

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (4): 660.doi: 10.7503/cjcu20141016
• Organic Chemistry • Previous Articles Next Articles
Received:2014-11-19
Online:2015-04-10
Published:2015-03-19
Contact:
ZHU Min
E-mail:hzzm60@163.com
Supported by:CLC Number:
TrendMD:
FANG Yingguo, ZHU Min. Synthesis of 3,5-Disubstituted Isoxazolines Mediated by Catalytic Alkyl Iodide†[J]. Chem. J. Chinese Universities, 2015, 36(4): 660.
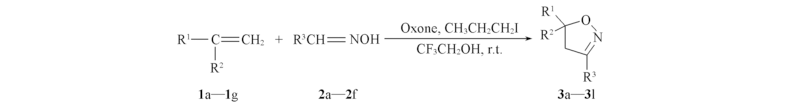
Scheme 1 Synthetic route of compound 3 3a: R1=R3=Ph, R2=H; 3b: R1=R3=Ph, R2=Me; 3c: R1=p-BrC6H4, R2=H, R3=Ph; 3d: R1=PhCH2, R2=H, R3=Ph; 3e: R1=n-C4H9, R2=H, R3=Ph; 3f: R1=MeCO2, R2=H, R3=Ph; 3g: R1=BrCH2, R2=H, R3=Ph; 3h: R1=Ph, R2=H, R3=p-MeC6H4; 3i: R1=Ph, R2=H, R3=p-MeOC6H4; 3j: R1=p-FC6H4, R2=H, R3= Ph; 3k: R1=o-HOC6H4, R2=H, R3= Ph; 3l: R1=o-C6H4, R2=H, R3= Ph
| Compd. | m.p.(ref.)/℃ | Compd. | m.p.(ref.)/℃ |
|---|---|---|---|
| 3a | 72—73(74—75[ | 3g | 60—62(59—61[ |
| 3b | 75—76(72—76[ | 3h | 96—97(97[ |
| 3c | 128—129(129.8—130.3[ | 3i | 103—105(103—104[ |
| 3d | 67—69(67.5—68.2[ | 3j | 88—90(91—92[ |
| 3e | 39—41(40—42[ | 3m | 136—138(138—139[ |
| 3f | 69—71(71—72.5[ |
Table 1 Melting point(m. p.) of 3,5-disubstituted isoxazolines 3a—3j, 3m
| Compd. | m.p.(ref.)/℃ | Compd. | m.p.(ref.)/℃ |
|---|---|---|---|
| 3a | 72—73(74—75[ | 3g | 60—62(59—61[ |
| 3b | 75—76(72—76[ | 3h | 96—97(97[ |
| 3c | 128—129(129.8—130.3[ | 3i | 103—105(103—104[ |
| 3d | 67—69(67.5—68.2[ | 3j | 88—90(91—92[ |
| 3e | 39—41(40—42[ | 3m | 136—138(138—139[ |
| 3f | 69—71(71—72.5[ |
| Entry | n(Styrene):n(RI)a:n(Oxidant)b | Solvent | Time/h | Yieldc(%) |
|---|---|---|---|---|
| 1 | 1.1:0.2:1.3 | CH3OH | 12 | 25 |
| 2 | 1.1:0.2:1.3 | CH3CH2OH | 12 | Trace |
| 3 | 1.1:0.2:1.3 | CH2Cl2 | 12 | 8 |
| 4 | 1.1:0.2:1.3 | THF | 12 | Trace |
| 5 | 1.1:0.2:1.3 | H2O | 12 | 65 |
| 6 | 1.1:0.2:1.3 | DMF | 12 | 5 |
| 7 | 1.1:0.2:1.3 | CH3CN | 12 | 6 |
| 8 | 1.1:0.2:1.3 | CF3CH2OH | 12 | 87 |
| 9 | 1.1:0:1.3 | CF3CH2OH | 12 | 0 |
| 10 | 1.5:0.2:1.3 | CF3CH2OH | 12 | 89 |
| 11 | 1.7:0.2:1.3 | CF3CH2OH | 12 | 93 |
| 12 | 2.0:0.2:1.3 | CF3CH2OH | 12 | 92 |
| 13 | 1.7:0.2:1.1 | CF3CH2OH | 12 | 65 |
| 14 | 1.7:0.2:1.5 | CF3CH2OH | 12 | 92 |
| 15 | 1.7:0.2:1.7 | CF3CH2OH | 12 | 93 |
| 16 | 1.7:0.2:2.0 | CF3CH2OH | 12 | 92 |
| 17 | 1.7:0.1:1.3 | CF3CH2OH | 12 | 83 |
| 18 | 1.7:0.3:1.3 | CF3CH2OH | 12 | 95 |
| 19 | 1.7:0.4:1.3 | CF3CH2OH | 12 | 94 |
| 20 | 1.7:0.2:1.3 | CF3CH2OH | 12 | 78 |
| 21 | 1.7:0.2:1.3 | CF3CH2OH | 12 | 93 |
| 22 | 1.7:0.2:1.3 | CF3CH2OH | 12 | 92 |
| 23 | 1.7:0.2:1.3 | CF3CH2OH | 12 | 71 |
| 24 | 1.7:0.2:1.3 | CF3CH2OH | 12 | 77 |
| 25 | 1.7:0.2:1.3 | CF3CH2OH | 3 | 43 |
| 26 | 1.7:0.2:1.3 | CF3CH2OH | 4 | 49 |
| 27 | 1.7:0.2:1.3 | CF3CH2OH | 6 | 56 |
| 28 | 1.7:0.2:1.3 | CF3CH2OH | 24 | 92 |
Table 2 Optimization of the reaction conditions
| Entry | n(Styrene):n(RI)a:n(Oxidant)b | Solvent | Time/h | Yieldc(%) |
|---|---|---|---|---|
| 1 | 1.1:0.2:1.3 | CH3OH | 12 | 25 |
| 2 | 1.1:0.2:1.3 | CH3CH2OH | 12 | Trace |
| 3 | 1.1:0.2:1.3 | CH2Cl2 | 12 | 8 |
| 4 | 1.1:0.2:1.3 | THF | 12 | Trace |
| 5 | 1.1:0.2:1.3 | H2O | 12 | 65 |
| 6 | 1.1:0.2:1.3 | DMF | 12 | 5 |
| 7 | 1.1:0.2:1.3 | CH3CN | 12 | 6 |
| 8 | 1.1:0.2:1.3 | CF3CH2OH | 12 | 87 |
| 9 | 1.1:0:1.3 | CF3CH2OH | 12 | 0 |
| 10 | 1.5:0.2:1.3 | CF3CH2OH | 12 | 89 |
| 11 | 1.7:0.2:1.3 | CF3CH2OH | 12 | 93 |
| 12 | 2.0:0.2:1.3 | CF3CH2OH | 12 | 92 |
| 13 | 1.7:0.2:1.1 | CF3CH2OH | 12 | 65 |
| 14 | 1.7:0.2:1.5 | CF3CH2OH | 12 | 92 |
| 15 | 1.7:0.2:1.7 | CF3CH2OH | 12 | 93 |
| 16 | 1.7:0.2:2.0 | CF3CH2OH | 12 | 92 |
| 17 | 1.7:0.1:1.3 | CF3CH2OH | 12 | 83 |
| 18 | 1.7:0.3:1.3 | CF3CH2OH | 12 | 95 |
| 19 | 1.7:0.4:1.3 | CF3CH2OH | 12 | 94 |
| 20 | 1.7:0.2:1.3 | CF3CH2OH | 12 | 78 |
| 21 | 1.7:0.2:1.3 | CF3CH2OH | 12 | 93 |
| 22 | 1.7:0.2:1.3 | CF3CH2OH | 12 | 92 |
| 23 | 1.7:0.2:1.3 | CF3CH2OH | 12 | 71 |
| 24 | 1.7:0.2:1.3 | CF3CH2OH | 12 | 77 |
| 25 | 1.7:0.2:1.3 | CF3CH2OH | 3 | 43 |
| 26 | 1.7:0.2:1.3 | CF3CH2OH | 4 | 49 |
| 27 | 1.7:0.2:1.3 | CF3CH2OH | 6 | 56 |
| 28 | 1.7:0.2:1.3 | CF3CH2OH | 24 | 92 |
| Compd. | Time/h | Yielda(%) | Compd. | Time/h | Yielda(%) |
|---|---|---|---|---|---|
| 3a | 12 | 93(88)b | 3h | 12 | 80(76)b |
| 3b | 12 | 60 | 3i | 12 | 73 |
| 3c | 12 | 81(92)b | 3j | 12 | 76 |
| 3d | 24 | 79(81)b | 3k | 24 | 62 |
| 3e | 24 | 65 | 3l | 24 | 61 |
| 3f | 12 | 85 | 3m | 24 | 48(8)b |
| 3g | 24 | 70 |
Table 3 Results of isoxazolines 3a—3m
| Compd. | Time/h | Yielda(%) | Compd. | Time/h | Yielda(%) |
|---|---|---|---|---|---|
| 3a | 12 | 93(88)b | 3h | 12 | 80(76)b |
| 3b | 12 | 60 | 3i | 12 | 73 |
| 3c | 12 | 81(92)b | 3j | 12 | 76 |
| 3d | 24 | 79(81)b | 3k | 24 | 62 |
| 3e | 24 | 65 | 3l | 24 | 61 |
| 3f | 12 | 85 | 3m | 24 | 48(8)b |
| 3g | 24 | 70 |
| [1] | Kozikowski A. P., Acc. Chem. Res., 1984, 17(12), 410—416 |
| [2] | Park K. K., Ko D. H., You Z., Khan M. O. F., Lee H. J., Steroids,2006, 71(3), 183—188 |
| [3] | Lamani R. S., Shetty N. S., Kamble R. R., Khazi I. A. M., Eur. J. Med. Chem., 2009, 44(7), 2828—2833 |
| [4] | Pulkkinen J. T., Honkakoski P., Perakyla M., Berczi I., Laatikainen R., J. Med. Chem., 2008, 51(12), 3562—3571 |
| [5] | Sammelson R. E., Ma T., Galietta L. J. V., Verkman A. S., Kurth M. J., Bioorg. Med. Chem. Lett., 2003, 13(15), 2509—2512 |
| [6] | Huisgen R., 1,3-Dipolar Cycloaddition Chemictry, Wiley, New York, 1984, 1/2, 55—92 |
| [7] | Torssell K.B. G., Nitrile Oxides, Nitrones, and Nitronates in Organic Synthesis, VCH, New York, 1988, 1—38 |
| [8] | Grundmann C.,Synthesis, 1970, (7), 344—359 |
| [9] | Larsen K. E., Torssell K. B. G., Tetrahedron,1984, 40(15), 2985—2988 |
| [10] | Amstrong S.K., Collington E. W., Knight J. G., Naylor A., Warren S., J. Chem. Soc., Perkin Trans. 1, 1993, (13), 1433—1447 |
| [11] | Lee G.A.,Synthesis, 1982, (6), 508—509 |
| [12] | Gi H. J., Xiang Y., Schinazi R. F., Zhao K., J. Org. Chem., 1997, 62(1), 88—92 |
| [13] | Kanemasa S., Matsuda H., Kamimura A., Kakinami T., Tetrahedron,2000, 56(8), 1057—1064 |
| [14] | Kumar V., Kaushik M. P., Tetrahedron Lett., 2006, 47(9), 1457—1460 |
| [15] | Dubrovskiy A. V., Larock R. C., Org. Lett., 2010, 12(6), 1180—1183 |
| [16] | Minakata S., Okumura S., Nagamachi T., Takeda Y., Org. Lett., 2011, 13(11), 2966—2969 |
| [17] | Jawalekar A. M., Reubsaet E., Rutjes F. P. J. T., van Delft F. L., Chem. Commun., 2011, 47(12), 3198—3200 |
| [18] | Medelsohn B. A., Lee D., Kim S., Teyssier F., Aulakh V. S., Ciufolini M. A., Org. Lett., 2009, 11(7), 1539—1542 |
| [19] | Das B., Holla H., Mahender G., Venkateswarlu K., Bangar B.P.,Synthesis, 2005, (10), 1572—1574 |
| [20] | Raihan M. J., Kavala V., Kuo C. W., Raju B. R., Yao C. F., Green Chem., 2010, 12(6), 1090—1096 |
| [21] | Chatterjee N., Pandit P., Halder S., Patra A., Maiti D. K., J. Org. Chem., 2008, 73(19), 7775—7778 |
| [22] | Richardson R. D., Wirth T., Angew. Chem. Int. Ed., 2006, 45(27), 4402—4404 |
| [23] | Dohi T., Kita Y.,Chem. Commun., 2009, (16), 2073—2085 |
| [24] | Uyanik M., Ishihara K.,Chem. Commun., 2009, (16), 2086—2099 |
| [25] | Ochiai M., Takeuchi Y., Katayama T., Sueda T., Miyamoto K., J. Am. Chem. Soc., 2005, 127(35), 12244—12245 |
| [26] | Fang Y. G., Xu C., Zhu M., Chem. J. Chinese Universities,2011, 32(12), 2782—2786 |
| (方应国, 徐翠, 朱敏. 高等学校化学学报, 2011, 32(12), 2782—2786) | |
| [27] | Zhu M., Li L., Zhang H., Xu D. M., Chem. J. Chinese Universities,2012, 33(9), 1995—1999 |
| (朱敏, 李立, 张慧, 徐冬梅. 高等学校化学学报, 2012, 33(9), 1995—1999) | |
| [28] | Zhu M., Jin J. C., Tong J. Y., Li Z. Y., Chem. J. Chinese Universities,2013, 34(2), 359—363 |
| (朱敏, 金建昌, 童建颖, 李志英. 高等学校化学学报, 2013, 34(2), 359—363) | |
| [29] | Yoshimura A., Middleton K. R., Todora A. D., Kastern B. J., Koski S. R., Maskaev A. V., Zhdankin V. V., Org. Lett., 2013, 15(15), 4010—4013 |
| [30] | Han L. Q., Zhang B. J., Xiang C. B., Yan J., Synthesis,2014, 46(2), 503—509 |
| [31] | Xiang C. B., Li T. T., Yan J., Synth. Commun., 2014, 44(5), 682—688 |
| [32] | Asensio G., Andreu C., Boix-Bernardini C., Mello R., Gonzalez-Nunez M. E., Org. Lett., 1999, 1(13), 2125—2128 |
| [33] | Yoshimura A., Zhu C. J., Middleton K. R., Todora A. D., Kastern B. J., Maskaev A. V, Zhdankin V. V., Chem. Commun., 2013, 49(17), 4800—4802 |
| [34] | Tokizane M., Sato K., Ohta T., Ito Y., Tetrahedron: Asymmetry,2008, 19(21), 2519—2528 |
| [35] | Kim B. H., Synth. Commun., 1987, 17(10), 1199—1206 |
| [36] | Osamu M., Hideo T., Masaichi L., Yoshikiyo U., Takeshi E.,J. Chem. Soc., Perkin Trans. 1, 1994, (4), 413—417 |
| [37] | Zlatopolskiy B. D., Kandler R., Kobus D., Mottaghyac F. M., Neumaier B., Chem. Commun., 2012, 48(57), 7134—7136 |
| [38] | Dohi T., Yamaoka N., Kita Y., Tetrahedron,2010, 66(31), 5775—5785 |
| [39] | Zefirov N. S., Zhdankin V. V., Makhon’kova G. V., Dan’kov Y. V., Koz’min A. S., J. Org. Chem., 1985, 50(11), 1872—1876 |
| [1] | GONG Yanxi, WANG Jianbing, CHAI Buyu, HAN Yuanchun, MA Yunfei, JIA Chaomin. Preparation of Potassium Doped g-C3N4 Thin Film Photoanode and Its Application in Photoelectrocatalytic Oxidation of Diclofenac Sodium in Water [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220005. |
| [2] | WANG Mingzhi, ZHENG Yanping, WENG Weizheng. Catalytic Methane Combustion over CeO2 Supported PdO and Ce1‒x Pd x O2‒δ Species [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210816. |
| [3] | CHEN Wangsong, LUO Lan, LIU Yuguang, ZHOU Hua, KONG Xianggui, LI Zhenhua, DUAN Haohong. Recent Progress in Photoelectrochemical H2 Production Coupled with Biomass-derived Alcohol/aldehyde Oxidation [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210683. |
| [4] | JIN Shaoqing, SUN Hongmin, YANG Weimin. Applications of Zeolite Catalysts in Chemical Industry [J]. Chem. J. Chinese Universities, 2021, 42(1): 217. |
| [5] | LI Xiao,XING Lisha,ZHAO Wanjun,WANG Yongzhao,ZHAO Yongxiang. Preparation and Characterization of Pd-Cu/hydroxyapatite Catalyst and Its Catalytic Performance for Room-temperature CO Oxidation in Humid Circumstances† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1600. |
| [6] | JIANG Yilan, YUAN Long, WANG Xiyang, HUANG Keke, FENG Shouhua. Effect of Defect Tuning on the Catalytic Behavior of Perovskite Structure Lanthanum Manganite† [J]. Chem. J. Chinese Universities, 2018, 39(3): 416. |
| [7] | SUN Kaifang, CAI Cheng, HOU Zongsheng, WANG Ying, REN Qizhi. Synthesis, Characterization and Catalytic Properties of Series of Water-soluble Sulfonated Porphyrins† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1117. |
| [8] | ZHANG Yuanyuan, LI Fenpei, LIU Xiangqing, CHEN Haiyan, LUO Ying, JING Lihong, LU Jiaxing, ZHANG Guirong. Methanol Electrocatalytic Oxidation on Pt/Poly(o-toluidine) Film/Activated Carbon Doped Graphite Carbon Paste Electrode† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2320. |
| [9] | ZHANG Jinshuai, YU Fengli, TAO Renqing, XIE Congxia, YUAN Bing, YU Shitao. Selective Oxidation of Cyclopentene to Glutaraldehyde Catalyzed by Heteropolyphosphatotungstate Ionic Liquids† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2248. |
| [10] | MA Yongjun, DING Jing, JIN Zhimei, TIE Zhenzhen, ZHOU Min. Inhibiting Effect of Chloride Ion for the Electro-oxidation Reaction of Four Organic Alcohols on a Chemically Modified Platinum Anode† [J]. Chem. J. Chinese Universities, 2015, 36(5): 864. |
| [11] | LUO Jinyuan, CHEN Linlin, WANG Yi, LI Hong. Fabrication and Performance of a Novel Visible Light-driven Fuel Cell Based on Photocatalytic Oxidation of Uric Acid by CdS Nanoparticles and Electrocatalytic Reduction of Oxygen by a Copper(Ⅱ) Complex† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2468. |
| [12] | ZHANG Ye, ZHOU Jiajia, WU Guisheng, MAO Dongsen, LU Guanzhong. Influence of the Surface Species over Co3O4 on the Formaldehyde Catalytic Oxidation Performance† [J]. Chem. J. Chinese Universities, 2014, 35(12): 2598. |
| [13] | DANG Guoju, WANG Miao, WANG Zhaoqing, LI Haiyan, ZHANG Quansheng. Electrocatalytic Oxidation of Dopamine at Graphene Modified Glass Carbon Electrode [J]. Chem. J. Chinese Universities, 2014, 35(12): 2680. |
| [14] | XIONG Ting, LIN Jianyun, SHANG Zhongjin, ZHANG Xiantu, LIN Xuan, TIAN Wei, ZHONG Qiling, REN Bin. Preparation of Pt Hollow Nanoparticles Using Ag as the Template and Electrocatalytic Performance for Methanol Oxidation† [J]. Chem. J. Chinese Universities, 2014, 35(11): 2460. |
| [15] | JIANG Yu-Liang, HAN Qiao-Rong, ZHOU Wen-Long, XU Zhu-Xiong, WANG Bing-Xiang. Synthseis and Antibacterial Activities of Some 3-(2-Hydroxy-4, 6-dimethoxyphenyl)-5-aryl-isoxazolines [J]. Chem. J. Chinese Universities, 2013, 34(9): 2120. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
