

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (11): 2311.doi: 10.7503/cjcu20150685
• Inorganic Chemistry • Previous Articles Next Articles
WANG Ning, SUN Qiming, YAN Yan, LIU Jiancong, YU Jihong*( ), XU Ruren
), XU Ruren
Received:2015-09-06
Online:2015-11-10
Published:2015-10-26
Contact:
YU Jihong
E-mail:jihong@jlu.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Ning, SUN Qiming, YAN Yan, LIU Jiancong, YU Jihong, XU Ruren. Organotemplate-free Synthesis and Proton Conduction Properties of a Layered Aluminophosphate Na4[Al4P4O18]·H2O†[J]. Chem. J. Chinese Universities, 2015, 36(11): 2311.
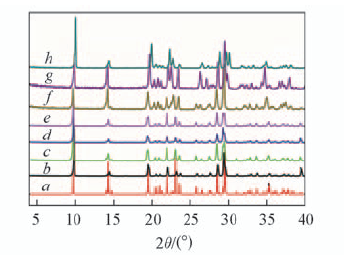
Fig.1 PXRD patterns of as-synthesized compound 1 and calcined samples as compared with the simulated XRD patterns a. Simulated; b. as-synthesized; calcination temperature/℃:c. 100; d. 200; e. 300; f. 400; g. 500; h. 600.
| Empirical formula | Al4P4O19H2Na4 | Calculated density/(Mg·m-3) | 2.694 |
|---|---|---|---|
| Crystal system | Monoclinic | Absorption coefficient/mm-1 | 0.940 |
| Space group | P21/c | F(000) | 616 |
| a/nm | 1.00887(9) | Limiting indices | -13≤h≤7, -11≤k≤11, -11≤l≤13 |
| b/nm | 0.86747(8) | Reflections collected/unique[R(int)=0.0270] | 5485/1935 |
| c/nm | 0.97580(9) | Refinement method | Full-matrix least-squares on F2 |
| α/(°) | 90 | Data/restraints/parameters | 1935/7/152 |
| β/(°) | 115.0160(10) | Goodness-of-fit on F2 | 1.041 |
| γ/(°) | 90 | Final R indices[I>2σ(I)] | R1=0.0307, wR2=0.0795 |
| V/nm3 | 0.77387(12) | R indices(all data) | R1=0.0386, wR2=0.0835 |
| Z | 2 |
Table 1 Crystal data and structure refinement parameters for compound 1
| Empirical formula | Al4P4O19H2Na4 | Calculated density/(Mg·m-3) | 2.694 |
|---|---|---|---|
| Crystal system | Monoclinic | Absorption coefficient/mm-1 | 0.940 |
| Space group | P21/c | F(000) | 616 |
| a/nm | 1.00887(9) | Limiting indices | -13≤h≤7, -11≤k≤11, -11≤l≤13 |
| b/nm | 0.86747(8) | Reflections collected/unique[R(int)=0.0270] | 5485/1935 |
| c/nm | 0.97580(9) | Refinement method | Full-matrix least-squares on F2 |
| α/(°) | 90 | Data/restraints/parameters | 1935/7/152 |
| β/(°) | 115.0160(10) | Goodness-of-fit on F2 | 1.041 |
| γ/(°) | 90 | Final R indices[I>2σ(I)] | R1=0.0307, wR2=0.0795 |
| V/nm3 | 0.77387(12) | R indices(all data) | R1=0.0386, wR2=0.0835 |
| Z | 2 |
| Atom | x | y | z | Ueq | Atom | x | y | z | Ueq |
|---|---|---|---|---|---|---|---|---|---|
| Al1 | 6752(1) | 2981(1) | 7649(1) | 10(1) | O6 | 7890(2) | -346(2) | 10647(2) | 21(1) |
| Al2 | 3834(1) | 3981(1) | 4444(1) | 10(1) | O7 | 3461(2) | -542(2) | 6108(2) | 15(1) |
| P1 | 3981(1) | 1123(1) | 6456(1) | 10(1) | O8 | 5577(2) | 4088(2) | 6003(2) | 12(1) |
| P2 | 8624(1) | 1262(1) | 10900(1) | 11(1) | O9 | 10259(2) | 1107(2) | 11597(2) | 24(1) |
| O1 | 3307(2) | 2031(2) | 4977(2) | 14(1) | O1W | -75(17) | 220(20) | 5430(40) | 43(5) |
| O2 | 5659(2) | 1229(2) | 7131(2) | 14(1) | O1W' | 0 | 0 | 5000 | 43(6) |
| O3 | 8109(2) | 2037(2) | 9368(2) | 18(1) | Na1 | 9545(1) | 7345(2) | 5822(2) | 32(1) |
| O4 | 8167(2) | 2807(2) | 6972(2) | 18(1) | Na2 | 2594(1) | 4450(1) | 7686(2) | 34(1) |
| O5 | 3481(2) | 1845(2) | 7602(2) | 14(1) |
Table 2 Atomic coordinates(×104) and equivalent isotropic displacement parameters(10 nm2) for compound 1
| Atom | x | y | z | Ueq | Atom | x | y | z | Ueq |
|---|---|---|---|---|---|---|---|---|---|
| Al1 | 6752(1) | 2981(1) | 7649(1) | 10(1) | O6 | 7890(2) | -346(2) | 10647(2) | 21(1) |
| Al2 | 3834(1) | 3981(1) | 4444(1) | 10(1) | O7 | 3461(2) | -542(2) | 6108(2) | 15(1) |
| P1 | 3981(1) | 1123(1) | 6456(1) | 10(1) | O8 | 5577(2) | 4088(2) | 6003(2) | 12(1) |
| P2 | 8624(1) | 1262(1) | 10900(1) | 11(1) | O9 | 10259(2) | 1107(2) | 11597(2) | 24(1) |
| O1 | 3307(2) | 2031(2) | 4977(2) | 14(1) | O1W | -75(17) | 220(20) | 5430(40) | 43(5) |
| O2 | 5659(2) | 1229(2) | 7131(2) | 14(1) | O1W' | 0 | 0 | 5000 | 43(6) |
| O3 | 8109(2) | 2037(2) | 9368(2) | 18(1) | Na1 | 9545(1) | 7345(2) | 5822(2) | 32(1) |
| O4 | 8167(2) | 2807(2) | 6972(2) | 18(1) | Na2 | 2594(1) | 4450(1) | 7686(2) | 34(1) |
| O5 | 3481(2) | 1845(2) | 7602(2) | 14(1) |
| Al1—O4 | 0.18147(18) | Al2—O1 | 0.19098(18) | P2—O6 | 0.15493(19) |
|---|---|---|---|---|---|
| Al1—O8 | 0.18163(18) | Al2—Al2#3 | 0.27720(14) | O4—P2#2 | 0.15384(18) |
| Al1—O2 | 0.18198(18) | P1—O7 | 0.15253(17) | O5—Al2#4 | 0.18248(18) |
| Al1—O7#1 | 0.18381(18) | P1—O1 | 0.15275(17) | O6—Al2#5 | 0.18002(19) |
| Al1—O3 | 0.18482(18) | P1—O2 | 0.15375(18) | O7—Al1#5 | 0.18381(18) |
| Al2—O8 | 0.17752(18) | P1—O5 | 0.15413(17) | O8—Al2#3 | 0.18895(17) |
| Al2—O6#1 | 0.18002(19) | P2—O9 | 0.15006(19) | O1W—O1W' | 0.050(4) |
| Al2—O5#2 | 0.18248(18) | P2—O3 | 0.15164(17) | O1W—O1W#6 | 0.099(9) |
| Al2—O8#3 | 0.18895(17) | P2—O4#4 | 0.15384(18) | O1W'—O1W#6 | 0.050(4) |
| O4—Al1—O8 | 92.40(8) | O6#1—Al2—O1 | 85.80(8) | O9—P2—O6 | 110.56(11) |
| O4—Al1—O2 | 107.89(9) | O5#2—Al2—O1 | 87.70(8) | O3—P2—O6 | 107.49(11) |
| O8—Al1—O2 | 95.95(8) | O8#3—Al2—O1 | 177.59(8) | O4#4—P2—O6 | 108.68(10) |
| O8—Al1—O7#1 | 90.01(8) | O6#1—Al2—Al2#3 | 117.05(7) | P1—O2—Al1 | 126.64(10) |
| O2—Al1—O7#1 | 122.63(9) | O5#2—Al2—Al2#3 | 114.53(7) | P2—O3—Al1 | 154.78(12) |
| O4—Al1—O3 | 84.21(8) | O8#3—Al2—Al2#3 | 39.33(5) | P2#2—O4—Al1 | 135.39(11) |
| O8—Al1—O3 | 173.26(9) | O1—Al2—Al2#3 | 138.29(7) | P1—O5—Al2#4 | 124.65(11) |
| O2—Al1—O3 | 90.64(8) | O7—P1—O1 | 107.72(9) | P2—O6—Al2#5 | 133.37(11) |
| O7#1—Al1—O3 | 87.62(8) | O7—P1—O2 | 111.38(10) | P1—O7—Al1#5 | 129.71(10) |
| O8—Al2—O6#1 | 127.69(9) | O1—P1—O2 | 110.14(10) | Al2—O8—Al1 | 140.33(10) |
| O8—Al2—O5#2 | 125.03(9) | O7—P1—O5 | 111.00(10) | Al2—O8—Al2#3 | 98.24(8) |
| O6#1—Al2—O5#2 | 107.27(9) | O1—P1—O5 | 108.93(10) | Al1—O8—Al2#3 | 121.43(9) |
| O8—Al2—O8#3 | 81.76(8) | O2—P1—O5 | 107.65(10) | O1W'—O1W—O1W#6 | 0.002(15) |
| O6#1—Al2—O8#3 | 95.32(8) | O9—P2—O3 | 109.84(11) | O1W—O1W'—O1W#6 | 180.00(2) |
| O5#2—Al2—O8#3 | 94.00(8) | O9—P2—O4#4 | 109.07(11) | ||
| O8—Al2—O1 | 95.87(8) | O3—P2—O4#4 | 111.19(10) |
Table 3 Selected bond lengths(nm) and bond angles(°) for compound 1*
| Al1—O4 | 0.18147(18) | Al2—O1 | 0.19098(18) | P2—O6 | 0.15493(19) |
|---|---|---|---|---|---|
| Al1—O8 | 0.18163(18) | Al2—Al2#3 | 0.27720(14) | O4—P2#2 | 0.15384(18) |
| Al1—O2 | 0.18198(18) | P1—O7 | 0.15253(17) | O5—Al2#4 | 0.18248(18) |
| Al1—O7#1 | 0.18381(18) | P1—O1 | 0.15275(17) | O6—Al2#5 | 0.18002(19) |
| Al1—O3 | 0.18482(18) | P1—O2 | 0.15375(18) | O7—Al1#5 | 0.18381(18) |
| Al2—O8 | 0.17752(18) | P1—O5 | 0.15413(17) | O8—Al2#3 | 0.18895(17) |
| Al2—O6#1 | 0.18002(19) | P2—O9 | 0.15006(19) | O1W—O1W' | 0.050(4) |
| Al2—O5#2 | 0.18248(18) | P2—O3 | 0.15164(17) | O1W—O1W#6 | 0.099(9) |
| Al2—O8#3 | 0.18895(17) | P2—O4#4 | 0.15384(18) | O1W'—O1W#6 | 0.050(4) |
| O4—Al1—O8 | 92.40(8) | O6#1—Al2—O1 | 85.80(8) | O9—P2—O6 | 110.56(11) |
| O4—Al1—O2 | 107.89(9) | O5#2—Al2—O1 | 87.70(8) | O3—P2—O6 | 107.49(11) |
| O8—Al1—O2 | 95.95(8) | O8#3—Al2—O1 | 177.59(8) | O4#4—P2—O6 | 108.68(10) |
| O8—Al1—O7#1 | 90.01(8) | O6#1—Al2—Al2#3 | 117.05(7) | P1—O2—Al1 | 126.64(10) |
| O2—Al1—O7#1 | 122.63(9) | O5#2—Al2—Al2#3 | 114.53(7) | P2—O3—Al1 | 154.78(12) |
| O4—Al1—O3 | 84.21(8) | O8#3—Al2—Al2#3 | 39.33(5) | P2#2—O4—Al1 | 135.39(11) |
| O8—Al1—O3 | 173.26(9) | O1—Al2—Al2#3 | 138.29(7) | P1—O5—Al2#4 | 124.65(11) |
| O2—Al1—O3 | 90.64(8) | O7—P1—O1 | 107.72(9) | P2—O6—Al2#5 | 133.37(11) |
| O7#1—Al1—O3 | 87.62(8) | O7—P1—O2 | 111.38(10) | P1—O7—Al1#5 | 129.71(10) |
| O8—Al2—O6#1 | 127.69(9) | O1—P1—O2 | 110.14(10) | Al2—O8—Al1 | 140.33(10) |
| O8—Al2—O5#2 | 125.03(9) | O7—P1—O5 | 111.00(10) | Al2—O8—Al2#3 | 98.24(8) |
| O6#1—Al2—O5#2 | 107.27(9) | O1—P1—O5 | 108.93(10) | Al1—O8—Al2#3 | 121.43(9) |
| O8—Al2—O8#3 | 81.76(8) | O2—P1—O5 | 107.65(10) | O1W'—O1W—O1W#6 | 0.002(15) |
| O6#1—Al2—O8#3 | 95.32(8) | O9—P2—O3 | 109.84(11) | O1W—O1W'—O1W#6 | 180.00(2) |
| O5#2—Al2—O8#3 | 94.00(8) | O9—P2—O4#4 | 109.07(11) | ||
| O8—Al2—O1 | 95.87(8) | O3—P2—O4#4 | 111.19(10) |
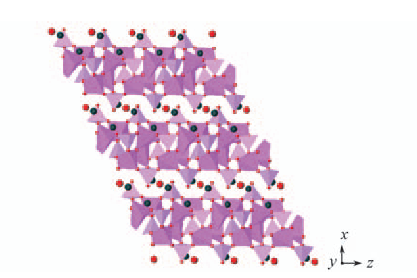
Fig.5 Polyhedral view of the structure of compound 1 showing interlayer stacking along [100] direction Na+ ions and H2O located between the layers are presented as balls. Na+ ions in blue; H2O in red.
| [1] | Li Y., Yu J. H., Chem. Rev., 2014, 114(14), 7268—7316 |
| [2] | Li J. Y., Corma A., Yu J. H., Chem. Soc. Rev., 2015, 44(6), 7112—7127 |
| [3] | Li Y., Yu J. H., Xu R. R., Angew. Chem. Int. Ed., 2013, 52(6), 1673—1677 |
| [4] | Wilson S. T., Lok B. M., Messina C. A., Cannan T. R., Flanigen E. M., J. Am. Chem. Soc., 1982, 104(4), 1146—1147 |
| [5] | Yu J. H., Xu R. R., Acc. Chem. Res., 2010, 43(9), 1195—1204 |
| [6] | Yu J. H., Xu R. R., Chem. Soc. Rev., 2006, 35(7), 593—604 |
| [7] | Yu J. H., Xu R. R., Acc. Chem. Res., 2003, 36(7), 481—490 |
| [8] | Yu J. H., Li J. Y., Xu R. R., Micropor. Mesopor. Mat., 2001, 48(1—3), 47—56 |
| [9] | Yan W. F., Yu J. H., Li Y., Shi Z., Xu R. R., J. Solid State Chem., 2002, 167(2), 282—288 |
| [10] | Yu J. H., Sugiyama K., Hiraga K., Togashi N., Terasaki O., Tanaka Y., Nakata S., Qiu S. L., Xu R. R., Chem. Mater., 1998, 10(11), 3636—3642 |
| [11] | Yu J. H., Williams I. D., J. Solid State Chem., 1998, 136(1), 141—144 |
| [12] | Lu H. Y., Tong X. Q., Yan Y., Yan W. F., Yu J. H., Xu R. R., Chem. J. Chinese Universities, 2013, 34(7), 1571—1575 |
| (卢慧英, 仝晓强, 颜岩, 闫文付, 于吉红, 徐如人. 高等学校化学学报, 2013, 34(7), 1571—1575) | |
| [13] | Tuel A., Gramlich V., Baerlocher C., J. Solid State Chem., 2004, 177(7), 2484—2493 |
| [14] | Shao L., Li Y., Wang X. F., Yu J. H., Chem. J. Chinese Universities, 2013, 34(8), 1806—1811 |
| (邵浪, 李乙, 王晓芳, 于吉红, 高等学校化学学报, 2013, 34(8), 1806—1811) | |
| [15] | Tuel A., Lorentz C., Gramlich V., Baerlocher C., J. Solid State Chem., 2005, 178(7), 2322—2331 |
| [16] | Zhou D., Chen L., Yu J. H., Li Y., Yan W. F., Deng F., Xu R. R., Inorg. Chem., 2005, 44(12), 4391—4397 |
| [17] | Wang M., Li J. Y., Li Y., Song X. W., Yu J. H., Xu R. R., Chem. J. Chinese Universities, 2005, 26(6), 1027—1029 |
| (王梅, 李激扬, 李乙, 宋晓伟, 于吉红, 徐如人. 高等学校化学学报, 2005, 26(6), 1027—1029) | |
| [18] | Marichal C., Chezeau J. M., Roux M., Patarin J., Jorda J. L., McCusker L. B., Baerlocher C., Pattison P., Micropor. Mesopor. Mat., 2006, 90(1), 5—15 |
| [19] | Wang M., Li J. Y., Pan Q. H., Yu J. H., Song X. W., Xu R. R., Solid State Sci., 2006, 8(9), 1079—1084 |
| [20] | Zhang M., Zhou D., Li J. Y., Yu J. H., Xu J., Deng F., Li G. H., Xu R. R., Inorg. Chem., 2007, 46(1), 136—140 |
| [21] | Chen P., Li J. Y., Xu J., Duan F. Z., Deng F., Xu R. R., Solid State Sci., 2009, 11(3), 622—627 |
| [22] | Li S. B., Dou Z. Y., He X. Q., Cui L. L., Zhang Y. T., Chem. J. Chinese Universities, 2013, 34(2), 319—323 |
| (李双宝, 窦志宇, 何兴权, 崔丽莉, 张誉腾. 高等学校化学学报, 2013, 34(2), 319—323) | |
| [23] | Tian Y., Wang S. R., Yan W. F., Xu R. R., Wang Y. R., Mu X. H., Chem. J. Chinese Universities, 2015, 36(3), 428—435 |
| (田野, 王淑荣, 闫文付, 徐如人, 王永睿, 慕旭宏. 高等学校化学学报, 2015, 36(3), 428—435) | |
| [24] | Yu J. H., Li J. Y., Sugiyama K., Togashi N., Terasaki O., Hiraga K., Zhou B., Qiu S. L., Xu R. R., Chem. Mater., 1999, 11(7), 1727—1732 |
| [25] | Wang Y. Y., Sun Y. J., Mu Y., Zhang C. Q., Li J. Y., Yu J. H., Chem. Commun., 2014, 50(97), 15400—15403 |
| [26] | Mu Y., Wang Y. Y., Li Y., Li J. Y., Yu J. H., Chem. Commun., 2015, 51(11), 2149—2151 |
| [27] | Sun Y. J., Yan Y., Wang Y. Y., Li Y., Li J. Y., Yu J. H., Chem. Commun., 2015, 51(45), 9317—9319 |
| [28] | Kongshaug K. O., Fjellvåg H., Lillerud. K. P., Micropor. Mesopor. Mat., 1999, 32(1/2), 17—28 |
| [29] | Felice V., Tavares A. C., Solid State Ionics, 2011, 194(1), 53—61 |
| [30] | Wu G. D., Zhang H. L., Zhou J. M., Huang A. S., Wan Q., J. Mater. Chem. C, 2013, 1(36), 5669—5674 |
| [31] | Mikhailenko S. D., Kaliaguine S., Ghali E., Micropor. Mat., 1997, 11(1/2), 37—44 |
| [32] | Yeung K. L., Han W., Catal. Today, 2014, 236, 182—205 |
| [33] | Liang X. Q., Zhang F., Feng W., Zou X. Q., Zhao C. J., Na H., Liu C., Sun F. X., Zhu G. S., Chem. Sci., 2013, 4(3), 983—992 |
| [1] | YAO Yiting, LYU Jiamin, YU Shen, LIU Zhan, LI Yu, LI Xiaoyun, SU Baolian, CHEN Lihua. Preparation of Hierarchical Microporous-mesoporous Fe2O3/ZSM-5 Hollow Molecular Sieve Catalytic Materials and Their Catalytic Properties for Benzylation [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220090. |
| [2] | CHEN Weiqin, LYU Jiamin, YU Shen, LIU Zhan, LI Xiaoyun, CHEN Lihua, SU Baolian. Preparation of Organic Hybrid Mesoporous Beta Zeolite for Alkylation of Mesitylene with Benzyl Alcohol [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220086. |
| [3] | LI Zhiguang, QI Guodong, XU Jun, DENG Feng. Role of Catalyst Acidity in Glucose Conversion over Sn-Al-β Zeolite as Studied by Solid-state NMR [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220138. |
| [4] | LI Jiafu, ZHANG Kai, WANG Ning, SUN Qiming. Research Progress of Zeolite-encaged Single-atom Metal Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220032. |
| [5] | MENG Xianglong, YANG Ge, GUO Hailing, LIU Chenguang, CHAI Yongming, WANG Chunzheng, GUO Yongmei. Synthesis of Nano-zeolite and Its Adsorption Performance for Hydrogen Sulfide [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210687. |
| [6] | WEI Lina, PENG Li, ZHU Feng, GU Pengfei, GU Xuehong. Preparation of Au-CeZr/FAU Catalytic Membranes for Preferential Oxidation of CO in H2-rich Stream [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220175. |
| [7] | LIANG Yu, LIU Huan, GONG Lige, WANG Chunxiao, WANG Chunmei, YU Kai, ZHOU Baibin. Synthesis and Supercapacitor Properties of Biimidazole-modified {SiW12O40} Hybrid [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210556. |
| [8] | LUO Qiangqiang, JIN Shaoqing, SUN Hongmin, YANG Weimin. Post-synthesis of Ti-MWW Zeolite via Titanium Incorporation in Liquid Acid Solution [J]. Chem. J. Chinese Universities, 2021, 42(9): 2742. |
| [9] | LI Yichuan, ZHU Guofu, WANG Yu, CHAI Yongming, LIU Chenguang, HE Shengbao. Effects of Substrate Surface Properties and Precursor Chemical Environment on In⁃situ Oriented Construction of Titanium Silicalite Zeolite Membranes [J]. Chem. J. Chinese Universities, 2021, 42(9): 2934. |
| [10] | ZHANG Xu, QUE Jiaqian, HOU Yuexin, LYU Jiamin, LIU Zhan, LEI Kunhao, YU Shen, LI Xiaoyun, CHEN Lihua, SU Baolian. Hierarchical Mesoporous-microporous TS-1 Single Crystal Catalysts for Epoxidation of Allyl Chloride [J]. Chem. J. Chinese Universities, 2021, 42(8): 2529. |
| [11] | WANG Lei, SUN Tantan, YAN Nana, MA Chao, LIU Xiaona, TIAN Peng, GUO Peng, LIU Zhongmin. Exploring Organic Structure-directing Agents Used for SAPO-34 to Synthesize SSZ-13 [J]. Chem. J. Chinese Universities, 2021, 42(6): 1716. |
| [12] | ZHANG Huishuang, GAO Yanxiao, WANG Qiuxian, LI Xiangnan, LIU Wenfeng, YANG Shuting. High-low Temperature Properties of Ni-rich LiNi0.6Co0.2Mn0.2O2 Cathode Material by Hydrothermal Synthesis with CTAB Assisted [J]. Chem. J. Chinese Universities, 2021, 42(3): 819. |
| [13] | WANG Ye, ZHANG Xiaosi, SUN Lijing, LI Bing, LIU Lin, YANG Miao, TIAN Peng, LIU Zhongyi, LIU Zhongmin. Morphology Control of SAPO Molecular Sieves under the Assistance of Organosilane [J]. Chem. J. Chinese Universities, 2021, 42(3): 683. |
| [14] | LI Jian, YU Mingming, SUN Yuan, FENG Wenhua, FENG Zhaochi, WU Jianfeng. Effect of Aqueous Solution pH on the Oxidation of Methane to Methanol at Low Temperature [J]. Chem. J. Chinese Universities, 2021, 42(3): 776. |
| [15] | LI Hongbin, ZHANG Shuai, LI Zheng, DING Changjiang, BEN Teng. Synthesis and Anisotropic Proton Conduction of Porous Organic Salt Single Crystal [J]. Chem. J. Chinese Universities, 2021, 42(10): 3047. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||