and Applications of Aptamer-drug Conjugates [J]. Chem. J. Chinese Universities, 2021, 42(11): 3367. |
| [14] | CHEN Weiju, CHEN Shiya, XUE Caoye, LIU Bo, ZHENG Jing. Fluorescent Probe for Hypoxia-triggered Imaging and Cancer Therapy [J]. Chem. J. Chinese Universities, 2021, 42(11): 3433. |
| [15] | KE Mengting, YUAN Jiangpei, ZHANG Heng, FANG Yu. Coordination Porous Polymers for Targeting Subcellular Organelles: Bio-imaging, Diagnosis and Therapy [J]. Chem. J. Chinese Universities, 2021, 42(11): 3295. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||