

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (12): 2491.doi: 10.7503/cjcu20150365
• Physical Chemistry • Previous Articles Next Articles
DUAN Xinli, ZHANG Xin*( ), LEI Ming*(
), LEI Ming*( )
)
Received:2015-05-08
Online:2015-12-10
Published:2015-11-17
Contact:
ZHANG Xin,LEI Ming
E-mail:zhangxin@mail.buct.edu.cn;leim@mail.buct.edu.cn
Supported by:CLC Number:
TrendMD:
DUAN Xinli, ZHANG Xin, LEI Ming. QM/MM Molecular Dynamics Simulation on the Mechanisms for the Hydrolytic Deamination of Nicotinamidase†[J]. Chem. J. Chinese Universities, 2015, 36(12): 2491.
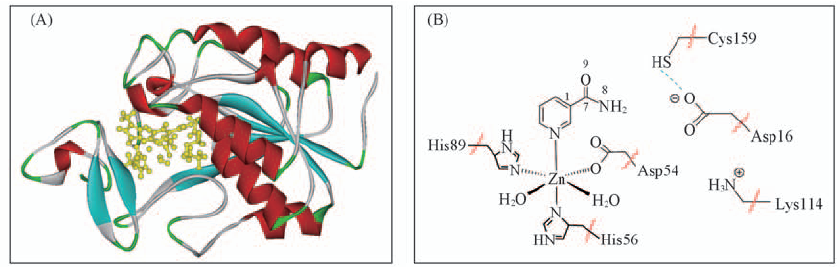
Fig.1 Crystal structure of nicotinamidase(A)(PDB entry: 2WTA) and details in QM part(B) (A) The active site with nicotinamide is highlighted in yellow; (B) QM/MM boundary is shown by the red break lines.
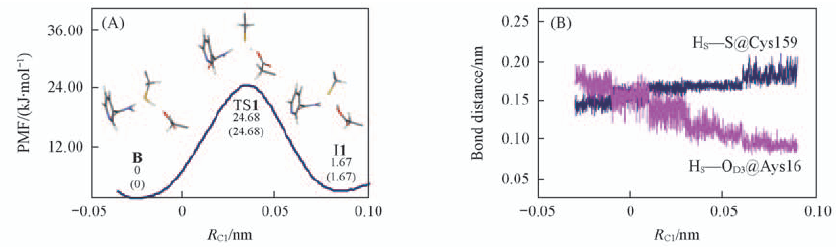
Fig.3 Free energy profile from the binding state B to the intermediate state(I1)(A) and fluctuations of the distances of the breaking and forming bonds in HS transfer process(B) RC1 refers to the distance difference between the HS—S@Cys159 and HS—OD3@Asp16.
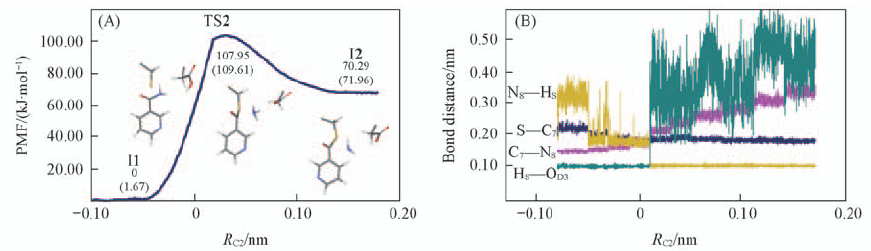
Fig.4 Free energy profile from the intermediate state(I1) to the intermediate state(I2) via a transition state(TS2)(A) and fluctuations of the distances of the breaking and forming bonds in nucleophilic attack of sulfur(B) RC2 refers to the distance difference between the C7—S@Cys159 and C7—N8.
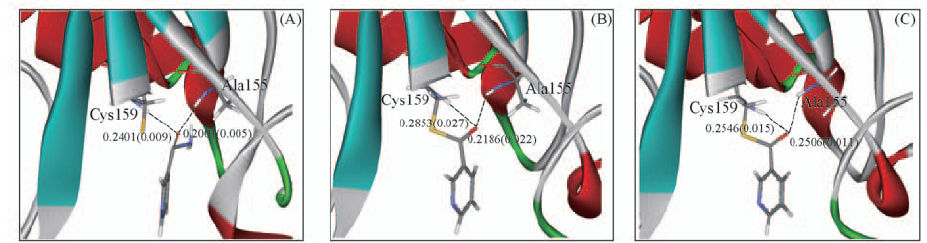
Fig.5 Snapshots of the micro-environments for QM part at the I1(A), TS2(B) and I2 states(C) Hydrogen bond lengths(nm) in step 2 are shown by the dash lines. The values in parentheses are standard deviations.
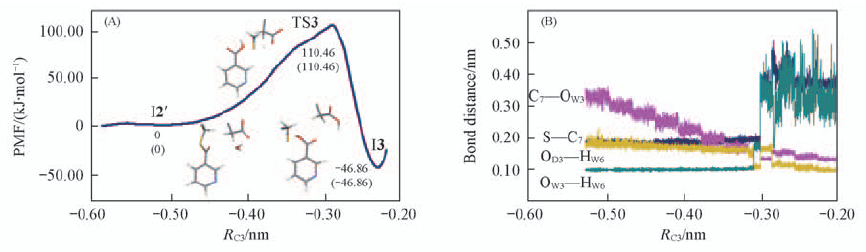
Fig.6 Free energy profile from the intermediate state(I2') to the intermediate state(I3) via a transition state(TS3)(A) and fluctuations of the distances of the breaking and forming bonds in nucleophilic attack of hydroxyl group(B) RC3 refers to the minus distance between the OW3—C7 and HW6—OD3.
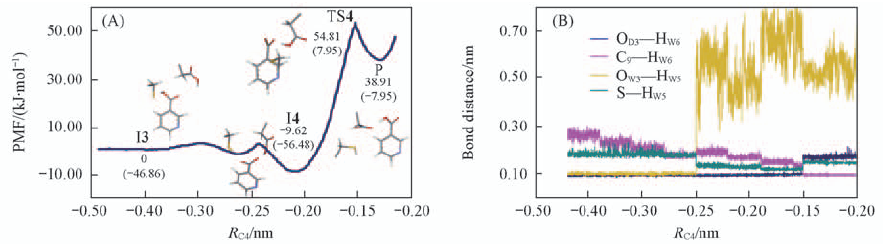
Fig.7 Free energy profile from the intermediate state(I3) to the product state(P) via a transition state(TS4)(A) and fluctuations of the distances of the breaking and forming bonds in nicotinic acid formation process(B) RC4 refers to the minus distance between the O9—HW6 and HW5—S.
| [1] | Jia Z. P., Hu X. G., Fang G. Y., Chem. J. Chinese Universities,2014, 35(2), 384—388 |
| (贾召鹏, 胡新根, 方国勇. 高等学校化学学报,2014, 35(2), 384—388) | |
| [2] | Knip M., Douek I. F., Moore W. P. T., Gillmor H. A., McLean A. E. M., Bingley P. J., Gale E. A. M., Diabetologia, 2000, 43(11), 1337—1345 |
| [3] | Belenky P., Bogan K. L., Brenner C., Trends Biochem. Sci., 2007, 32(1), 12—19 |
| [4] | Niren N. M., Cutis., 2006, 77(S1), 11—16 |
| [5] | Tallman J. F., Paul S. M., Skolnick P., Gallager D. W., Science, 1980, 207(4428), 274—281 |
| [6] | Damian D. L., Patterson C. R. S., Stapelberg M., Park J., Barnetson R. S. C., Halliday G. M., J. Invest. Dermatol., 2007, 128(2), 447—454 |
| [7] | Hakozaki T., Minwalla L., Zhuang J., Chhoa M., Matsubara A., Miyamoto K., Greatens A., Hillebrand G. G., Bissett D. L., Boissy R. E., Br. J. Dermatol., 2002, 147(1), 20—31 |
| [8] | Gerdes S. Y., Scholle M. D., D’Souza M., Bernal A., Baev M. V., Farrell M., Kurnasov O. V., Daugherty M. D., Mseeh F., Polanuyer B. M., J. Bacteriol., 2002, 184(16), 4555—4572 |
| [9] | Boshoff H. I. M., Mizrahi V., J. Bacteriol., 1998, 180(22), 5809—5814 |
| [10] | Zhang H., Deng J. Y., Bi L. J., Zhou Y. F., Zhang Z. P., Zhang C. G., Zhang Y., Zhang X. E., FEBS J., 2008, 275(4), 753—762 |
| [11] | Scorpio A., Zhang Y., Nat. Med., 1996, 2(6), 662—667 |
| [12] | Ghislain M., Talla E., François J. M., Yeast, 2002, 19(3), 215—224 |
| [13] | Hu G., Taylor A. B., McAlister-Henn L., Hart P. J., Arch. Biochem. Biophys., 2007, 461(1), 66—75 |
| [14] | Zerez C., Roth E., Schulman S., Tanaka K., Blood, 1990, 75(8), 1705—1710 |
| [15] | Sheng X., Liu Y., Org. Biomol. Chem., 2014, 12(8), 1265—1277 |
| [16] | Fyfe P. K., Rao V. A., Zemla A., Cameron S., Hunter W. N., Angewandte Chemie., 2009, 121(48), 9340—9343 |
| [17] | French J. B., Cen Y., Vrablik T. L., Xu P., Allen E., Hanna-Rose W., Sauve A. A., Biochemistry, 2010, 49(49), 10421—10439 |
| [18] | Zhang F. D., Liu Y., Xu J. C., Li S. J., Wang X. N., Sun Y., Zhao X. L., Chem. J. Chinese Universities,2015, 36(6), 1156—1165 |
| (张法达, 刘轶, 徐京城, 李生娟, 汪秀南, 孙玥, 赵新洛. 高等学校化学学报,2015, 36(6), 1156—1165) | |
| [19] | Dong L., Yi Z. S., Wu Z. W., Wang H. Y., Zhang A. Q., Chem. J. Chinese Universities,2015, 36(3), 516—522 |
| (董露, 易忠胜, 伍智蔚, 王海洋, 张爱茜. 高等学校化学学报,2015, 36(3), 516—522) | |
| [20] | French J. B., Cen Y., Sauve A. A., Ealick S. E., Biochemistry, 2010, 49(40), 8803—8812 |
| [21] | Zhang X., Wu R., Song L., Lin Y., Lin M., Cao Z., Wu W., Mo Y., J. Comput. Chem., 2009, 30(15), 2388—2401 |
| [22] | Zhang X., Lei M., J. Theor. Comput. Chem., 2013, 12(8), 1341002-1—14 |
| [23] | Yuan T., Zhang X., Hu Z., Wang F., Lei M., Biopolymers, 2012, 97(12), 998—1009 |
| [24] | Zhao L. J., Zhang L. R., Lei M., Sci. China Chem., 2013, 56(11), 1550—1563 |
| [25] | Stewart J. J. P., J. Comput. Chem., 1989, 10(2), 209—220 |
| [26] | Stewart J. J. P., J. Mol. Model., 2007, 13(11), 1173—1213 |
| [27] | Brothers E. N., Suarez D., Deerfield D. W., Merz K. M., J. Comput. Chem., 2004, 25(14), 1677—1692 |
| [28] | Gao J. L., Amara P., Alhambra C., Field M. J., J. Phys. Chem. A,1998, 102(24), 4714—4721 |
| [29] | MacKerell A. D., Bashford D., Bellott M., Dunbrack R. L., Evanseck J. D., Field M. J., Fischer S., Gao J., Guo H., Ha S., Joseph-McCarthy D., Kuchnir L., Kuczera K., Lau F. T. K., Mattos C., Michnick S., Ngo T., Nguyen D. T., Prodhom B., Reiher W. E., Roux B., Schlenkrich M., Smith J. C., Stote R., Straub J., Watanabe M., Wiorkiewicz-Kuczera J., Yin D., Karplus M., J. Phys. Chem. B,1998, 102(18), 3586—3616 |
| [30] | Brooks B. R., Bruccoleri R. E., Olafson B. D., States D. J., Swaminathan S., Karplus M., J. Comput. Chem., 1983, 4(2), 187—217 |
| [31] | Van Gunsteren W. F., Berendsen H. J. C., Mol. Phys., 1977, 34(5), 1311—1327 |
| [32] | Torrie G. M., Valleau J. P., J. Chem. Phys., 1977, 23(2), 187—199 |
| [1] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| [2] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [3] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [4] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [5] | CHANG Yunfei, LIAO Mingyi, WEN Jiaming. Reduction Performance and Mechanism of Liquid Terminated-carboxyl Fluoroelastomers Using NaBH4/MCl x Reduction System [J]. Chem. J. Chinese Universities, 2022, 43(6): 20210835. |
| [6] | REN Nana, XUE Jie, WANG Zhifan, YAO Xiaoxia, WANG Fan. Effects of Thermodynamic Data on Combustion Characters of 1,3-Butadiene [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220151. |
| [7] | GAO Zhiwei, LI Junwei, SHI Sai, FU Qiang, JIA Junru, AN Hailong. Analysis of Gating Characteristics of TRPM8 Channel Based on Molecular Dynamics [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220080. |
| [8] | ZHANG Shiyu, HE Runhe, LI Yongbing, WEI Shijun, ZHANG Xingxiang. Fabrication of Lithium-sulfur Battery Cathode with Radiation Crosslinked Low Molecular Weight of Polyacrylonitrile and the Mechanism of Sulfur Storage [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210632. |
| [9] | BI Gening, XIAO Xiaohua, LI Gongke. Development and Validation of Multiple Physical Fields Coupling Model for Microwave-assisted Extraction [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210739. |
| [10] | SUN Cuihong, LYU Liqiang, LIU Ying, WANG Yan, YANG Jing, ZHANG Shaowen. Mechanism and Kinetics on the Reaction of Isopropyl Nitrate with Cl, OH and NO3 Radicals [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210591. |
| [11] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [12] | WEN Zhiguo, QIAO Zaiyin, TIAN Chong, MAXIM Borzov, NIE Wanli. Catalytic Activity and Reaction Mechanism of FLPs for the Reduction of Enamine [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220555. |
| [13] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [14] | MENG Fanwei, GAO Qi, YE Qing, LI Chenxi. Potassium Poisoning Mechanism of Cu-SAPO-18 Catalyst for Selective Catalytic Reduction of NOx by Ammonia [J]. Chem. J. Chinese Universities, 2021, 42(9): 2832. |
| [15] | YANG Yiying, ZHU Rongxiu, ZHANG Dongju, LIU Chengbu. Theoretical Study on Gold-catalyzed Cyclization of Alkynyl Benzodioxin to 8-Hydroxy-isocoumarin [J]. Chem. J. Chinese Universities, 2021, 42(7): 2299. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||